The Landscape and Clinical Application of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
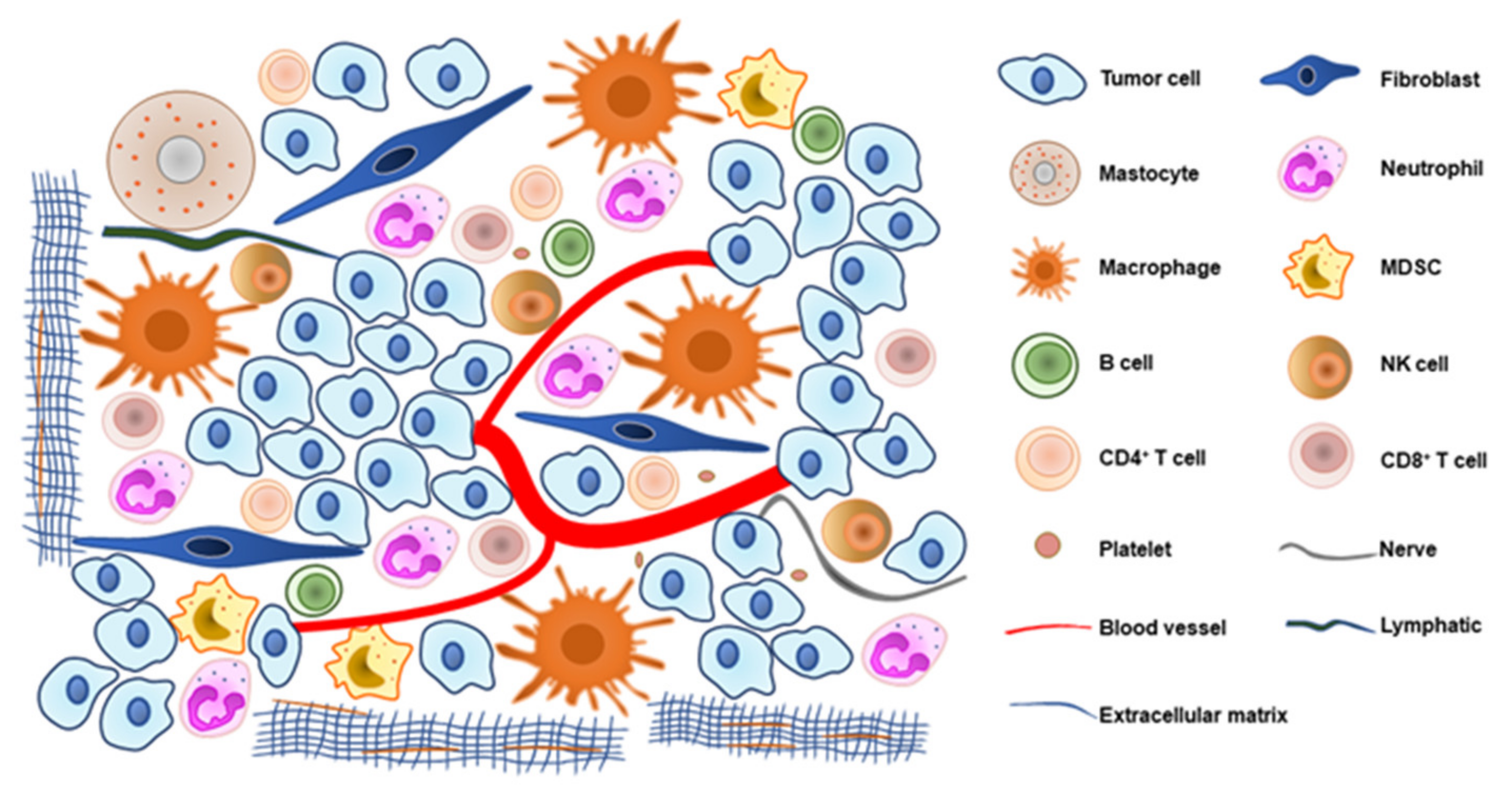
1. Introduction
2. Innate Immune Cells
3. Adaptive Immune Cells
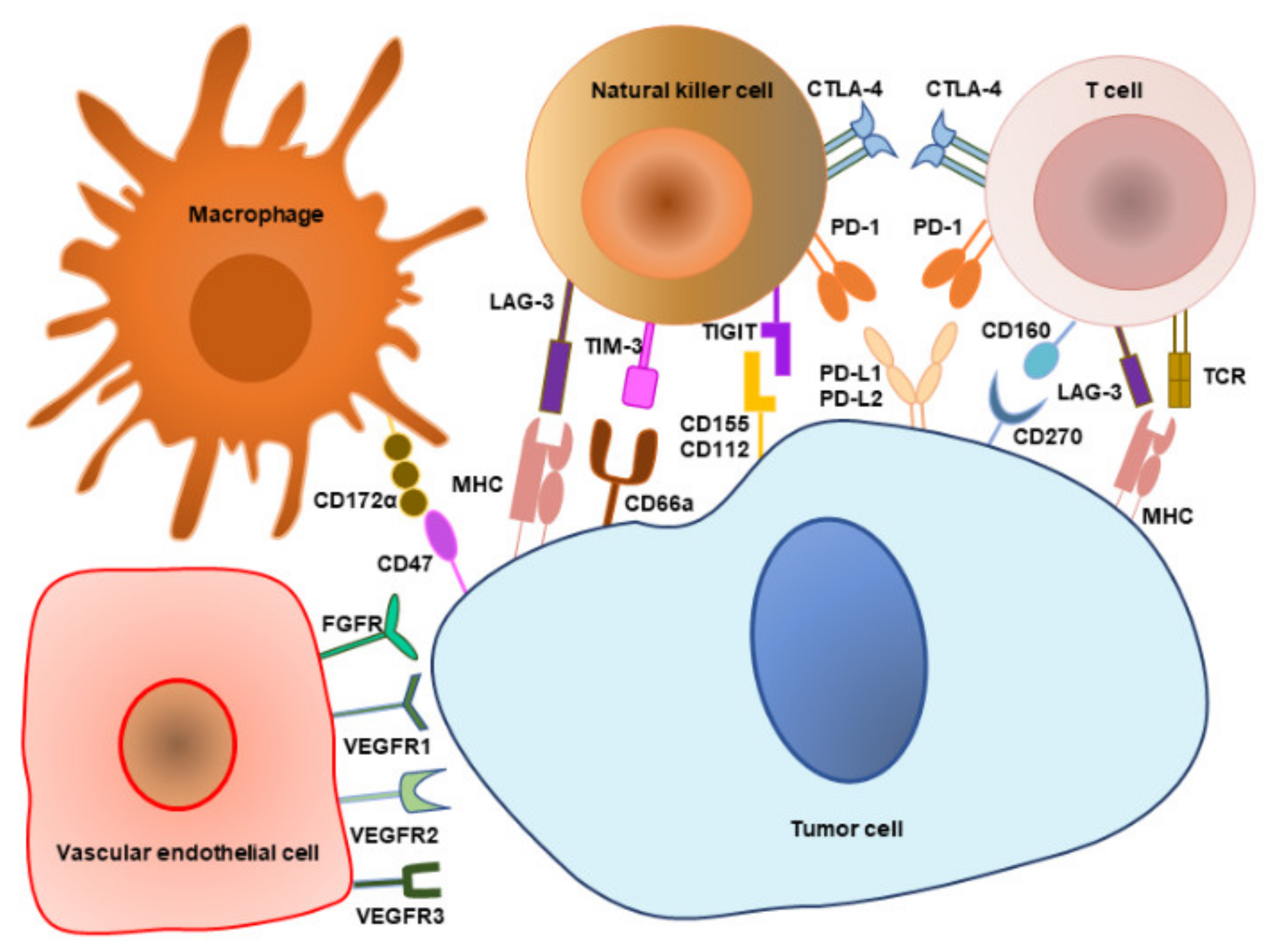
4. Immune Checkpoints
5. Vasculature and Lymphatic Factors
6. Microbial Community
7. Other Cells and Molecules in TME
8. TME-Associated Combination Treatment
9. TME-Associated Clinical Application Prospects
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Yang, L.; Lin, P.C. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin. Cancer Biol. 2017, 47, 185–195. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Pardoll, D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020, 367, 6477. [Google Scholar] [CrossRef]
- Heinhuis, K.M.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.H.; Schellens, J.H.M. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019, 30, 219–235. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couvelard, A.; Cros, J. An update on the development of concepts, diagnostic criteria, and challenging issues for neuroendocrine neoplasms across different digestive organs. Virchows Arch. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Ghosh, S. Lifting the innate immune barriers to antitumor immunity. J. Immunother. Cancer 2020, 8, e000695. [Google Scholar] [CrossRef] [PubMed]
- Centonze, G.; Lagano, V.; Sabella, G.; Mangogna, A.; Garzone, G.; Filugelli, M.; Belmonte, B.; Cattaneo, L.; Crisafulli, V.; Pellegrinelli, A.; et al. Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Ferrata, M.; Schad, A.; Zimmer, S.; Musholt, T.J.; Bahr, K.; Kuenzel, J.; Becker, S.; Springer, E.; Roth, W.; Weber, M.M.; et al. PD-L1 Expression and Immune Cell Infiltration in Gastroenteropancreatic (GEP) and Non-GEP Neuroendocrine Neoplasms with High Proliferative Activity. Front. Oncol. 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, S.; Abbassi, R.; Griesmann, H.; Sipos, B.; Wiese, D.; Rexin, P.; Blank, A.; Perren, A.; Haybaeck, J.; Huttelmaier, S.; et al. Therapeutic targeting of tumor-associated macrophages in pancreatic neuroendocrine tumors. Int. J. Cancer 2018, 143, 1806–1816. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, S.; Liu, C.; Yang, J.; Lin, Q.; Zheng, S.; Chen, C.; Zhou, Q.; Chen, R. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef]
- Harimoto, N.; Hoshino, K.; Muranushi, R.; Hagiwara, K.; Yamanaka, T.; Ishii, N.; Tsukagoshi, M.; Igarashi, T.; Tanaka, H.; Watanabe, A.; et al. Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology 2019, 19, 897–902. [Google Scholar] [CrossRef]
- Wei, I.H.; Harmon, C.M.; Arcerito, M.; Cheng, D.F.; Minter, R.M.; Simeone, D.M. Tumor-associated macrophages are a useful biomarker to predict recurrence after surgical resection of nonfunctional pancreatic neuroendocrine tumors. Ann. Surg. 2014, 260, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Michelakos, T.; Deshpande, V.; Arora, K.S.; Yamada, T.; Ting, D.T.; Taylor, M.S.; Castillo, C.F.; Warshaw, A.L.; Lillemoe, K.D.; et al. Role of Tumor-Associated Macrophages in the Clinical Course of Pancreatic Neuroendocrine Tumors (PanNETs). Clin. Cancer Res. 2019, 25, 2644–2655. [Google Scholar] [CrossRef]
- Xu, S.S.; Li, H.; Li, T.J.; Li, S.; Xia, H.Y.; Long, J.; Wu, C.T.; Wang, W.Q.; Zhang, W.H.; Gao, H.L.; et al. Neutrophil Extracellular Traps and Macrophage Extracellular Traps Predict Postoperative Recurrence in Resectable Nonfunctional Pancreatic Neuroendocrine Tumors. Front. Immunol. 2021, 12, 577517. [Google Scholar] [CrossRef] [PubMed]
- Schiavo Lena, M.; Partelli, S.; Castelli, P.; Andreasi, V.; Smart, C.E.; Pisa, E.; Bartolomei, M.; Bertani, E.; Zamboni, G.; Falconi, M.; et al. Histopathological and Immunophenotypic Changes of Pancreatic Neuroendocrine Tumors after Neoadjuvant Peptide Receptor Radionuclide Therapy (PRRT). Endocr. Pathol. 2020, 31, 119–131. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef] [PubMed]
