Simple Summary
One of the important efforts in the treatment of cancers is to achieve targeted drug delivery by nanocarriers to be more effective and reduce adverse effects. However, due to the adverse responses of nanocarriers in clinical trials due to the very weak EPR effects, doubts have been raised in this regard. In this study, an attempt has been made to take a critical look at EPR approaches to enable the convergence of previous papers and the EPR critics to reach an appropriate therapeutic path. Although the effectiveness of EPR is highly variable due to the complex microenvironment of the tumor, there is high hope for cancer treatment by describing new strategies to overcome the challenges of EPR effect. Furthermore, in this paper an attempt was made to provide a reliable path for future to develop cancer therapeutics based on EPR effect.
Abstract
The enhanced permeability and retention (EPR) effect in cancer treatment is one of the key mechanisms that enables drug accumulation at the tumor site. However, despite a plethora of virus/inorganic/organic-based nanocarriers designed to rely on the EPR effect to effectively target tumors, most have failed in the clinic. It seems that the non-compliance of research activities with clinical trials, goals unrelated to the EPR effect, and lack of awareness of the impact of solid tumor structure and interactions on the performance of drug nanocarriers have intensified this dissatisfaction. As such, the asymmetric growth and structural complexity of solid tumors, physicochemical properties of drug nanocarriers, EPR analytical combination tools, and EPR description goals should be considered to improve EPR-based cancer therapeutics. This review provides valuable insights into the limitations of the EPR effect in therapeutic efficacy and reports crucial perspectives on how the EPR effect can be modulated to improve the therapeutic effects of nanomedicine.
1. Introduction
Despite various approaches to control solid tumor growth, such as surgery, chemotherapy, radiation therapy, and thermotherapy, solid tumors are still the leading cause of death in cancer patients [1]. The need for better clinical outcomes in patients with cancer has led researchers to reconsider therapeutic strategies. To overcome these issues, the structural characteristics of solid tumors, drug nanocarrier transport in tumor vessels and the interstitium, selective drug delivery systems, and analytical tools should be carefully considered to develop cancer therapeutics [2,3]. Although it has long been thought that drug nanocarriers will facilitate cancer therapeutics by directing drugs to solid tumors, nanomedicine development has not yet achieved promising clinical outcomes. Numerous reports indicate that virus-/inorganic nanoparticles-/organic nanoparticles-based nanocarriers could improve the efficacy and safety of anticancer drugs through potential targeting, mitigated drug release in non-target tissues, and pH-sensitive drug release in the tumor microenvironment (TME) [4,5,6,7]. Tumor vascular permeability and the enhanced permeability and retention (EPR) mechanism in macromolecular drug delivery to solid tumors have been reported as successful strategies for cancer therapeutics [8,9,10]. However, the low EPR effect in human solid tumors [8], poor tumor extravasation [11] and infiltration [11], and a diversity in the tumor-immune microenvironment (TIME) [12] has resulted in the full realization of EPR-mediated cancer therapeutics [13].
Today, simulation models have reported the dynamics of tumor vessel cooption and have promoted the treatment of solid tumors [14,15]. However, different results were obtained from different strategies aimed at investigating EPR-mediated drug delivery to cancer cells. Different results can be related to the animal species used, the extent of intratumoral ECM content, an unorganized tumor vasculature, the diverse properties of drug nanocarriers, variable fluid velocities, the types of malignant cells in the TIME despite having the exact origin, and the heterogeneous tissues of a solid tumor [16,17,18,19,20,21]. Despite the certainty of the EPR-mediated tumor targeting and cancer nanomedicine treatment efficacy [22], the negative side of the EPR effect in cancer nano-therapy is derived from the heterogeneous microvascular networks of solid tumors, which results in heterogeneous distributions of nanomedicines. Therefore, a full exploration of the EPR effect in solid tumors and issues associated with dynamics, such as pathophysiological and pathoanatomic characteristics, drug nanocarrier formulations, physicochemical factors, and EPR analytical methodology, can provide useful information in the development of EPR-based therapeutics.
In this regard, Nichols and Bae [9] discussed that the EPR approach in the therapeutic activities of animal tumors is more prominent than in human tumors due to significant differences in tumor structure and function [9]. Furthermore, Maeda, et al. [23] reported that the use of EPR in the imaging process based on the presence of nano-probes and nanocarriers in the tumor for a short time (a few minutes) is more appropriate than for therapeutic activities. However, they showed that the EPR effect varies based on the physicochemical properties of the nanocarriers [23]. Moreover, Danhier [17] discussed some factors that result in EPR pitfalls in clinical trials, the future of EPR, and the required changes in drug nanocarriers. Recently, Wu [22] reported that despite the meager success of the EPR effect in clinical trials, it can be enhanced by regulating the physicochemical properties of nanocarriers, treatment duration, and the type of anti-cancer agents. At the same time, Izci, et al. [24] explained the advantages and challenges of EPR, the EPR enhancement mechanisms, and the study of independent EPR-based drug delivery strategies.
Although these review articles have comprehensively discussed the EPR-based therapeutics of solid tumors, people have not surveyed EPR-based tumor targeting with a focus on fluid flow and convection/diffusion mechanisms. Therefore, this review discusses changes in structure as the tumor grows and how fluid flow, convection/diffusion, and the physicochemical properties of nanocarriers affect the EPR-based transport of nanocarriers in solid tumors. Additionally, we discuss the need for improved novelty methodologies in analyzing the EPR effect.
2. Effective Vascular Structures for EPR in Solid Tumors
Solid tumors, unlike hematologic cancers, have a structure similar to normal tissue, including the parenchyma, which contains neoplastic cells and the surrounding stroma [25,26]. The stroma of solid tumors is critical for cancer cell survival and metabolism through extracellular matrix components and blood vessels [27,28]. Despite the similarity of the main components of the stromal extracellular matrix of solid tumors and normal tissue, solid tumors have different stromal patterns based on early- or late-forming blood vessels, which play an important role in therapeutic efficacy [29,30]. For example, the highest ratio of stroma to tumor cells is found in gastric and pancreatic cancer tissues [31,32], while medullary breast carcinoma and lymphomas have the lowest stromal content [27]. Hence, solid tumor therapy with one type of drug-based nanoplatform could not result in comparable clinical efficacy between these tumor types. Changes in the heterogeneity and aggressiveness of solid tumors are derived from the extent of interactions between tumor cell populations [33]. Targeting stromal cell-mediated pro-angiogenic signals in the TME is a key therapeutic strategy. The heterogeneity of angiogenesis and blood vessel maturation in human tumors can be regarded as a critical factor, resulting in conflicting outcomes in therapeutic studies. Based on the growth of solid tumors, it is possible to observe mature and immature vessels within the same tumor, resulting in different EPR effects. In fact, six groups of vessels in solid tumors can be observed with various sizes and shapes [34] (Figure 1A), which include (1) mother vessels (very large, thin-walled, permeable, with weak pericytes), (2) glomeruli microvascular (poor organization of proliferated endothelial cells, pericytes, and basement membranes), (3) capillaries (including primary and microvascular glomeruloid vessels), (4) vascular malformations (often with asymmetric coverage of smooth muscle cells and or tissue), (5) feeding arteries (large vessels with complete capillary structure and often torturous), and (6) drainage veins (very large with complete capillary structure). Late detection of solid tumors in humans, unlike in model animals in which cancer is screened in the early stages, can also increase vascular growth, maturation, and diversity, which can serve as potential barriers to drug delivery in solid tumors [24]. It is well known that biological sex and vascular development affect vascular architecture and permeability [35]. Therefore, the impairment of newly formed blood vessels (types 1 to 4) during tumor angiogenesis could not lead to improved efficacy of conventional anticancer therapies. Moreover, during blood vessel maturation in solid tumors, the vessels show a variety of complexities that affect fluid flow resistance and may even generate reversed fluid flows [36]. Additionally, the asymmetric vascular growth, vasoconstriction, and solid stress found in desmoplastic tumors create altered physical forces around the blood vessels [16,37,38] (Figure 1B), which induce low red blood cell fluidity and increased flow resistance, which can induce hypoxia and acidosis. Furthermore, angiogenesis and vascular remodeling in normal tissues follow the law of diameter reduction in smaller branches, which is not seen in solid tumors [39]. Therefore, the heterogeneity of solid tumor microvascular networks could result in alterations in vascular permeability and interstitial fluid flow, which act as key biological barriers to cancer drug delivery and efficacy in solid tumors. The overgrowth of solid tumors and a fibrotic response with increased ECM deposition lead to the introduction of mechanical barriers, resulting in altered interstitial fluid flows [40,41]. Heterogeneity of vascular fluid flow due to physical barriers due to increased cell proliferation in some solid tumor areas can reduce the efficiency of drug nanocarriers, especially in dimensions larger than 100 nm, intratumoral transport, and the EPR effect [20,41].
