Peripheral Blood Mononuclear Cells Predict Therapeutic Efficacy of Immunotherapy in NSCLC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PBMC Isolation
2.2. Flow Cytometry
2.3. Statistical Methods
3. Results
3.1. Study Group Population
3.2. Control Group Population
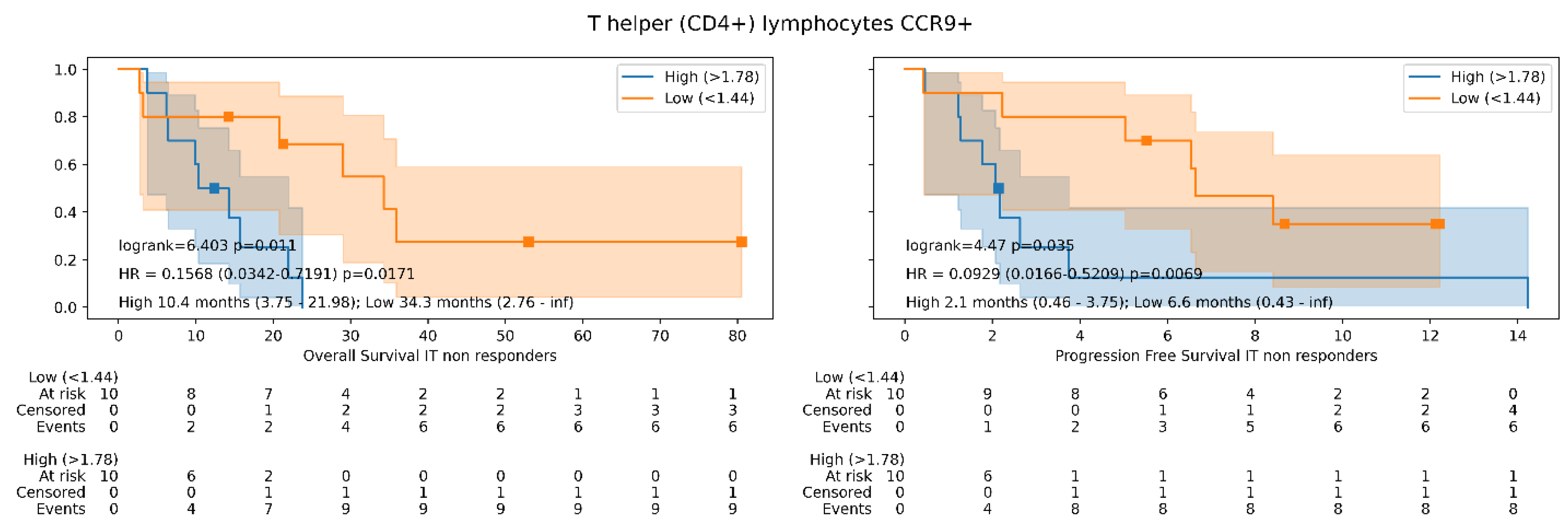
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Mezquita, L.; Ferrara, R.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Auclin, E.; et al. Integrating circulating biomarkers in the immune checkpoint inhibitor treatment in lung cancer. Cancers 2020, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J. Clin. Oncol. 2019, 37, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Romero-Laorden, N.; Sanchez-Torres, J.M.; Ramos-Levi, A.M.; Pacheco-Barcia, V.; Ballesteros, A.I.; Arranz, R.; Lorenzo, A.; Gullon, P.; Garrido, A.; et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology 2020, 9, 1751548. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Leví, A.; Arranz, R.; Lorenzo, A.; Gullón, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Rogado, J.; Pacheco-Barcia, V.; Toquero, P.; Vera, B.; Mondejar, R.; Romero Laorden, N.; Ballesteros Garcia, A.I.; Donnay, O.; Colomer Bosch, R.; Sánchez-Torres, J.M. Are neutrophil-tolymphocyte ratio (NLR) and neutrophil count percentage (NCP) predictors of nivolumab outcome and toxicity in NSCLC? J. Thorac. Oncol. 2018, 13 (Suppl. 4), S1–S139. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Zhang, H.-Y.; Du, W.; Qin, Z.; Yao, Y.; Mao, Y.; Zhou, L. Upregulation of chemokine receptor CCR10 is essential for glioma proliferation, invasion and patient survival. Oncotarget 2014, 5, 6576–6583. [Google Scholar] [CrossRef]
- Ren, L.; Yu, Y.; Wang, L.; Zhu, Z.; Lu, R.; Yao, Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget 2016, 7, 75763–75773. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Du, H.; Huang, H.; Wang, C.; Wang, P.; Zha, Z.; Wu, Y.; Liu, X.; Weng, C.; Fang, X.; et al. CCR9 Promotes Migration and Invasion of Lung Adenocarcinoma Cancer Stem Cells. Int. J. Med. Sci. 2020, 17, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Klimowicz, A.C.; Kopciuk, K.; Petrillo, S.K.; Konno, M.; Hao, D.; Muzik, H.; Stolte, E.; Boland, W.; Morris, D.; et al. CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Wald, O.; Izhar, U.; Amir, G.; Avniel, S.; Bar-Shavit, Y.; Wald, H.; Weiss, I.D.; Galun, E.; Peled, A. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J. Immunol. 2006, 177, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef] [PubMed]