- Akkari, L.; Gocheva, V.; Kester, J.C.; Hunter, K.E.; Quick, M.L.; Sevenich, L.; Wang, H.W.; Peters, C.; Tang, L.H.; Klimstra, D.S.; et al. Distinct functions of macrophage-derived and cancer cell-derived cathepsin Z combine to promote tumor malignancy via interactions with the extracellular matrix. Genes Dev. 2014, 28, 2134–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harney, A.S.; Karagiannis, G.S.; Pignatelli, J.; Smith, B.D.; Kadioglu, E.; Wise, S.C.; Hood, M.M.; Kaufman, M.D.; Leary, C.B.; Lu, W.P.; et al. The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2(Hi) Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors. Mol. Cancer Ther. 2017, 16, 2486–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyonteck, S.M.; Gadea, B.B.; Wang, H.W.; Gocheva, V.; Hunter, K.E.; Tang, L.H.; Joyce, J.A. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene 2012, 31, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.D.; Basturk, O.; Thirabanjasak, D.; Hruban, R.H.; Klimstra, D.S.; Bagci, P.; Altinel, D.; Adsay, V. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod. Pathol. 2011, 24, 1612–1619. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, W.Q.; Gao, H.L.; Xu, S.S.; Li, S.; Li, T.J.; Han, X.; Xu, H.X.; Li, H.; Jiang, W.; et al. Tumor-Infiltrating Neutrophils Predict Poor Survival of Non-Functional Pancreatic Neuroendocrine Tumor. J. Clin. Endocrinol. Metab. 2020, 105, 2217–2228. [Google Scholar] [CrossRef]
- Debien, V.; Davidson, G.; Baltzinger, P.; Kurtz, J.E.; Severac, F.; Imperiale, A.; Pessaux, P.; Addeo, P.; Bachellier, P.; Su, X.; et al. Involvement of Neutrophils in Metastatic Evolution of Pancreatic Neuroendocrine Tumors. Cancers 2021, 13, 2771. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Corna, G.; Moresco, M.; Coltella, N.; Restuccia, U.; Maggioni, D.; Raccosta, L.; Lin, C.Y.; Invernizzi, F.; Crocchiolo, R.; et al. 24-Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc. Natl. Acad. Sci. USA 2016, 113, E6219–E6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhang, Y.; Chen, L.; Lin, Y.; He, Q.; Zeng, Y.; Chen, M.; Chen, J. Myeloid-derived suppressor cells in gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2021, 71, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bao, X.; Zhang, R.; Ding, Y.; Zhang, M.; Li, B.; Zhang, H.; Li, X.; Tong, Z.; Liu, L.; et al. Depiction of the genomic and genetic landscape identifies CCL5 as a protective factor in colorectal neuroendocrine carcinoma. Br. J. Cancer 2021, 125, 994–1002. [Google Scholar] [CrossRef]
- Kosaloglu, Z.; Zornig, I.; Halama, N.; Kaiser, I.; Buchhalter, I.; Grabe, N.; Eils, R.; Schlesner, M.; Califano, A.; Jager, D. Identification of immunotherapeutic targets by genomic profiling of rectal NET metastases. Oncoimmunology 2016, 5, e1213931. [Google Scholar] [CrossRef] [Green Version]
- Soucek, L.; Buggy, J.J.; Kortlever, R.; Adimoolam, S.; Monclus, H.A.; Allende, M.T.; Swigart, L.B.; Evan, G.I. Modeling pharmacological inhibition of mast cell degranulation as a therapy for insulinoma. Neoplasia 2011, 13, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Al-Toubah, T.; Schell, M.J.; Cives, M.; Zhou, J.M.; Soares, H.P.; Strosberg, J.R. A Phase II Study of Ibrutinib in Advanced Neuroendocrine Neoplasms. Neuroendocrinology 2020, 110, 377–383. [Google Scholar] [CrossRef]
- Mo, S.; Zong, L.; Chen, X.; Chang, X.; Lu, Z.; Yu, S.; Chen, J. High Mast Cell Density Predicts a Favorable Prognosis in Patients with Pancreatic Neuroendocrine Neoplasms. Neuroendocrinology 2021. [Google Scholar] [CrossRef]
- Szabo, P.A.; Levitin, H.M.; Miron, M.; Snyder, M.E.; Senda, T.; Yuan, J.; Cheng, Y.L.; Bush, E.C.; Dogra, P.; Thapa, P.; et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 2019, 10, 4706. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Babala, N.; Melief, C.J.M.; Kastenmuller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Bosch, F.; Bruwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Zhu, Q.; Zahurak, M.; Bhaijee, F.; Xu, H.; Engle, E.L.; Kotte, A.; Pawlik, T.M.; Anders, R.A.; De Jesus-Acosta, A. Prognostic Implications of the Immune Tumor Microenvironment in Patients with Pancreatic and Gastrointestinal Neuroendocrine Tumors. Pancreas 2021, 50, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tsuchikawa, T.; Nakamura, T.; Sato, N.; Tamoto, E.; Okamura, K.; Shichinohe, T.; Hirano, S. Impact of the tumor microenvironment in predicting postoperative hepatic recurrence of pancreatic neuroendocrine tumors. Oncol. Rep. 2014, 32, 2753–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, A.; Bowden, M.; Zhang, S.; Masugi, Y.; Thorner, A.R.; Herbert, Z.T.; Zhou, C.W.; Brais, L.; Chan, J.A.; Hodi, F.S.; et al. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas 2018, 47, 1123–1129. [Google Scholar] [CrossRef]