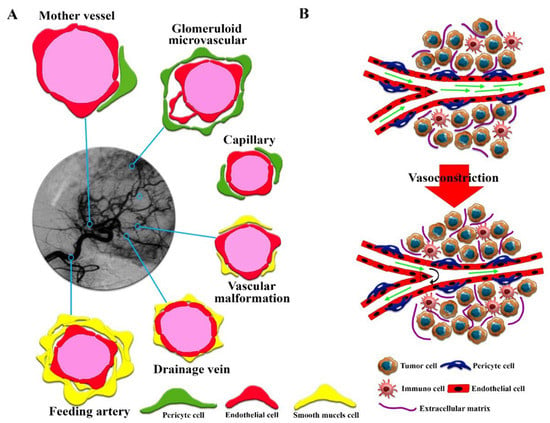
Figure 1.
(A) Abnormally patterned vascular vessels in solid tumors. Six types of blood vessels with different characteristics can be identified. (B) A description of the overall structure of solid tumors and the solid stress phenomenon caused by tumor tissue growth that reduces fluid flow and even reverses fluid flow in the tumor.
Regardless of the stages of vascular formation in solid tumors, which have been studied by Fang et al. [42], exploring the molecular characteristics of tumor vessels can provide a more accurate understanding of the active or inactive delivery of drug nanocarriers. Unlike normal vascular architecture with a homogeneous distribution of endothelial cells surrounded by pericytes, the heterogeneous vasculature of a solid tumor results in different permeability models [43]. This morphological abnormality not only provides greater vascular permeability in solid tumors but also leads to increased interstitial pressure in the tumor tissue, which generates multiple distribution patterns of drug nanocarriers [44]. It seems that determining the balance between vascular resistance and vascular leakage of solid tumors can be used as a basis for drug delivery mediated by the EPR effect to increase therapeutic efficacy in interstitial fluid. Basically, momentary changes in fluid flow with vascular resistance, and vascular leakage with vascular wall pore diameters ranging from 50–100 nm (tumor vessels with poor permeability) to 500–1000 nm (high permeability vessels) are explained. Overall, the EPR effect is expected to be more effective during the early phases of cancer growth or peritumors than late phases due to improved leakage behavior and the rate of fluid flow. This result could advance future therapeutics for solid tumors with a different approach.
3. EPR-Mediated Drug Delivery to Solid Tumors
In recent years, various perspectives on anticancer nanomedicine have been developed based on nanocarrier intratumoral transport pathways [22,45,46,47]. The structural complexities of solid tumors that affect drug delivery pathways remain unclear [15]. As such, the blood flow complexities mentioned in Section 2, such as obstruction of fluid flow in the deeper parts of the tumor, tumor heterogeneity due to different types of vessels that cause interstitial fluid pressure, diversity of ECM of solid tumors, and lack of lymphatic drainage vessels, have caused a variety of therapeutic challenges based on the intratumoral transport and drug efficacy [20,25,48]. Regardless of the method and location of the injection, the drug nanocarriers introduced in vivo are distributed intratumorally using two basic concepts. Convection is the transfer of drugs by a moving fluid, such as blood or interstitial fluid, in which case the distance between the fluid and the cancer cell is critical [49], and diffusion is proportional to the concentration gradient, which is effective at short intervals of drug injection) [50]. Meanwhile, the intratumoral transport of drug nanocarriers in solid tumors is a function of both phenomena, based on fluid flow in the solid tumor, which strongly depends on the tumor size [51]. In addition, diffusion is more prevalent in the case of smaller-sized drug nanocarriers in small vessels as well as in the intercellular spaces [52,53] similar to the pulmonary tissue vessels [54]. However, convection is more prevalent in the case of larger drug nanocarriers present in a solid tumor core [55] and large vessels [53,56]. In this regard, it has been determined that the specific entry of therapeutic payloads into tumors is inhibited when diffusion is involved in the intratumoral transport of nanocarriers due to the increase of interstitial pressure in solid tumors [57,58,59]. Hence, diffusion can sometimes be considered an obstacle to yielding novel, effective therapies for solid tumors. Furthermore, if the interstitial fluid pressure stops as a function of tumor solid stress [60], or fluid flow in small vessels is reduced [61], EPR-mediated drug delivery could fail. Thus, the changes in fluid velocity in different parts of solid tumors based on their heterogeneous development and progression can be considered a very important indicator of the efficacy of EPR-mediated drug delivery in solid tumors. As the role of interstitial fluid pressure [59] and tumor solid stress [62] in modulating fluid velocity is very important, it is expected that the intratumoral transport of nanocarriers and the EPR effect in the peritumoral areas will be greater than that of the core of solid tumors. In addition, the change in the direction of fluid flow in solid tumors [63,64] due to the irregular structure of vessels can result in the mitigation of convection/diffusion-enhanced delivery of drugs for the treatment of solid tumors. By reversing the flow, the viscosity of the fluid in solid tumors is expected to be increased several times that of normal tissue due to an increase in hematocrit [65]. Therefore, this phenomenon can effectively manipulate convection and diffusion events in interstitial transport in solid tumors mediated by EPR.
Tumor vessel development and maturation can also lead to the formation of an integrated structure of endothelial cells and the presence of pericytes, which changes the mechanism of intratumoral transport of drug nanocarriers due to an alteration in the ratios of convection/diffusion status [58,64]. Although reducing the number and size of vascular wall pores moderates the passive transport of drug nanocarriers into the interstitial space, computational models predict that even without vessel pores/gaps due to decreased interstitial fluid retention and reduced leakage into vessel lumen with higher uptake through intercellular and linked vesicles effectively increases the tumor delivery of macromolecular drugs based on the EPR effect (Figure 2) [64].