| Immune Cell Subset | Immune Biomarkers Analyzed | |||
|---|---|---|---|---|
| T helper lymphocytes | ADAM8 | CD210 | GRK2 | IL6R |
| CD3, CD4 | β7 | CD47 | IFNΥ | PSGL1 |
| CCR10 | CTLA-4 | IL15Ra | SLAN | |
| CCR9 | CXCR4 | IL17 | Tie2 | |
| TSP1 | ||||
| T cytotoxic lymphocytes | ADAM8 | CD244 | IFNΥ | PD1 |
| CD3, CD8 | β7 | CD47 | IL15Ra | PSGL1 |
| CCR10 | CTLA-4 | IL17 | SLAN | |
| CCR9 | CXCR4 | IL6R | Tie2 | |
| CD210 | GRK2 | LAG3 | TIM3 | |
| TSP1 | ||||
| Myeloid cells | ADAM8 | CD123 | GRK2 | SLAN |
| CD14, CD11c, HLA II | β7 | CD210 | IL15Ra | Tie2 |
| CCR10 | CD47 | IL6R | TSP1 | |
| CCR9 | CXCR4 | PSGL1 | ||
| B Lymphocytes | CD210 | CD244 | IL6R | |
| CD19 | β7 | CD47 | PSGL1 | |
| CCR10 | CXCR4 | SLAN | ||
| CCR9 | GRK2 | Tie2 | ||
| CD210 | IL15Ra | TSP1 | ||
| Natural killer cells | ADAM8 | CD244 | Tie2 | SLAN |
| CD56 | β7 | CD47 | KIR | Tie2 |
| CCR10 | CXCR4 | NKG2A | TSP1 | |
| CCR9 | GRK2 | NKG2C | ||
| CD210 | IL15Ra | PSGL1 | ||
| Immunotherapy NSCLC Cohort | Non-Immunotherapy Cohort | p Value | |
|---|---|---|---|
| Age, median (range) | 69 (50–85) | 68 (43–88) | 0.6 |
| Sex | |||
| Women | 3 (7.7%) | 16 (40%) | |
| Men | 36 (92.3%) | 24 (60%) | 0.001 |
| Tobacco exposure, N (%) | 39 (100%) | 23 (57.5%) | - |
| BMI, median (range) | 25.12 (16.6–34.0) | 23.37 (16.8–31.5) | 0.4 |
| Overweight *, N (%) | 16 (41.0%) | 10 (25%) | 0.2 |
| HIV, N (%) | 1 (2.6%) | 1 (2.5%) | 1 |
| High comorbidities (Charlson index), N (%) | 7 (17.9%) | 5 (12.5%) | 0.49 |
| Liver metastasis, N (%) | 6 (15.3%) | 22 (55%) | <0.001 |
| CNS metastasis, N (%) | 9 (23.1%) | 3 (7.5%) | 0.06 |
| Previous treatments, median (range) | 1 (0–3) | 0 (0–2) | <0.001 |
| Objective response, N (%) | 15 (38.4%) | 14 (35%) | 0.8 |
| IrAEs, N (%) | 14 (35.8%) | - | - |
| Steroid’s consumption, N (%) | 8 (20.5%) | 0 (0%) | 0.002 |
| Hemoglobin, g/dL, median (range) | 13.0 (7.4–17.4) | 12.4 (9.1–16.3) | 0.7 |
| Neutrophils, 103/mcL, median (range) | 6.7 (2.4–54.0) | 6.1 (2.0–15.4) | 0.6 |