- Busse, A.; Mochmann, L.H.; Spenke, C.; Arsenic, R.; Briest, F.; Johrens, K.; Lammert, H.; Sipos, B.; Kuhl, A.A.; Wirtz, R.; et al. Immunoprofiling in Neuroendocrine Neoplasms Unveil Immunosuppressive Microenvironment. Cancers 2020, 12, 3448. [Google Scholar] [CrossRef]
- De Reuver, P.R.; Mehta, S.; Gill, P.; Andrici, J.; D’Urso, L.; Clarkson, A.; Mittal, A.; Hugh, T.J.; Samra, J.S.; Gill, A.J. Immunoregulatory Forkhead Box Protein p3-Positive Lymphocytes Are Associated with Overall Survival in Patients with Pancreatic Neuroendocrine Tumors. J. Am. Coll. Surg. 2016, 222, 281–287. [Google Scholar] [CrossRef]
- Sciammarella, C.; Luce, A.; Riccardi, F.; Mocerino, C.; Modica, R.; Berretta, M.; Misso, G.; Cossu, A.M.; Colao, A.; Vitale, G.; et al. Lanreotide Induces Cytokine Modulation in Intestinal Neuroendocrine Tumors and Overcomes Resistance to Everolimus. Front. Oncol. 2020, 10, 1047. [Google Scholar] [CrossRef]
- Tanno, L.; Naheed, S.; Dunbar, J.; Tod, J.; Lopez, M.A.; Taylor, J.; Machado, M.; Green, B.; Ashton-Key, M.; Chee, S.J.; et al. Analysis of Immune Landscape in Pancreatic and Ileal Neuroendocrine Tumours Demonstrates an Immune Cold Tumour Microenvironment. Neuroendocrinology 2021, 112, 370–383. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, W.Q.; Han, X.; Gao, H.L.; Xu, S.S.; Li, S.; Li, T.J.; Xu, H.X.; Li, H.; Ye, L.Y.; et al. Infiltrating pattern and prognostic value of tertiary lymphoid structures in resected non-functional pancreatic neuroendocrine tumors. J. Immunother. Cancer 2020, 8, e001188. [Google Scholar] [CrossRef]
- Sautes-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016, 7, 407. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, V.H.; Rodriguez, A.B.; Mauldin, I.S.; Woods, A.N.; Peske, J.D.; Slingluff, C.L., Jr. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J. Immunol. 2018, 200, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosery, V.; Reis, H.; Savvatakis, K.; Kowall, B.; Stuschke, M.; Paul, A.; Dechene, A.; Yang, J.; Zhao, B.; Borgers, A.; et al. Antitumor immune response is associated with favorable survival in GEP-NEN G3. Endocr. Relat. Cancer 2021, 28, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Gonzalez, R.S.; Das, S.; Berlin, J.; Shi, C. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: A potential target for anti-PD-1/PD-L1 therapy. Hum. Pathol. 2017, 70, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive Genomic Profiling of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Clin. Cancer Res. 2020, 26, 5943–5951. [Google Scholar] [CrossRef]
- Milione, M.; Miceli, R.; Barretta, F.; Pellegrinelli, A.; Spaggiari, P.; Tagliabue, G.; Centonze, G.; Paolino, C.; Mangogna, A.; Kankava, K.; et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J. Pathol. Clin. Res. 2019, 5, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, E.; Armentano, R.; Valentini, A.M.; Chieppa, M.; Caruso, M.L. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis. 2017, 8, e3004. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.; Al Diffalha, S.; Coppola, D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr. Relat. Cancer 2019, 26, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.W.; Fu, X.L.; Jiang, Y.S.; Chen, X.J.; Tao, L.Y.; Yang, J.Y.; Huo, Y.M.; Liu, W.; Zhang, J.F.; Liu, P.F.; et al. Clinical significance of programmed death 1/programmed death ligand 1 pathway in gastric neuroendocrine carcinomas. World J. Gastroenterol. 2019, 25, 1684–1696. [Google Scholar] [CrossRef]
- Taboada, R.; Claro, L.; Felismino, T.; de Jesus, V.H.; Barros, M.; Riechelmann, R.P. Clinicopathological and molecular profile of grade 3 gastroenteropancreatic neuroendocrine neoplasms. J. Neuroendocrinol. 2022, 34, e13099. [Google Scholar] [CrossRef]