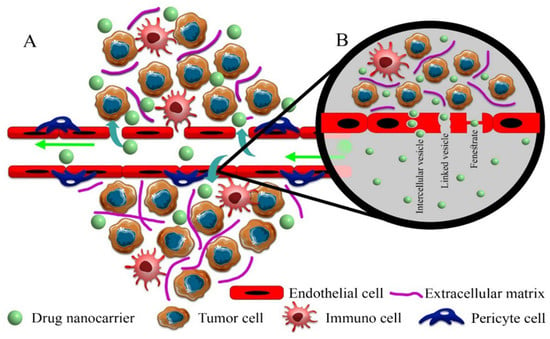
Figure 2.
Proposed pathways for drug nanocarriers to enter solid tumors. (A) Paracellular process: in this pathway, drug nanocarriers passively enter the extracellular space of solid tumors through intercellular gaps with dimensions up to 2000 nm, which are very important in the EPR effect. (B) Transcellular process: drug nanocarriers in mature vessels without common gaps in solid tumors actively enter the extracellular space of the solid tumor through vesicles (endocytosis-exocytosis) and pores. The fenestrate pathway does not have a complete incision, and a diaphragm separates the internal space from the lumen of the vessel. In the linked vesicle path, interconnected vesicles cause the transfer of drug nanocarriers.
However, due to capillary growth and the reduction of leaky regions of the tumor vasculature, the passive transport of drug nanocarriers is significantly reduced. Increasing the thickness and density between the vascular lumen and the interstitial space due to vascular maturation along with greater ECM and collagen accumulation can dramatically reduce the potential tumor-targeted drug delivery based on EPR-effect [66]. Therefore, an updated view of the transport of drug nanocarriers based on the EPR effect due to the heterogeneous vasculature of a solid tumor is needed.
4. Physicochemical Properties of Nanoscales Affecting EPR
Numerous reports have indicated the effect of the size, shape, and surface charge of drug nanocarriers on the transport of drugs based on the EPR effect in cancer [67,68,69,70]. However, most accumulation, retention, and drug transfer kinetics in solid tumors have been investigated in animal models without accessing human data. Therefore, despite various reports on the therapeutic advantages of drug nanocarriers for the treatment of tumors (Table 1), significant advances have still not been observed in clinical trials. Of course, the use of other treatment modalities, such as photothermal therapy [71], photodynamic therapy [72], magnetic field therapy [73], sonodynamic therapy [74], has been able to boost the efficacy of drug nanocarriers in tumor therapy. However, the heterogeneity of solid tumors and the effect on EPR-mediated passive drug targeting result in a non-uniform distribution of the drug in solid tumors [75], which increases the likelihood of local cancer recurrence.

Table 1.
Summary of in vivo pharmacokinetic (PK) studies of conventional chemotherapeutics (CC) and targeted anticancer agents (TAA).
4.1. Size of Drug Nanocarriers
Drug delivery through the EPR effect is related to the biocompatibility of drugs and their molecular size to escape renal clearance. Reports indicate that drugs with molecular weights of over 40 kDa can increase the EPR effect on solid tumors and other organs by increasing their shelf life in the blood. Therefore, in order to increase the shelf life of drugs less than 40 kDa, the combination of drugs with polymers or serum proteins, such as albumin, is recommended [7]. Nevertheless, despite the direct and uniform effect of drug molecular size and drug nanocarrier size on EPR via increasing shelf life more than a few hours, nanocarrier size directly affects EPR by changing their transport mechanisms to the interstitial space based on fluid velocity, and inducing accumulation in the tumor site by reducing leakage into the vessel lumen [7,81]. If the fluctuations in fluid flow in the vessels are minimized, small-sized drug nanocarriers (20–70 nm) tend to penetrate further into solid tumor cores compared to larger ones [67,82]. Thus, the size of the spherical nanocarriers plays a key role in EPR-based intracellular targeted delivery of drugs. Therefore, the transport of smaller-sized and larger-sized spherical nanocarriers becomes a function of convection and diffusion, respectively [53]. Based on this fact, in the case of larger nanocarriers, it is necessary to accelerate the fluid velocity to overcome interstitial pressure, which prevents deep penetration of nanocarriers into tumor tissue [83]. However, by using the ligand-receptor binding approach in targeting nanocarriers, the effect of nanoparticle size and shape on EPR-based drug delivery can be moderated by enhancing the penetration of nanocarriers into tumors without affecting fluid flow [24]. Hence, the success of EPR-based nanocarriers in the interstitial space is strongly dependent on the size of the nano-based platform and interstitial fluid flow velocity. In addition to vascular flows, the dense matrix in the interstitial space of tumors [84] dramatically reduced the penetration of larger-sized drug nanocarriers (150–200 nm) compared to smaller-sized drug counterparts (20–70 nm) [85]. In addition, drug nanocarriers below 10 nm are not potential candidates for efficient tumor-targeted drug delivery based on the EPR effect in solid tumors due to rapid clearance by kidneys and macrophages located in the liver, lung, and pancreas [86].
As a result, to improve the EPR effect in solid tumors to predict the therapeutic efficacy of nano-based drugs, the lack of rapid clearance, increased permeability in solid tumors, and the ability to use combined therapeutic modalities should be considered.
4.2. Shape and Surface Charge of the Drug Nanocarriers
Other factors, including the shape and surface charge of drug nanocarriers based on changing the nature of transport, improving shelf life, and intracellular permeability, can affect the EPR-based therapeutics of solid tumors. It has been observed that non-spherical nanocarriers can have higher diffusivity than spherical nanocarriers as a result of increased interactions with the vessel wall through improved radial thrust force deriving from rapid pressure changes [7,87,88]. Thus, the fluid provided a higher radial thrust force by applying multilateral force to different surfaces of non-spherical nanocarriers (Figure 3B). In contrast, spherical drug nanocarriers show convection-enhanced delivery of targeted drug penetration into solid tumors (Figure 3A) [89]. Based on radial thrust, Chauhan, et al. [90] reported that rod nanocarriers were retained 4 times higher than spherical nanocarriers in solid tumors (Figure 3B). In addition, in a 3D spheroid model, it was recognized that cylindrical nanocarriers with 100 nm height and 325 nm diameter have maximum delivery to solid tumors and a more uniform penetration pattern than their nanorod counterparts, while much smaller non-spherical nanocarriers penetrated deeper and more uniformly compared to larger non-spherical nanocarriers [91]. Therefore, the deformation of nanocarriers based on diffusion and convection results in heterogeneous distributions of nanomedicines in solid tumors. However, the combination of the size and shape factors of drug nanocarriers can exhibit different responses based on the various behaviors of drug nanocarriers with respect to interstitial fluid flow in tumors. For instance, it has been reported that spherical drug nanocarriers with dimensions less than 70 nm have a higher radial thrust force than spherical nanocarriers with dimensions greater than 130 nm [92]. Furthermore, non-spherical nanocarriers have a longer half-life due to less uptake by macrophages, which can increase the probability of deep penetration into the tumor [93]. The non-spherical shape of nanocarriers also improves drug-mediated endocytosis due to increased interactions between endothelial cells and drug nanocarriers [88]. Together, this will result in a more effective penetration of anticancer drugs through tumor tissue. However, due to the negative charge of vessels [94], EPR-based drug delivery by nanocarriers can be manipulated by using a positive charge on nanocarriers through modulation of radial thrust [95] based on electrostatic interactions. For example, Campbell, et al. [96] and Krasnici, et al. [97] showed that liposomal nanocarriers with dimensions of ~150 nm and positively charged surface area could accumulate 1.5 and 7 times higher than that of anionic and highly anionic liposomal nanocarriers in the solid tumor, respectively. However, their results showed that cationic liposomes did not potentially penetrate the intratumoral space compared to the other two samples. Therefore, the possibility of low penetration of drug nanocarriers in the interstitial space due to the potential interaction of positively charged nanocarriers with the vascular wall is also conceivable [98]. Nevertheless, the application of a positive charge faces serious challenges due to its high toxicity to non-target tissues and rapid clearance through opsonization [99,100]. The use of chemical surface modification approaches for drug nanocarriers, such as the use of compounds that are positively charged in the acidic environment of the tumor [101], can improve the surface charge drawbacks of nanocarriers.