| Lymphocytes, 103/µL, median (range) | 1.7 (0.6–5.6) | 1.4 (0.3–3.8) | 0.04 |
| Platelets, 103/mcL, median (range) | 280.0 (135.0–721.0) | 256.5 (103.0–633.0) | 0.2 |
| LDH, U/L, median (range) | 201 (115–662) | 167 (167–167) | <0.001 |
| NSCLC Immunotherapy Treatment Group | Non-Immunotherapy Treatment Control Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers | N | Median | Range | Percentile 55 (n Patients, %) | Percentile 45 (n Patients, %) | N | Median | Range | Percentile 55 (n Patients, %) | Percentile 45 (n Patients, %) |
| CD3+CD4+ | 36 | 25.06 | 1.3–60.4 | N = 16 23.5% | N = 16 18.8% | 37 | 27.22 | 3.5–64.6 | N = 17 26.4% | N = 17 20.0% |
| CD3+CD4+CCR9+ | 36 | 5.10 | 0.4–57.6 | N = 16 1.7% | N = 17 1.3% | 37 | 5.06 | 0.4–70.4 | N = 17 1.6% | N = 17 1.4% |
| CD3+CD4+CCR10+ | 36 | 5.59 | 0.4–59.3 | N = 16 2.8% | N = 16 2.2% | 37 | 7.15 | 0.4–83.1 | N = 17 3.7% | N = 17 2.4% |
| CD3+CD8+CXCR4+ | 36 | 50.95 | 27–98.1 | N = 16 73.7% | N = 16 72.2% | 37 | 48.97 | 27.0–98.1 | N = 17 75.9% | N = 17 73.6% |
| CD11c+CD14-MHCII+CD123+ | 36 | 71.36 | 22.9–95.5 | N = 16 79.8% | N = 16 75.8% | 37 | 61.67 | 22.9–95.5 | N = 17 70.7% | N = 18 68.1% |
| CD56+CCR9+ | 36 | 3.19 | 0.2–50.5 | N = 16 1.4% | N = 16 1.2% | 37 | 4.00 | 0.2–50.5 | N = 17 1.5% | N = 17 1.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogado, J.; Pozo, F.; Troule, K.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Mondejar, R.; Donnay, O.; Ballesteros, A.; Pacheco-Barcia, V.; Aspa, J.; et al. Peripheral Blood Mononuclear Cells Predict Therapeutic Efficacy of Immunotherapy in NSCLC. Cancers 2022, 14, 2898. https://doi.org/10.3390/cancers14122898
Rogado J, Pozo F, Troule K, Sánchez-Torres JM, Romero-Laorden N, Mondejar R, Donnay O, Ballesteros A, Pacheco-Barcia V, Aspa J, et al. Peripheral Blood Mononuclear Cells Predict Therapeutic Efficacy of Immunotherapy in NSCLC. Cancers. 2022; 14(12):2898. https://doi.org/10.3390/cancers14122898
Chicago/Turabian StyleRogado, Jacobo, Fernando Pozo, Kevin Troule, José Miguel Sánchez-Torres, Nuria Romero-Laorden, Rebeca Mondejar, Olga Donnay, Anabel Ballesteros, Vilma Pacheco-Barcia, Javier Aspa, and et al. 2022. "Peripheral Blood Mononuclear Cells Predict Therapeutic Efficacy of Immunotherapy in NSCLC" Cancers 14, no. 12: 2898. https://doi.org/10.3390/cancers14122898