- Fraune, C.; Simon, R.; Hube-Magg, C.; Makrypidi-Fraune, G.; Kluth, M.; Buscheck, F.; Amin, T.; Viol, F.; Fehrle, W.; Dum, D.; et al. Homogeneous MMR Deficiency Throughout the Entire Tumor Mass Occurs in a Subset of Colorectal Neuroendocrine Carcinomas. Endocr. Pathol. 2020, 31, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Ying, H.; Li, J.; Gao, Y.; Sun, Z.; Li, J.; Bai, C.; Cheng, Y.; Wu, H. Immune Checkpoint Markers in Neuroendocrine Carcinoma of the Digestive System. Front. Oncol. 2020, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.E.; Puccini, A.; Grothey, A.; Raghavan, D.; Goldberg, R.M.; Xiu, J.; Korn, W.M.; Weinberg, B.A.; Hwang, J.J.; Shields, A.F.; et al. Landscape of Tumor Mutation Load, Mismatch Repair Deficiency, and PD-L1 Expression in a Large Patient Cohort of Gastrointestinal Cancers. Mol. Cancer Res. 2018, 16, 805–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinato, D.J.; Vallipuram, A.; Evans, J.S.; Wong, C.; Zhang, H.; Brown, M.; Dina, R.E.; Trivedi, P.; Akarca, A.U.; Marafioti, T.; et al. Programmed Cell Death Ligand Expression Drives Immune Tolerogenesis across the Diverse Subtypes of Neuroendocrine Tumours. Neuroendocrinology 2021, 111, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z.; et al. Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial. Clin. Cancer Res. 2020, 26, 2337–2345. [Google Scholar] [CrossRef] [Green Version]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M.; et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results from the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- MacFarlane, A.W.T.; Yeung, H.M.; Alpaugh, R.K.; Dulaimi, E.; Engstrom, P.F.; Dasari, A.; Campbell, K.S.; Vijayvergia, N. Impacts of pembrolizumab therapy on immune phenotype in patients with high-grade neuroendocrine neoplasms. Cancer Immunol. Immunother. 2021, 70, 1893–1906. [Google Scholar] [CrossRef]
- Yap, T.A.; Parkes, E.E.; Peng, W.; Moyers, J.T.; Curran, M.A.; Tawbi, H.A. Development of Immunotherapy Combination Strategies in Cancer. Cancer Discov. 2021, 11, 1368–1397. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [Green Version]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Al-Toubah, T.; Halfdanarson, T.; Gile, J.; Morse, B.; Sommerer, K.; Strosberg, J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open 2022, 7, 100364. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Lee, J.B.; Ha, S.J.; Kim, H.R. Clinical Insights into Novel Immune Checkpoint Inhibitors. Front. Pharmacol. 2021, 12, 681320. [Google Scholar] [CrossRef]
- Yuan, Z.; Gardiner, J.C.; Maggi, E.C.; Huang, S.; Adem, A.; Bagdasarov, S.; Li, G.; Lee, S.; Slegowski, D.; Exarchakis, A.; et al. B7 immune-checkpoints as targets for the treatment of neuroendocrine tumors. Endocr. Relat. Cancer 2021, 28, 135–149. [Google Scholar] [CrossRef]
- Krampitz, G.W.; George, B.M.; Willingham, S.B.; Volkmer, J.P.; Weiskopf, K.; Jahchan, N.; Newman, A.M.; Sahoo, D.; Zemek, A.J.; Yanovsky, R.L.; et al. Identification of tumorigenic cells and therapeutic targets in pancreatic neuroendocrine tumors. Proc. Natl. Acad. Sci. USA 2016, 113, 4464–4469. [Google Scholar] [CrossRef] [Green Version]
- Imam, R.; Chang, Q.; Black, M.; Yu, C.; Cao, W. CD47 expression and CD163(+) macrophages correlated with prognosis of pancreatic neuroendocrine tumor. BMC Cancer 2021, 21, 320. [Google Scholar] [CrossRef]
- Young, K.; Lawlor, R.T.; Ragulan, C.; Patil, Y.; Mafficini, A.; Bersani, S.; Antonello, D.; Mansfield, D.; Cingarlini, S.; Landoni, L.; et al. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut 2021, 70, 1904–1913. [Google Scholar] [CrossRef]
- Ono, K.; Shiozawa, E.; Ohike, N.; Fujii, T.; Shibata, H.; Kitajima, T.; Fujimasa, K.; Okamoto, N.; Kawaguchi, Y.; Nagumo, T.; et al. Immunohistochemical CD73 expression status in gastrointestinal neuroendocrine neoplasms: A retrospective study of 136 patients. Oncol. Lett. 2018, 15, 2123–2130. [Google Scholar] [CrossRef]
- Katsuta, E.; Tanaka, S.; Mogushi, K.; Shimada, S.; Akiyama, Y.; Aihara, A.; Matsumura, S.; Mitsunori, Y.; Ban, D.; Ochiai, T.; et al. CD73 as a therapeutic target for pancreatic neuroendocrine tumor stem cells. Int. J. Oncol. 2016, 48, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesely, C.; Wong, Y.N.S.; Childs, A.; Akarca, A.U.; Dhami, P.; Vaikkinen, H.; Conde, L.; Herrero, J.; Ogunbiyi, O.; Gander, A.; et al. Systematic Evaluation of the Immune Environment of Small Intestinal Neuroendocrine Tumours. Clin. Cancer Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Cigrovski Berkovic, M.; Cacev, T.; Catela Ivkovic, T.; Marout, J.; Ulamec, M.; Zjacic-Rotkvic, V.; Kapitanovic, S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Weng, X.F.; Xie, X.S.; Lian, N.Z.; Qiu, S.L.