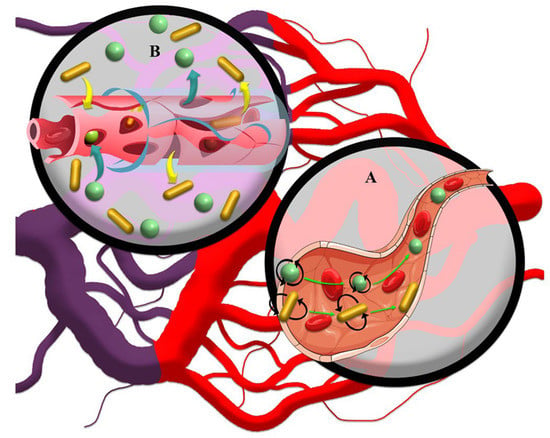
Figure 3.
(A) Regardless of size, spherical drug nanocarriers tend to penetrate the solid tumor core based on the convection current. The forces exerted on the moving rod-like drug nanocarriers cause them to marginalize and further attach to the vessel wall and incomplete vessel structure, which has many gaps for leakage of drug nanocarriers. (B) Based on high interstitial pressure in solid tumors, rod-like drug nanocarriers show a further EPR effect in the interstitial space than spherical drug nanocarriers due to their lower tendency to move.
5. Challenges and Common Approaches to EPR Analysis
To achieve improved EPR-based drug delivery in solid tumors by nanocarriers, strategies such as the development of potential drug nanocarriers, injection time periods of drug nanocarriers, imaging of solid tumors, evaluation of pharmacokinetic and pharmacodynamic parameters, and qualitative and quantitative analysis of cancer specimens should be considered (Figure 4). The most common imaging techniques used during the treatment process are MRI, SPECT/CT and PET/CT imaging techniques in humans and sometimes in animal models and fluorescence imaging in in vitro models. Staining methods for microscopic observations and electron microscopy in qualitative techniques and atomic absorption, magnetic separation, flow cytometry, chromatography, and centrifuges in quantitative methods are the most common analytical strategies. The inconsistency of evaluation methods between human and animal models and between in vitro and in vivo methods has made it difficult and sometimes impossible to compare outcomes.
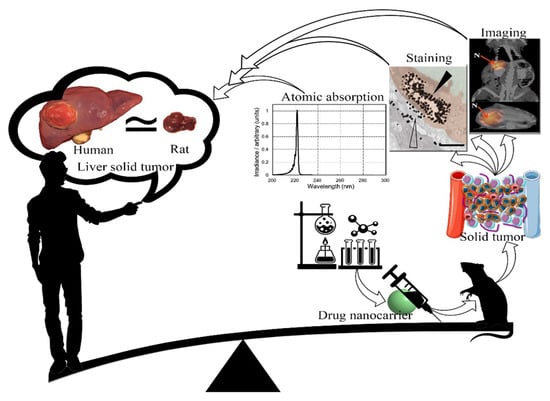
Figure 4.
Schematic view of the production process of drug nanocarriers for the treatment of solid tumors in animal models and their generalization to humans. It seems that, due to dissatisfaction with the research achievements in the treatment of solid tumors mediated by an EPR effect, it is necessary to update the experimental strategies in this field.
By reviewing the literature, we can see that the main challenge in EPR-based trial approaches is the lack of standardization of animal models used in clinical assays for humans. For instance, humans simultaneously face challenges such as metabolic problems (obesity, diabetes, stress, high cholesterol or blood pressure, and age-related vascular problems), an unhealthy diet, and stressful lifestyles that are not present in animal models. Additionally, the different sizes of animals, especially rodents compared to humans, alter the pharmacokinetic and pharmacodynamic processes, which is often not considered. The resulting distribution, stability, and retention of drug nanocarriers are closely related to the above parameters. Moreover, tumor induction in mice with intensified growth patterns and extraordinarily large sizes relative to body mass compared to humans can alter EPR results by altering tumor cell population, tumor spatial structures, and intrinsic difference in fluid flow [102]. The rate of blood flow in mouse tissues is approximately 800 times lower than that of humans. For example, the blood flow in mouse C3 and human lymphosarcoma tumors is 5.4 mL/100 g per min [103] and 40–64 mL/100 g per min [104], respectively. Since tumor blood flow affects the penetration of nanocarriers and the delivery of EPR-based antitumor drugs based on the slower blood flow rate in mice than in humans, a more appropriate situation for the treatment of mouse tumors is conceivable. Nonetheless, in many research activities to investigate the EPR effect in solid tumors, this factor has been neglected, and the best solution is to use animal models with more similar body weights as humans.
The second error that should be investigated is the lack of standardized information on the injection times of drug nanocarriers and duration of therapy, i.e., the treatment schedule, without adopting it to human conditions. Although the use of drug nanocarriers is modified based on the weight to match the effects of the drug in mice and humans, the injection periods and duration of treatment used in research activities generally depend on the durability of the drug and tumor size. These factors result in fundamental differences in the rate of clearance of drug nanocarriers between humans and animals based on fluid velocity, clearance, and solid tumor size. Therefore, due to the different stability of drug nanocarriers in different species, it is expected that the process of evaluating EPR effects in animal and human solid tumors will differ.
Another important challenge is the lack of evaluation of the net efficacy of the EPR effect on the intratumoral accumulation of drug nanocarriers. EPR-based assays are generally performed based on the administration of a specific dose of drug nanocarriers intravenously or orally and the analysis of subsequent retention in tumor tissues. Based on the clearance challenges mentioned in the previous sections, it seems that the administration of drug nanocarriers through tumor-associated arteries can truly reveal the net retention efficiency in solid tumors. For this strategy, the direct injection of drug nanocarriers into the tumor-associated arteries by imaging-guided catheter placement can be used. Explaining the net efficacy of drug nanocarriers in increasing EPR could influence the opinion of researchers to use a variety of drug nanocarriers to treat solid tumors by modifying the production process. Nevertheless, few drug nanocarriers have been reported for arterial direct injection into solid tumors [105,106].
Other obvious errors in EPR detection processes include the method of collecting samples based on the structural status of solid tumors and the accuracy of sample preparation. As mentioned above, the penetration efficacy of drug nanocarriers in the solid tumor core is lower than that of the peritumoral region. Thus, it is more desirable in therapeutic approaches to make a detailed distinction between different areas of the tumor in the EPR examination to explain the effect of drug nanocarriers on therapeutic pathways in a more accurate and reproducible way. In addition, it seems that complete blood withdrawal should be performed to prevent the report of drug nanocarriers in the blood, as nanocarriers are retained from solid tumor samples.
