Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression
Abstract
:Simple Summary
Abstract
1. Introduction
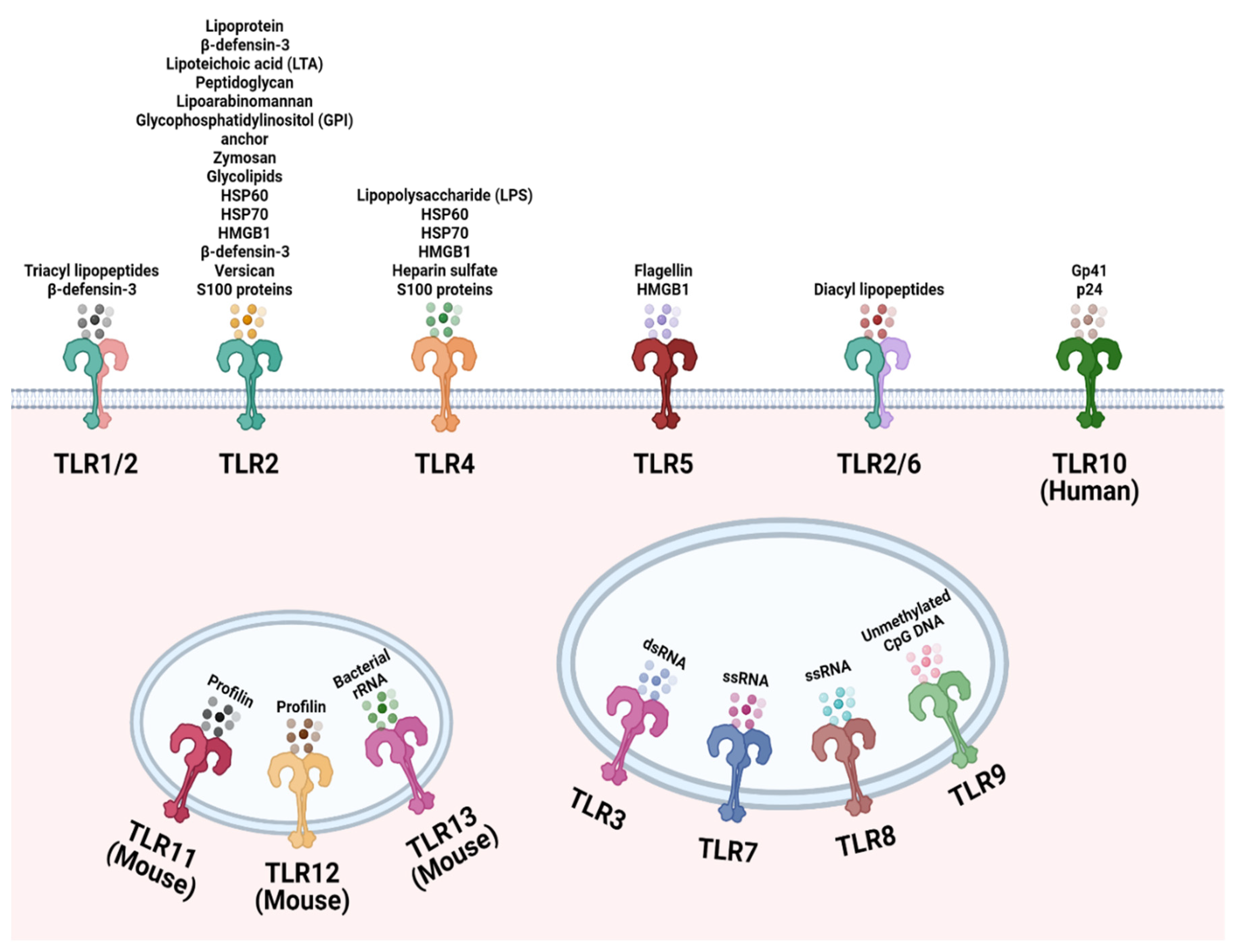
2. TLR Localization and Recognition of Microbial Ligands