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. CDK5RAP3 inhibits angiogenesis in gastric neuroendocrine carcinoma by modulating AKT/HIF-1alpha/VEGFA signaling. Cancer Cell Int. 2019, 19, 282. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; He, J.Y.; Xu, J.; Hu, S.Y.; Mo, B.H.; Shu, Q.X.; Chen, C.; Gong, Y.Z.; Zhao, X.L.; Xie, G.F.; et al. Vascular NRP2 triggers PNET angiogenesis by activating the SSH1-cofilin axis. Cell Biosci. 2020, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Altendorf-Hofmann, A.; Jacob, S.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Kirchner, T.; Angele, M.K.; et al. Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor. J. Clin. Med. 2020, 9, 3368. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Paez-Ribes, M.; Carrasco, P.; Martin, L.; Soler, A.; Martinez-Lozano, M.; Pons, R.; Llena, J.; Palomero, L.; Graupera, M.; et al. Antitumor Effects of Anti-Semaphorin 4D Antibody Unravel a Novel Proinvasive Mechanism of Vascular-Targeting Agents. Cancer Res. 2019, 79, 5328–5341. [Google Scholar] [CrossRef] [Green Version]
- Keklikoglou, I.; Kadioglu, E.; Bissinger, S.; Langlois, B.; Bellotti, A.; Orend, G.; Ries, C.H.; De Palma, M. Periostin Limits Tumor Response to VEGFA Inhibition. Cell Rep. 2018, 22, 2530–2540. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, E.; Ignazzi, A.; De Michele, F.; Caruso, M.L. PDGFRalpha expression as a novel therapeutic marker in well-differentiated neuroendocrine tumors. Cancer Biol. Ther. 2019, 20, 423–430. [Google Scholar] [CrossRef]
- Puliani, G.; Sesti, F.; Anastasi, E.; Verrico, M.; Tarsitano, M.G.; Feola, T.; Campolo, F.; Di Gioia, C.R.T.; Venneri, M.A.; Angeloni, A.; et al. Angiogenic factors as prognostic markers in neuroendocrine neoplasms. Endocrine 2022, 76, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, N.; Kadioglu, E.; Keklikoglou, I.; Wyser Rmili, C.; Leow, C.C.; De Palma, M. Role of angiopoietin-2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 2014, 8, 696–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Remirez, M.; Del Puerto-Nevado, L.; Fernandez Acenero, M.J.; Ebrahimi-Nik, H.; Cruz-Ramos, M.; Garcia-Garcia, L.; Solanes, S.; Banos, N.; Molina-Roldan, E.; Garcia-Foncillas, J.; et al. Strong Antitumor Activity of Bevacizumab and Aflibercept in Neuroendocrine Carcinomas: In-Depth Preclinical Study. Neuroendocrinology 2020, 110, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, L.; Bai, C.; Wang, W.; Li, J.; Yu, X.; Li, Z.; Li, E.; Yuan, X.; Chi, Y.; et al. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1489–1499. [Google Scholar] [CrossRef]
- Capdevila, J.; Fazio, N.; Lopez, C.; Teule, A.; Valle, J.W.; Tafuto, S.; Custodio, A.; Reed, N.; Raderer, M.; Grande, E.; et al. Lenvatinib in Patients with Advanced Grade 1/2 Pancreatic and Gastrointestinal Neuroendocrine Tumors: Results of the Phase II TALENT Trial (GETNE1509). J. Clin. Oncol. 2021, 39, 2304–2312. [Google Scholar] [CrossRef]
- Chang, T.M.; Chu, P.Y.; Hung, W.C.; Shan, Y.S.; Lin, H.Y.; Huang, K.W.; Chang, J.S.; Chen, L.T.; Tsai, H.J. c-Myc promotes lymphatic metastasis of pancreatic neuroendocrine tumor through VEGFC upregulation. Cancer Sci. 2021, 112, 243–253. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, L.; Song, Y.L.; Xu, S.; Hua, Z.; Fang, N.; Zhai, M.; Liu, H.; Fang, Q.; Deng, T.; et al. Pseudo-hemorrhagic region formation in pancreatic neuroendocrine tumors is a result of blood vessel dilation followed by endothelial cell detachment. Oncol. Lett. 2018, 15, 4255–4261. [Google Scholar] [CrossRef] [Green Version]
- Massironi, S.; Facciotti, F.; Cavalcoli, F.; Amoroso, C.; Rausa, E.; Centonze, G.; Cribiu, F.M.; Invernizzi, P.; Milione, M. Intratumor Microbiome in Neuroendocrine Neoplasms: A New Partner of Tumor Microenvironment? A Pilot Study. Cells 2022, 11, 692. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.M.; Li, X.X.; Lu, L.; Yang, G.H.; Lei, Z.X.; You, L.J.; Cui, X.B.; Lu, S.C.; Zhai, Z.Y.; et al. Faecal microbiome and metabolic signatures in rectal neuroendocrine tumors. Theranostics 2022, 12, 2015–2027. [Google Scholar] [CrossRef]