Although the mentioned challenges in controlling the concentration of drug nanocarriers in solid tumors of animal models can be relaxed due to ethical sensitivities, possible toxicities, high cost, and the complexity of experiments, the use of the above methods in clinical practice faces serious challenges.
6. Convergence of Theories to Reduce Conceptual Shortcomings in EPR
For almost 45 years, EPR theory has been pursued by researchers as an important and reliable effect in cancer therapy. However, in recent years, a group of researchers have hypothesized the ineffectiveness of the EPR effect in cancer therapy [17]. They believe that the different outcomes in cancer treatment derived from animal and human models are a challenge to EPR effect-based cancer therapeutics due to differences in body structure and solid tumor morphologies. At first glance, based on Section 2 and Section 3, this hypothesis is logical. However, could the potential pitfall for the improvement of tumor uptake by nanocarriers be considered a criterion for accepting/rejecting the EPR effect hypothesis? Could the previous results of EPR effect-based tumor targeting and cancer nanomedicine treatment efficacy be advanced with further strategies? The results of several studies demonstrate that the retention of drug nanocarriers by increasing the imbalance in the inflow (through vascular wall disorders) and fluid output (due to defects in the tumor lymphatic system) increases the killing of tumor cells in solid tumors [9,107]. Although the lack of reliable human data has challenged the EPR effect-based anticancer nanomedicine drug targeting, assuming that clinical outcomes are always a function of solid tumor heterogeneity [108], physicochemical features of drug nanocarriers [109], and therapeutic designs with combined modalities (photo-therapy, thermal-therapy, magnetic-therapy, etc.) [24], it is not possible to precisely attribute the rates of treatment failure to one of the existing therapeutic factors. Nevertheless, the convergence of the different theories can serve as a platform for optimizing cancer treatment strategies.
The first new cancer treatment strategy based on the EPR effect is to change the perspective of EPR effect-based therapeutic approaches. In the treatment process, the transfer of drug nanocarriers from the cancer stroma to malignant cells is essential [110]. As seen in several papers, the high accumulation of drug nanocarriers in normal tissues can lead to undesirable toxicity through drug release and catalytic activity [111]. With this new perspective, it can be hoped that the actual accumulation of drug nanocarriers into solid tumors will result in a promising therapeutic target for cancer treatment, especially by enhanced tumor penetration of the drug. Therefore, to enhance therapeutic activities in solid tumors, accepting the EPR effect, along with recruiting combined therapeutic modalities to trigger the penetration of drug nanocarriers into malignant cells, can result in improved nano-based precision carriers for targeting therapeutic and diagnostic agents in the treatment of cancer. However, the accumulation of drug nanocarriers in solid tumors, especially in peritumoral areas, could be an EPR-independent strategy. Therefore, based on the nature of the nanocarriers and drug availability, a new delivery nanoplatform could be developed for tracking and treating poorly vascularized solid tumors.
Another strategy to modulate EPR is the manipulation of drug nanocarriers through surface charge modifications, cluster size, flexible shape, and surface coatings for greater stability, with or without external inducers, to augment the permeability of tumor-associated vessels [6,112,113]. Modifications applied to drug nanocarriers to enhance the EPR effect usually do not result in potential active targeting. Since the effect of EPR indicates a high accumulation of targeted nanocarriers in the stromal tissue of solid tumors, overtaking the inflow on outflow of drug nanocarriers can lead to inactive or semi-active accumulation of nanocarriers in solid tumors using the EPR effect.
Competition is also one of the most important strategies in the development of therapeutic nanomedicine for solid tumors. Indeed, the main competitors of EPR-based treatment of solid tumors are the liver, spleen, lung, and kidney. Therefore, reducing the clearance of drug nanocarriers by modulating the physicochemical properties of nanocarriers matched to the TME of solid tumors can improve EPR-based tumor targeting and cancer nanomedicine treatment efficacy. As mentioned in Section 3, altering the physicochemical properties of drug nanocarriers can alter penetration into tumor tissue. For example, binding molecules or coatings of drug nanocarriers can modulate the renal filtration rate [114], or natural and synthetic polymers can be used to minimize uptake by the liver [115]. On the other hand, the different morphological structures of solid tumors are key factors in designing and developing potential targeting drug nanocarriers. This means that EPR effect-based drug delivery is much more effective in primary tumors with vascular structures 1 to 4 than in mature tumors with vascular structures 5 to 6 [116]. Therefore, quantifying the morphological structures of solid tumors by various methods, such as imaging and histopathology, can highlight the ability of the EPR effect to improve the efficacy of anticancer nanomedicine. For instance, it has been determined that in micro-metastatic tumor cell clusters the possibility of EPR effect-based drug delivery is much lower than that of other tumors [117].
Standardization of the methodology for analyzing the EPR effect in solid tumors can enable researchers to develop the function of EPR effect-based treatment of solid tumors. According to Section 2 and Section 5, exploring the morphology of solid tumors and their development can provide useful information about the EPR effect-based tumor delivery of nanodrugs, intracellular infiltration, cancer cell death, and changes in the physiological behavior of the tumor. It should be noted that the main cause of EPR effect-based dependent or independent treatment of solid tumors is due to the intratumoral morphological heterogeneity of solid tumors and the different methodologies used in EPR effect analysis. Therefore, due to the presence of different methodologies, along with the variable structure of the solid tumor, reports on the EPR effect-based treatment of tumors are conflicting.
The timetable of treatment interventions is a critical challenge that can limit the EPR effect on the treatment of solid tumors. The review of Park, Choi, Chang, Um, Ryu, and Kwon [46] confirms the alliance of the EPR effect and the reduction of hypoxia levels in solid tumors based on greater oxygen permeability using drug nanocarriers [118]. Reducing the level of hypoxia by the EPR effect increases the possibility of the potential treatment of solid tumors [119,120]. However, different responses to solid tumors have been observed with increasing oxygen levels [121]. Furthermore, due to the lack of sufficient information on the relationship between physiological changes in solid tumors with decreasing hypoxia and metastatic status, the role of the EPR effect in macromolecular therapeutics is still unknown in detail [122,123].
7. Conclusions
Although therapeutic strategies used in solid tumors based on the EPR effect in animal activities are more successful than in human activities, a successful EPR-based treatment process can be established by increasing the structural insight of solid tumors in patients, changing the methods of manufacturing drug nanocarriers, and improving EPR analysis approaches. Finally, we emphasize that addressing one-dimensional issues rather than multidimensional concepts eliminates the possibility of integrating factors that affect the treatment of solid tumors. This eventually leads to more expensive and sophisticated approaches that divert public access from promising treatments. The current effort is to overcome the misconceptions of traditional EPR-related nanomedicine with the convergence of different ideas.
Author Contributions
M.S., W.C.C., A.A., R.T., H.M., S.S., S.H.B., Z.E. and M.A. conceptualized, conducted the bibliographic search, and wrote the manuscript. J.P.G., M.F. and T.L.M.t.H. designed, supervised, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nayak, P.P.; Narayanan, A.; Badekila, A.K.; Kini, S. Nanomedicine in cancer clinics: Are we there yet? Curr. Pathobiol. Rep. 2021, 9, 43–55. [Google Scholar] [CrossRef]
- Desa, D.E. Understanding the Role of Collagen Fiber Internal Structure in Solid Tumor Metastasis and Chemoresistance Using Second-harmonic Generation Imaging; University of Rochester: Rochester, NY, USA, 2021. [Google Scholar]
- Peng, C.; Yu, M.; Hsieh, J.T.; Kapur, P.; Zheng, J. Correlating Anticancer Drug Delivery Efficiency with Vascular Permeability of Renal Clearable Versus Non-renal Clearable Nanocarriers. Angew. Chem. 2019, 131, 12204–12208. [Google Scholar] [CrossRef]
- Zheng, Y.; Hasan, A.; Babadaei, M.M.N.; Behzadi, E.; Nouri, M.; Sharifi, M.; Falahati, M. Exosomes: Multiple-targeted multifunctional biological nanoparticles in the diagnosis, drug delivery, and imaging of cancer cells. Biomed. Pharmacother. 2020, 129, 110442. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; You, Y.; Yang, C.; Niu, Y.; Zhang, X. Numerical simulation of transport and adhesion of thermogenic nano-carriers in microvessels. Soft Matter 2020, 16, 10345–10357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Lin, R.; Li, H.J.; He, W.l.; Du, J.Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1519. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, C.; Fan, W.; Jiang, H.; Xiang, J.; Qiu, N.; Piao, Y.; Xie, T.; Luo, Y.; Li, Z. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials 2020, 240, 119902. [Google Scholar] [CrossRef]
- Derks, S.; de Klerk, L.; Xu, X.; Fleitas, T.; Liu, K.; Liu, Y.; Dietlein, F.; Margolis, C.; Chiaravalli, A.; Da Silva, A. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann. Oncol. 2020, 31, 1011–1020. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Sefidgar, M.; Soltani, M.; Raahemifar, K.; Sadeghi, M.; Bazmara, H.; Bazargan, M.; Mousavi Naeenian, M. Numerical modeling of drug delivery in a dynamic solid tumor microvasculature. Microvasc. Res. 2015, 99, 43–56. [Google Scholar] [CrossRef]
- Voutouri, C.; Kirkpatrick, N.D.; Chung, E.; Mpekris, F.; Baish, J.W.; Munn, L.L.; Fukumura, D.; Stylianopoulos, T.; Jain, R.K. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc. Natl. Acad. Sci. USA 2019, 116, 2662–2671. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing function of tumor vessels: Progress, opportunities, and challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T. Recent progress of nanocarrier-based therapy for solid malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef]
- Jin, Z.-H. Oscillatory interstitial fluid pressure and velocity in a solid tumor with partial surface fluid leakage. Microvasc. Res. 2021, 133, 104097. [Google Scholar] [CrossRef]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug delivery systems modulate tumor vessels to increase the enhanced permeability and retention effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Falahati, M.; Sharifi, M.; Ten Hagen, T.L. Explaining chemical clues of metal organic framework-nanozyme nano-/micro-motors in targeted treatment of cancers: Benchmarks and challenges. J. Nanobiotechnol. 2022, 20, 153. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, J.; Li, Q.; Li, L.; Jia, Y.; Geng, F.; Zhou, J.; Yin, T. Tumor microenvironment remodeling-based penetration strategies to amplify nanodrug accessibility to tumor parenchyma. Adv. Drug Deliv. Rev. 2021, 172, 80–103. [Google Scholar] [CrossRef]
- Sharifi, M.; Bai, Q.; Babadaei, M.M.N.; Chowdhury, F.; Hassan, M.; Taghizadeh, A.; Derakhshankhah, H.; Khan, S.; Hasan, A.; Falahati, M. 3D bioprinting of engineered breast cancer constructs for personalized and targeted cancer therapy. J. Control. Release 2021, 333, 91–106. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumor stroma, tumor blood vessels, and antiangiogenesis therapy. Cancer J. 2015, 21, 237–243. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, A.; Attar, F.; Babadaei, M.M.N.; Zeinabad, H.A.; Salehi, M.; Alizadeh, M.; Hassan, M.; Derakhshankhah, H.; Hamblin, M.R.; et al. Diagnostic and drug release systems based on microneedle arrays in breast cancer therapy. J. Control. Release 2021, 338, 341–357. [Google Scholar] [CrossRef]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954. [Google Scholar] [CrossRef] [Green Version]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer associated fibroblasts: The architects of stroma remodeling. Proteomics 2018, 18, 1700167. [Google Scholar] [CrossRef]
- Van Pelt, G.W.; Sandberg, T.P.; Morreau, H.; Gelderblom, H.; van Krieken, J.H.J.M.; Tollenaar, R.A.E.M.; Mesker, W.E. The tumour–stroma ratio in colon cancer: The biological role and its prognostic impact. Histopathology 2018, 73, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.; Burri, D.; Le Sueur, C.; Lemant, J.; Viossat, Y.; Kather, J.N.; Beerenwinkel, N. Spatial structure governs the mode of tumour evolution. Nat. Ecol. Evol. 2022, 6, 207–217. [Google Scholar] [CrossRef]
- Lin, F.; Shelton, S.E.; Espíndola, D.; Rojas, J.D.; Pinton, G.; Dayton, P.A. 3-D ultrasound localization microscopy for identifying microvascular morphology features of tumor angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics 2017, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.T.; Shirazi, J.; Comber, E.M.; Eschenburg, C.; Gleghorn, J.P. Fabrication of centimeter-scale and geometrically arbitrary vascular networks using in vitro self-assembly. Biomaterials 2019, 189, 37–47. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef]
- Rieger, H.; Welter, M. Integrative models of vascular remodeling during tumor growth. Wiley Interdiscip. Rev. Syst. Biol. Med. 2015, 7, 113–129. [Google Scholar] [CrossRef]
- Herman, A.B.; Savage, V.M.; West, G.B. A Quantitative Theory of Solid Tumor Growth, Metabolic Rate and Vascularization. PLoS ONE 2011, 6, e22973. [Google Scholar] [CrossRef]
- Less, J.R.; Skalak, T.C.; Sevick, E.M.; Jain, R.K. Microvascular architecture in a mammary carcinoma: Branching patterns and vessel dimensions. Cancer Res. 1991, 51, 265–273. [Google Scholar]
- Sandha, K.K.; Shukla, M.K.; Gupta, P.N. Recent Advances in Strategies for Extracellular Matrix Degradation and Synthesis Inhibition for Improved Therapy of Solid Tumors. Curr. Pharm. Des. 2020, 26, 5456–5467. [Google Scholar] [CrossRef]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control. Release 2015, 201, 78–89. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Ganss, R. Tumour vessel remodelling: New opportunities in cancer treatment. Vasc. Biol. 2020, 2, R35–R43. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pérez, M.-J.; Priego, E.-M.; Bueno, O.; Martins, M.S.; Canela, M.-D.; Liekens, S. Blocking Blood Flow to Solid Tumors by Destabilizing Tubulin: An Approach to Targeting Tumor Growth. J. Med. Chem. 2016, 59, 8685–8711. [Google Scholar] [CrossRef] [Green Version]