- Yachida, S.; Totoki, Y.; Noe, M.; Nakatani, Y.; Horie, M.; Kawasaki, K.; Nakamura, H.; Saito-Adachi, M.; Suzuki, M.; Takai, E.; et al. Comprehensive Genomic Profiling of Neuroendocrine Carcinomas of the Gastrointestinal System. Cancer Discov. 2022, 12, 692–711. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Leja, J.; Yu, D.; Nilsson, B.; Gedda, L.; Zieba, A.; Hakkarainen, T.; Akerstrom, G.; Oberg, K.; Giandomenico, V.; Essand, M. Oncolytic adenovirus modified with somatostatin motifs for selective infection of neuroendocrine tumor cells. Gene Ther. 2011, 18, 1052–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Jin, C.; Leja, J.; Majdalani, N.; Nilsson, B.; Eriksson, F.; Essand, M. Adenovirus with hexon Tat-protein transduction domain modification exhibits increased therapeutic effect in experimental neuroblastoma and neuroendocrine tumors. J. Virol. 2011, 85, 13114–13123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Nagasato, M.; Rin, Y.; Henmi, M.; Ino, Y.; Yachida, S.; Ohki, R.; Hiraoka, N.; Tagawa, M.; Aoki, K. Strong antitumor efficacy of a pancreatic tumor-targeting oncolytic adenovirus for neuroendocrine tumors. Cancer Med. 2017, 6, 2385–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Schaller, M.; Fehrenbacher, B.; Krieg, A.; Sipos, B.; Lauer, U.M. The Oncolytic Herpes Simplex Virus Talimogene Laherparepvec Shows Promising Efficacy in Neuroendocrine Cancer Cell Lines. Neuroendocrinology 2019, 109, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Kloker, L.D.; Berchtold, S.; Smirnow, I.; Beil, J.; Krieg, A.; Sipos, B.; Lauer, U.M. Oncolytic vaccinia virus GLV-1h68 exhibits profound antitumoral activities in cell lines originating from neuroendocrine neoplasms. BMC Cancer 2020, 20, 628. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125 Pt 23, 5591–5596. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, Y.; Guo, C.; Li, X.; Cui, W.; Wang, Z.; Chen, X. Microscopic Invasion of Nerve Is Associated with Aggressive Behaviors in Pancreatic Neuroendocrine Tumors. Front. Oncol. 2021, 11, 630316. [Google Scholar] [CrossRef]
- Xu, S.S.; Xu, H.X.; Wang, W.Q.; Li, S.; Li, H.; Li, T.J.; Zhang, W.H.; Liu, L.; Yu, X.J. Tumor-infiltrating platelets predict postoperative recurrence and survival in resectable pancreatic neuroendocrine tumor. World J. Gastroenterol. 2019, 25, 6248–6257. [Google Scholar] [CrossRef]
- Werner, R.A.; Weich, A.; Higuchi, T.; Schmid, J.S.; Schirbel, A.; Lassmann, M.; Wild, V.; Rudelius, M.; Kudlich, T.; Herrmann, K.; et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors—A Triple Tracer Comparative Approach. Theranostics 2017, 7, 1489–1498. [Google Scholar] [CrossRef]
- Rodriguez Laval, V.; Pavel, M.; Steffen, I.G.; Baur, A.D.; Dilz, L.M.; Fischer, C.; Detjen, K.; Prasad, V.; Pascher, A.; Geisel, D.; et al. Mesenteric Fibrosis in Midgut Neuroendocrine Tumors: Functionality and Radiological Features. Neuroendocrinology 2018, 106, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Laskaratos, F.M.; Levi, A.; Schwach, G.; Pfragner, R.; Hall, A.; Xia, D.; von Stempel, C.; Bretherton, J.; Thanapirom, K.; Alexander, S.; et al. Transcriptomic Profiling of In Vitro Tumor-Stromal Cell Paracrine Crosstalk Identifies Involvement of the Integrin Signaling Pathway in the Pathogenesis of Mesenteric Fibrosis in Human Small Intestinal Neuroendocrine Neoplasms. Front. Oncol. 2021, 11, 629665. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, K.; Karakatsanis, A.; Stalberg, P.; Norlen, O.; Hellman, P. Clinical signs of fibrosis in small intestinal neuroendocrine tumours. Br. J. Surg. 2017, 104, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Laskaratos, F.M.; Rombouts, K.; Caplin, M.; Toumpanakis, C.; Thirlwell, C.; Mandair, D. Neuroendocrine tumors and fibrosis: An unsolved mystery? Cancer 2017, 123, 4770–4790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazevic, A.; Zandee, W.T.; Franssen, G.J.H.; Hofland, J.; van Velthuysen, M.F.; Hofland, L.J.; Feelders, R.A.; de Herder, W.W. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr. Relat. Cancer 2018, 25, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Bruewer, K.; D’Anastasi, M.; Ilhan, H.; Knoesel, T.; Pratschke, S.; Thomas, M.; Rentsch, M.; Guba, M.; Werner, J.; et al. Neuroendocrine tumors of the small intestine causing a desmoplastic reaction of the mesentery are a more aggressive cohort. Surgery 2018, 164, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [Green Version]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-angiogenic agents—Overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Grierson, P.M.; Picus, J.; Lockhart, A.C.; Roth, B.J.; Liu, J.; Morton, A.; Chan, E.; Huffman, J.; Liang, C.; et al. Vorolanib (X-82), an oral anti-VEGFR/PDGFR/CSF1R tyrosine kinase inhibitor, with everolimus in solid tumors: Results of a phase I study. Investig. New Drugs 2021, 39, 1298–1305. [Google Scholar] [CrossRef]
- Hobday, T.J.; Qin, R.; Reidy-Lagunes, D.; Moore, M.J.; Strosberg, J.; Kaubisch, A.; Shah, M.; Kindler, H.L.; Lenz, H.J.; Chen, H.; et al. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J. Clin. Oncol. 2015, 33, 1551–1556. [Google Scholar] [CrossRef]
- Gile, J.J.; Liu, A.J.; McGarrah, P.W.; Eiring, R.A.; Hobday, T.J.; Starr, J.S.; Sonbol, M.B.; Halfdanarson, T.R. Efficacy of Checkpoint Inhibitors in Neuroendocrine Neoplasms: Mayo Clinic Experience. Pancreas 2021, 50, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Rodriguez-Antona, C.; Lopez, C.; Alonso-Gordoa, T.; Benavent, M.; Capdevila, J.; Teule, A.; Custodio, A.; Sevilla, I.; Hernando, J.; et al. Sunitinib and Evofosfamide (TH-302) in Systemic Treatment-Naive Patients with Grade 1/2 Metastatic Pancreatic Neuroendocrine Tumors: The GETNE-1408 Trial. Oncologist 2021, 26, 941–949. [Google Scholar] [CrossRef]
- Inoue, M.; Kim, M.; Inoue, T.; Tait, M.; Byrne, T.; Nitschke, M.; Murer, P.; Cha, H.; Subramanian, A.; De Silva, N.; et al. Oncolytic vaccinia virus injected intravenously sensitizes pancreatic neuroendocrine tumors and metastases to immune checkpoint blockade. Mol. Ther. Oncolytics 2022, 24, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.L.; Murphy, K.M. Dendritic cells in cancer immunology. Cell. Mol. Immunol. 2022, 19, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.; Tsokos, M.G.; Ding, Y.; Malek, T.R.; Klatzmann, D.; Tsokos, G.C. Regulatory T cells in the treatment of disease. Nat. Rev. Drug Discov. 2018, 17, 823–844. [Google Scholar] [CrossRef]
- Vitale, G.; Carra, S.; Ferrau, F.; Guadagno, E.; Faggiano, A.; Colao, A.; Nike. Gastroenteropancreatic neuroendocrine neoplasms and inflammation: A complex cross-talk with relevant clinical implications. Crit Rev. Oncol. Hematol. 2020, 146, 102840. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Subramaniam, P.S.; Tang, L.H.; Grunn, A.; Aburi, M.; Rieckhof, G.; Komissarova, E.V.; Hagan, E.A.; Bodei, L.; Clemons, P.A.; et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat. Genet 2018, 50, 979–989. [Google Scholar] [CrossRef]
- Weber, E.W.; Maus, M.V.; Mackall, C.L. The Emerging Landscape of Immune Cell Therapies. Cell 2020, 181, 46–62. [Google Scholar] [CrossRef]
- Feng, Z.; He, X.; Zhang, X.; Wu, Y.; Xing, B.; Knowles, A.; Shan, Q.; Miller, S.; Hojnacki, T.; Ma, J.; et al. Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues. Nat. Cancer 2022, 3, 581–594. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Longo, S.K.; Guo, M.G.; Ji, A.L.; Khavari, P.A. Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet 2021, 22, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Kalloger, S.E.; Aird, J.J.; Lee, M.K.C.; Rushton, C.; Mungall, K.L.; Mungall, A.J.; Gao, D.; Chow, C.; Xu, J.; et al. Proteotranscriptomic classification and characterization of pancreatic neuroendocrine neoplasms. Cell Rep. 2021, 37, 109817. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
| NCT Number | Conditions | Drugs | Targets | Phases | Primary Endpoints |
|---|---|---|---|---|---|
| NCT03517488 | Progressive NECs | XmAb®20717 | PD-1/CTLA-4 bispecific antibody | 1 | Safety, tolerability |
| NCT03012620 | Progressive NETs | Pembrolizumab | PD-1 | 2 | Objective response rate |
| NCT03352934 | Progressive NECs | Avelumab | PD-L1 | 2 | Disease control rate |
| NCT03278379 | Progressive NETs with G2/3 | Avelumab | PD-L1 | 2 | Overall response rate |
| NCT03591731 | Progressive NECs | Nivolumab +/− ipilimumab | PD-1, CTLA-4 | 2 | Objective response rate |
| NCT03420521 | Progressive well-differentiated nonfunctional NETs | Nivolumab, ipilimumab | PD-1, CTLA-4 | 2 | Objective response rate |
| NCT03095274 | Progressive NENs | Durvalumab, tremelimumab | PD-L1, CTLA-4 | 2 | Clinical benefit rate |
| NCT Number | Conditions | Drugs | Phases | Primary Endpoints |
|---|---|---|---|---|
| NCT02549937 | Progressive NETs | Surufatinib | 1, 2 | Dose-limiting toxicity incidence, progression-free survival |