- Belotti, D.; Pinessi, D.; Taraboletti, G. Alternative vascularization mechanisms in tumor resistance to therapy. Cancers 2021, 13, 1912. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073. [Google Scholar] [CrossRef]
- Khan, S.; Sharifi, M.; Bloukh, S.H.; Edis, Z.; Siddique, R.; Falahati, M. In vivo guiding inorganic nanozymes for biosensing and therapeutic potential in cancer, inflammation and microbial infections. Talanta 2021, 224, 121805. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, Y.; An, Y.; Kim, B.Y.S. Remodeling Tumor Vasculature to Enhance Delivery of Intermediate-Sized Nanoparticles. ACS Nano 2015, 9, 8689–8696. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, C.-H. Convection enhanced delivery of liposome encapsulated doxorubicin for brain tumour therapy. J. Control. Release 2018, 285, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarized External Magnets To Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano 2020, 14, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Gedroyc, W.; Xu, X.Y. The effect of tumour size on drug transport and uptake in 3-D tumour models reconstructed from magnetic resonance images. PLoS ONE 2017, 12, e0172276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirthl, B.; Kremheller, J.; Schrefler, B.A.; Wall, W.A. Extension of a multiphase tumour growth model to study nanoparticle delivery to solid tumours. PLoS ONE 2020, 15, e0228443. [Google Scholar] [CrossRef] [Green Version]
- Moradi Kashkooli, F.; Soltani, M.; Momeni, M.M.; Rahmim, A. Enhanced Drug Delivery to Solid Tumors via Drug-Loaded Nanocarriers: An Image-Based Computational Framework. Front. Oncol. 2021, 11, 655781. [Google Scholar] [CrossRef]
- Henry, F.S.; Tsuda, A. Onset of alveolar recirculation in the developing lungs and its consequence on nanoparticle deposition in the pulmonary acinus. J. Appl. Physiol. 2016, 120, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Burke, C.W.; Alexander, E.; Timbie, K.; Kilbanov, A.L.; Price, R.J. Ultrasound-activated Agents Comprised of 5FU-bearing Nanoparticles Bonded to Microbubbles Inhibit Solid Tumor Growth and Improve Survival. Mol. Ther. 2014, 22, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Raeesi, V.; Chan, W.C.W. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale 2016, 8, 12524–12530. [Google Scholar] [CrossRef]
- Sefidgar, M.; Soltani, M.; Raahemifar, K.; Bazmara, H.; Nayinian, S.M.M.; Bazargan, M. Effect of tumor shape, size, and tissue transport properties on drug delivery to solid tumors. J. Biol. Eng. 2014, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Bordeau, B.M.; Balthasar, J.P. Strategies to enhance monoclonal antibody uptake and distribution in solid tumors. Cancer Biol. Med. 2021, 18, 649–664. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Y.; Fu, M.; Feng, Y.; Lin, G.; Kong, D.; Jiang, B. Simulation study of the effects of interstitial fluid pressure and blood flow velocity on transvascular transport of nanoparticles in tumor microenvironment. Comput. Methods Programs Biomed. 2020, 193, 105493. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef] [Green Version]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: From mathematical modeling to bench to bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durymanov, M.O.; Rosenkranz, A.A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mpekris, F.; Voutouri, C.; Papageorgis, P.; Stylianopoulos, T. Stress alleviation strategy in cancer treatment: Insights from a mathematical model. ZAMM-J. Appl. Math. Mech. 2018, 98, 2295–2306. [Google Scholar] [CrossRef]
- Sevick, E.M.; Jain, R.K. Viscous resistance to blood flow in solid tumors: Effect of hematocrit on intratumor blood viscosity. Cancer Res. 1989, 49, 3513–3519. [Google Scholar]
- Nizzero, S.; Ziemys, A.; Ferrari, M. Transport barriers and oncophysics in cancer treatment. Trends Cancer 2018, 4, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Wang, Z.; Sun, X.; Song, J.; Jacobson, O.; Niu, G.; Kiesewetter, D.O.; Chen, X. Size dependent kinetics of gold nanorods in EPR mediated tumor delivery. Theranostics 2016, 6, 2039. [Google Scholar] [CrossRef] [Green Version]
- Bort, G.; Lux, F.; Dufort, S.; Crémillieux, Y.; Verry, C.; Tillement, O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics 2020, 10, 1319. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Wang, H.; Li, Q.; Li, X.; Zhou, C.; Xu, J.; Chai, Y.; Liang, X.; Xiong, L.; et al. Tumor Chemo-Radiotherapy with Rod-Shaped and Spherical Gold Nano Probes: Shape and Active Targeting Both Matter. Theranostics 2019, 9, 1893–1908. [Google Scholar] [CrossRef]
- Smith, B.R.; Kempen, P.; Bouley, D.; Xu, A.; Liu, Z.; Melosh, N.; Dai, H.; Sinclair, R.; Gambhir, S.S. Shape Matters: Intravital Microscopy Reveals Surprising Geometrical Dependence for Nanoparticles in Tumor Models of Extravasation. Nano Lett. 2012, 12, 3369–3377. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, M.; Jafari, S.; Hasan, A.; Paray, B.A.; Gong, G.; Zheng, Y.; Falahati, M. Antimetastatic Activity of Lactoferrin-Coated Mesoporous Maghemite Nanoparticles in Breast Cancer Enabled by Combination Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3574–3584. [Google Scholar] [CrossRef]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor microenvironment triple-responsive nanoparticles enable enhanced tumor penetration and synergetic chemo-photodynamic therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic Fields and Cancer: Epidemiology, Cellular Biology, and Theranostics. Int. J. Mol. Sci. 2022, 23, 1339. [Google Scholar] [CrossRef]
- Wang, X.; Wu, M.; Li, H.; Jiang, J.; Zhou, S.; Chen, W.; Xie, C.; Zhen, X.; Jiang, X. Enhancing Penetration Ability of Semiconducting Polymer Nanoparticles for Sonodynamic Therapy of Large Solid Tumor. Adv. Sci. 2022, 9, 2104125. [Google Scholar] [CrossRef]
- De Maar, J.S.; Sofias, A.M.; Porta Siegel, T.; Vreeken, R.J.; Moonen, C.; Bos, C.; Deckers, R. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics 2020, 10, 1884–1909. [Google Scholar] [CrossRef]
- Ho, K.S.; Aman, A.M.; Al-awar, R.S.; Shoichet, M.S. Amphiphilic micelles of poly (2-methyl-2-carboxytrimethylene carbonate-co-D, L-lactide)-graft-poly (ethylene glycol) for anti-cancer drug delivery to solid tumours. Biomaterials 2012, 33, 2223–2229. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, U.; Asthana, A.; Jain, N.K. Dextran conjugated dendritic nanoconstructs as potential vectors for anti-cancer agent. Biomaterials 2009, 30, 3588–3596. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Mishra, P.; Jain, N.K. Long circulating PEGylated poly (D,L-lactide-co-glycolide) nanoparticulate delivery of Docetaxel to solid tumors. J. Drug Target. 2008, 16, 424–435. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, A.; Majumder, S.; Lariya, N.; Khaya, A.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J. Control. Release 2010, 148, 359–367. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Tang, W.-L.; MacCallum, N.W.; Li, S.-D. Preclinical pharmacokinetic, biodistribution, and anti-cancer efficacy studies of a docetaxel-carboxymethylcellulose nanoparticle in mouse models. Biomaterials 2012, 33, 1445–1454. [Google Scholar] [CrossRef]