| NCT04579679 | Progressive NETs | Surufatinib | 2 | Disease control rate |
| NCT03457844 | Progressive NETs with G3 and NECs | Anlotinib | 2 | Progression-free survival |
| NCT04524208 | Locally unresectable or metastatic NENs with Ki67 of 20–60% | Cabozantinib | 2 | Disease control rate |
| NCT04412629 | Progressive NETs with G3 and NECs | Cabozantinib | 2 | Objective response rate |
| NCT01466036 | Locally unresectable or metastatic, well-differentiated, pancreatic NETs | Cabozantinib | 2 | objective response rate |
| NCT03375320 | Locally unresectable or metastatic NETs with G1/2 | Cabozantinib S-malate | 3 | Progression-free survival |
| NCT Number | Conditions | Drugs | Phases | Primary Endpoints |
|---|---|---|---|---|
| NCT05015621 | Progressive NECs | Surufatnib, toripalimab | 3 | Overall survival |
| NCT04207463 | Late NETs with G1/2 | Anlotinib, penpulimab, | 2 | Overall response rate |
| NCT04400474 | Progressive NETs with G3 and NECs | Cabozantinib, atezolizumab | 2 | Objective response rate |
| NCT04197310 | Locally unresectable or metastatic well-differentiated, non-pancreatic NETs | Cabozantinib, nivolumab | 2 | Objective response rate |
| NCT04079712 | Progressive poorly-differentiated NETs and NECs | Cabozantinib s-malate, nivolumab, ipilimumab | 2 | Overall response rate |
| NCT03290079 | Progressive well-differentiated NETs of small intestinal and colorectal origin | Lenvatinib, pembrolizumab | 2 | Objective response rate |
| NCT03074513 | Progressive NETs with G1/2 | Bevacizumab, atezolizumab | 2 | Objective response rate |
| NCT Number | Conditions | Drugs | Combinations | Phases | Primary Endpoints |
|---|---|---|---|---|---|
| NCT05048901 | Progressive NETs | Cabozantinib | Lanreotide | 1,2 | Maximal tolerated dose, progression-free survival |
| NCT04427787 | Locally unresectable or metastatic, well-differentiated NETs | Cabozantinib | Lanreotide | 2 | Objective response rate, safety |
| NCT04893785 | Progressive NETs and large cells NECs with Ki67< 55% | Cabozantinib | Temozolomide | 2 | Overall response rate |
| NCT02230176 | Progressive pancreatic NETs | Sunitinib | 177Lu-dota0-Tyr3-octreotate | 2 | Progression-free survival |
| NCT03950609 | Locally unresectable or metastatic NETs with G1/2 | Lenvatinib | Everolimus | 2 | Radiographic response rate, objective response rate |
| NCT02820857 | Progressive NECs | Bevacizumab | FOLFIRI | 2 | proportion of patients alive 6 months after treatment |
| NCT04705519 | Progressive NECs | Bevacizumab | Nab-paclitaxel | 2 | Overall Survival |
| NCT01782443 | Progressive NETs with G1/2 | Ziv-aflibercept | Octreotide LAR | 2 | Progression-free survival |
| NCT04525638 | Locally unresectable, recurrent or metastatic NETs with G3 and NECs | Nivolumab | 177Lu-dotatate | 2 | Overall response rate |
| NCT03980925 | Locally unresectable or metastatic, NETs with G3 and NECs | Nivolumab | Carboplatin, etoposide | 2 | Overall survival |
| NCT03728361 | Progressive NECs | Nivolumab | Temozolomide | 2 | Objective response rate |
| NCT05058651 | Small cell NECs | Atezolizumab | Platinum drug (cisplatin or carboplatin) and etoposide | 2, 3 | Overall survival |
| NCT03457948 | Progressive NETs | Pembrolizumab | 177Lu-dota0-Tyr3-octreotate, or arterial embolization, or yttrium-90 microsphere radioembolization | 2 | Overall response rate |
| NCT03136055 | Progressive NECs | Pembrolizumab | Irinotecan, paclitaxel | 2 | Overall response rate |
| NCT03043664 | Progressive NETs with G1/2 | Pembrolizumab | Lanreotide | 1,2 | Overall response rate |
| NCT02489903 | Progressive NETs with G3 and NECs | RRx-001 | Platinum based doublet regimen | 2 | Overall survival |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Ye, C.; Chen, R.; Li, Q.; Ruan, J. The Landscape and Clinical Application of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2022, 14, 2911. https://doi.org/10.3390/cancers14122911
Xu S, Ye C, Chen R, Li Q, Ruan J. The Landscape and Clinical Application of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2022; 14(12):2911. https://doi.org/10.3390/cancers14122911
Chicago/Turabian StyleXu, Shuaishuai, Chanqi Ye, Ruyin Chen, Qiong Li, and Jian Ruan. 2022. "The Landscape and Clinical Application of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Neoplasms" Cancers 14, no. 12: 2911. https://doi.org/10.3390/cancers14122911