- Sheth, V.; Wang, L.; Bhattacharya, R.; Mukherjee, P.; Wilhelm, S. Strategies for delivering nanoparticles across tumor blood vessels. Adv. Funct. Mater. 2021, 31, 2007363. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popović, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Tang, L.; Gabrielson, N.P.; Uckun, F.M.; Fan, T.M.; Cheng, J. Size-Dependent Tumor Penetration and in Vivo Efficacy of Monodisperse Drug–Silica Nanoconjugates. Mol. Pharm. 2013, 10, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, B.; Poon, W.; Zhang, Y.-N.; Lin, Z.P.; Kingston, B.R.; Tavares, A.J.; Zhang, Y.; Chen, J.; Valic, M.S.; Syed, A.M. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 2020, 19, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Coclite, A.; Mollica, H.; Ranaldo, S.; Pascazio, G.; De Tullio, M.; Decuzzi, P. Predicting different adhesive regimens of circulating particles at blood capillary walls. Microfluid. Nanofluid. 2017, 21, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurney, P.; Agarwal, R.; Singh, V.; Choi, D.; Roy, K.; Sreenivasan, S.V.; Shi, L. Unique size and shape-dependent uptake behaviors of non-spherical nanoparticles by endothelial cells due to a shearing flow. J. Control. Release 2017, 245, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, V.P.; Popović, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M.G.; Jain, R.K. Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angew. Chem. Int. Ed. 2011, 50, 11417–11420. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Jurney, P.; Raythatha, M.; Singh, V.; Sreenivasan, S.V.; Shi, L.; Roy, K. Effect of Shape, Size, and Aspect Ratio on Nanoparticle Penetration and Distribution inside Solid Tissues Using 3D Spheroid Models. Adv. Healthc. Mater. 2015, 4, 2269–2280. [Google Scholar] [CrossRef]
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101. [Google Scholar] [CrossRef]
- Jindal, A.B. The effect of particle shape on cellular interaction and drug delivery applications of micro- and nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Voutouri, C.; Polydorou, C.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Hyaluronan-Derived Swelling of Solid Tumors, the Contribution of Collagen and Cancer Cells, and Implications for Cancer Therapy. Neoplasia 2016, 18, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Stylianopoulos, T.; Soteriou, K.; Fukumura, D.; Jain, R.K. Cationic Nanoparticles Have Superior Transvascular Flux into Solid Tumors: Insights from a Mathematical Model. Ann. Biomed. Eng. 2013, 41, 68–77. [Google Scholar] [CrossRef]
- Campbell, R.B.; Fukumura, D.; Brown, E.B.; Mazzola, L.M.; Izumi, Y.; Jain, R.K.; Torchilin, V.P.; Munn, L.L. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002, 62, 6831–6836. [Google Scholar]
- Krasnici, S.; Werner, A.; Eichhorn, M.E.; Schmitt-Sody, M.; Pahernik, S.A.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Naujoks, K. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer 2003, 105, 561–567. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Rattan, R.; Bhattacharjee, S.; Zong, H.; Swain, C.; Siddiqui, M.A.; Visovatti, S.H.; Kanthi, Y.; Desai, S.; Pinsky, D.J.; Goonewardena, S.N. Nanoparticle-macrophage interactions: A balance between clearance and cell-specific targeting. Bioorgan. Med. Chem. 2017, 25, 4487–4496. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Li, Z.Y.; Zhu, J.Y.; Han, K.; Zeng, Z.Y.; Hong, W.; Li, W.X.; Jia, H.Z.; Liu, Y.; Zhuo, R.X. Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef]
- Petersen, G.H.; Alzghari, S.K.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 2016, 232, 255–264. [Google Scholar] [CrossRef]
- Siracka, E.; Pappova, N.; Pipa, V.; Durkovský, J. Changes in blood flow of growing experimental tumor determined by the clearance of 133Xe. Neoplasma 1979, 26, 173–177. [Google Scholar]
- Wilson, C.B.; Lammertsma, A.A.; McKenzie, C.G.; Sikora, K.; Jones, T. Measurements of blood flow and exchanging water space in breast tumors using positron emission tomography: A rapid and noninvasive dynamic method. Cancer Res. 1992, 52, 1592–1597. [Google Scholar]
- Xu, Z.; Kleinstreuer, C. Direct nanodrug delivery for tumor targeting subject to shear-augmented diffusion in blood flow. Med. Biol. Eng. Comput. 2018, 56, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Lu, W.; Zhang, R.; Xiong, C.; Ensor, J.; Nazario, J.; Jackson, J.; Shaw, C.; Dixon, K.A.; Miller, J. Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model. Mol. Imaging Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Maeda, H.; Fang, J. Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opin. Drug Deliv. 2021, 19, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Luo, Z.; Dai, L.; Guo, X.; Cai, K. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today 2017, 15, 56–90. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Morgan, J.T.; Stewart, W.G.; McKee, R.A.; Gleghorn, J.P. The mechanosensitive ion channel TRPV4 is a regulator of lung development and pulmonary vascular stabilization. Cell. Mol. Bioeng. 2018, 44, 309–320. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive Tumor Targeting of Renal-Clearable Luminescent Gold Nanoparticles: Long Tumor Retention and Fast Normal Tissue Clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Sadoqi, M.; Shao, J. Biodistribution of indocyanine green-loaded nanoparticles with surface modifications of PEG and folic acid. Int. J. Pharm. 2012, 436, 25–31. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Liu, Y.; Liu, X.; Wu, Y.; Fan, J.; Zhao, L.; Cao, Y. Mathematical modeling of the heterogeneous distributions of nanomedicines in solid tumors. Eur. J. Pharm. Biopharm. 2019, 142, 153–164. [Google Scholar] [CrossRef]
- Peiris, P.M.; Toy, R.; Doolittle, E.; Pansky, J.; Abramowski, A.; Tam, M.; Vicente, P.; Tran, E.; Hayden, E.; Camann, A.; et al. Imaging Metastasis Using an Integrin-Targeting Chain-Shaped Nanoparticle. ACS Nano 2012, 6, 8783–8795. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, E.-J.; Han, J.; Lee, H.; Shin, W.S.; Kim, H.M.; Bhuniya, S.; Kim, J.S.; Hong, K.S. Hypoxia-directed and activated theranostic agent: Imaging and treatment of solid tumor. Biomaterials 2016, 104, 119–128. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Fang, J.; Islam, R.; Islam, W.; Yin, H.; Subr, V.; Etrych, T.; Ulbrich, K.; Maeda, H. Augmentation of EPR Effect and Efficacy of Anticancer Nanomedicine by Carbon Monoxide Generating Agents. Pharmaceutics 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Sceneay, J.; Gödde, N.; Kinwel, T.; Ham, S.; Thompson, E.W.; Humbert, P.O.; Möller, A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene 2018, 37, 4214–4225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Suo, C.; Zheng, C.; Zhang, H. Hypoxia and metabolism in metastasis. In Hypoxia Cancer Metastasis; Springer: Cham, Switzerland, 2019; Volume 1136, pp. 87–95. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).