3. Expression of TLRs on Tumor Cells and Its Clinical Relevance
4. Microbial Derived TLR Agonists and Their Role in Cancer Immunotherapy
5. Bacterial-Derived TLR Agonists
6. Viral-Derived TLR Agonists
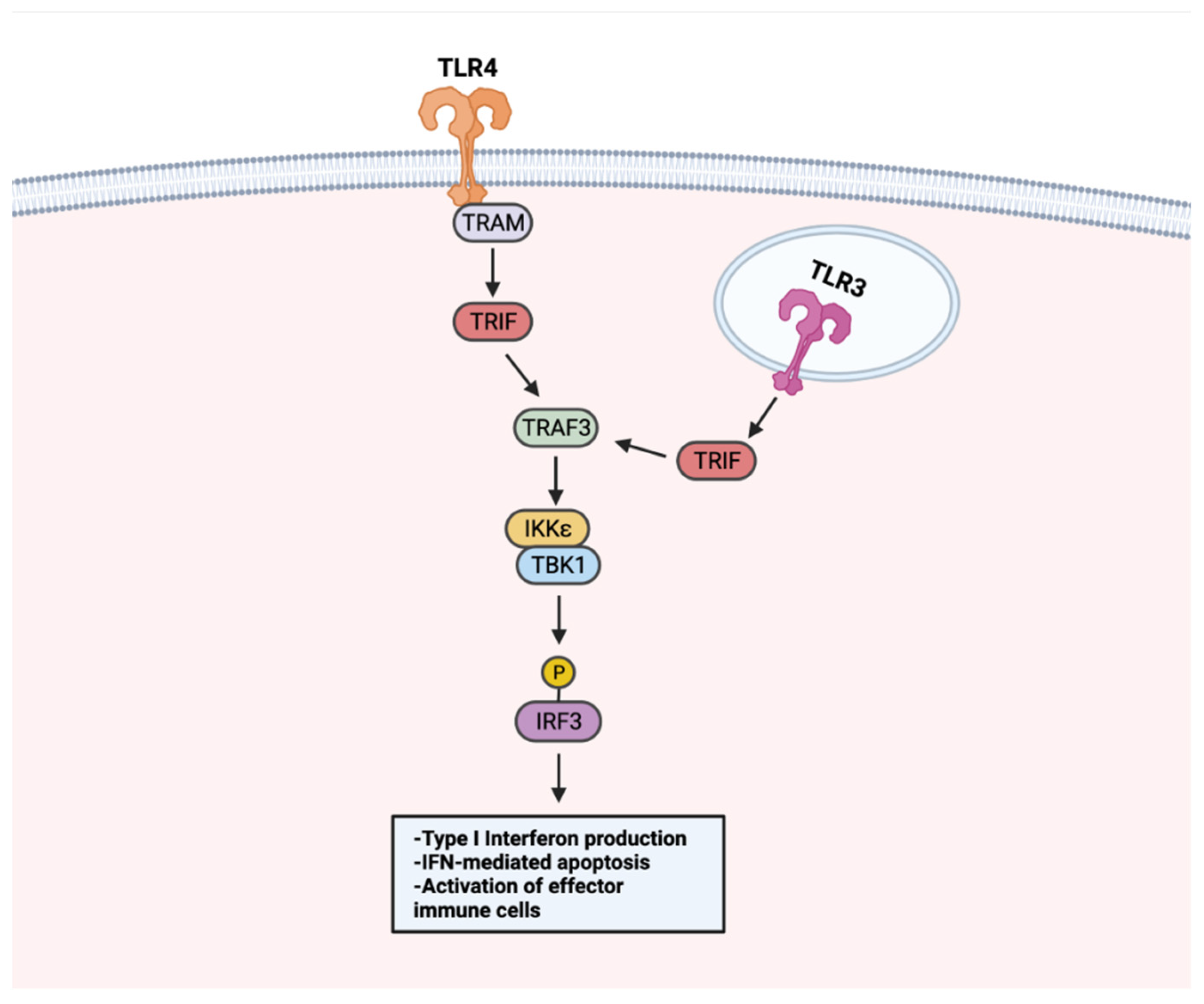
7. TLR–TLR Cross-Talk and the Modulation of Immune Response
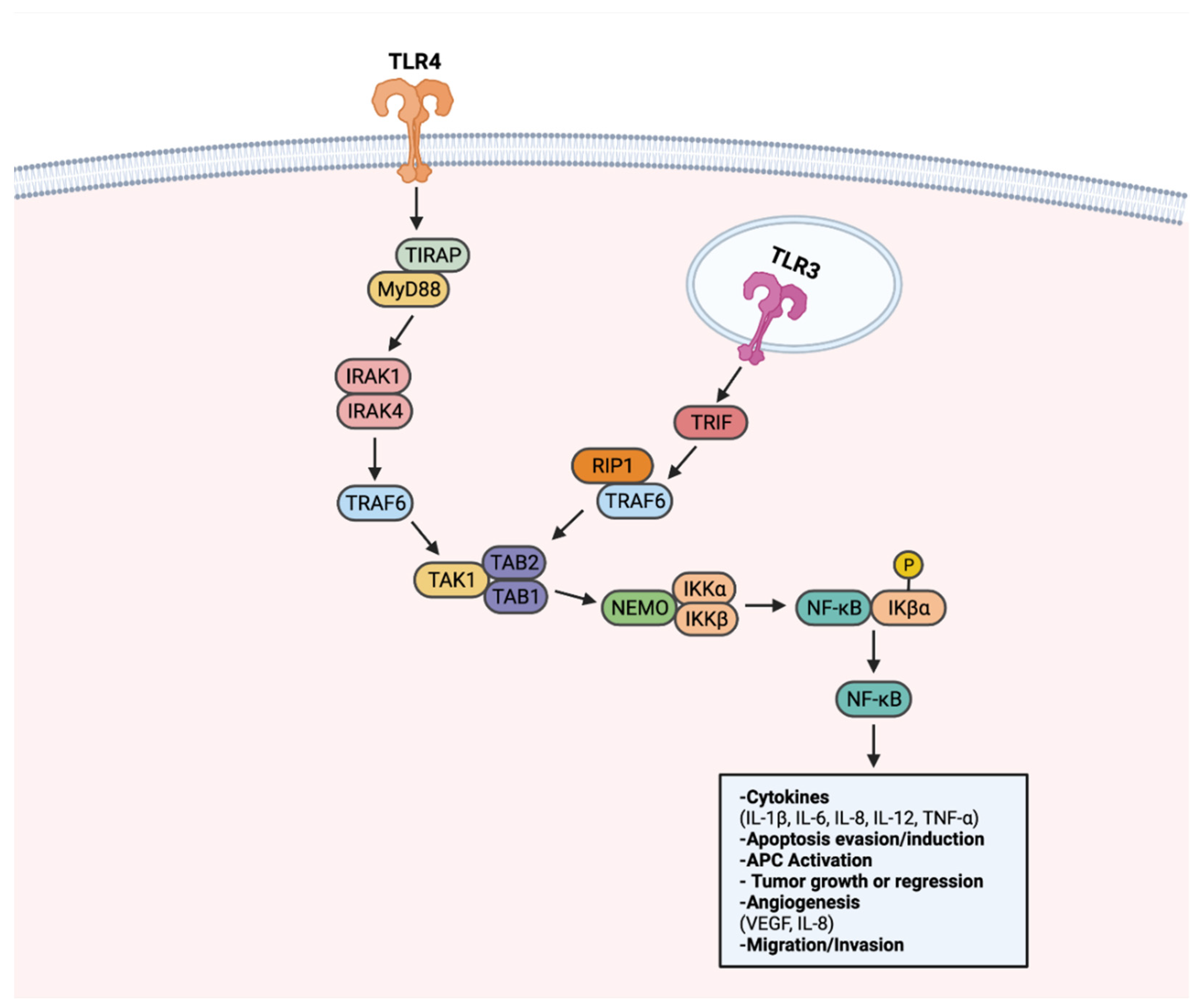
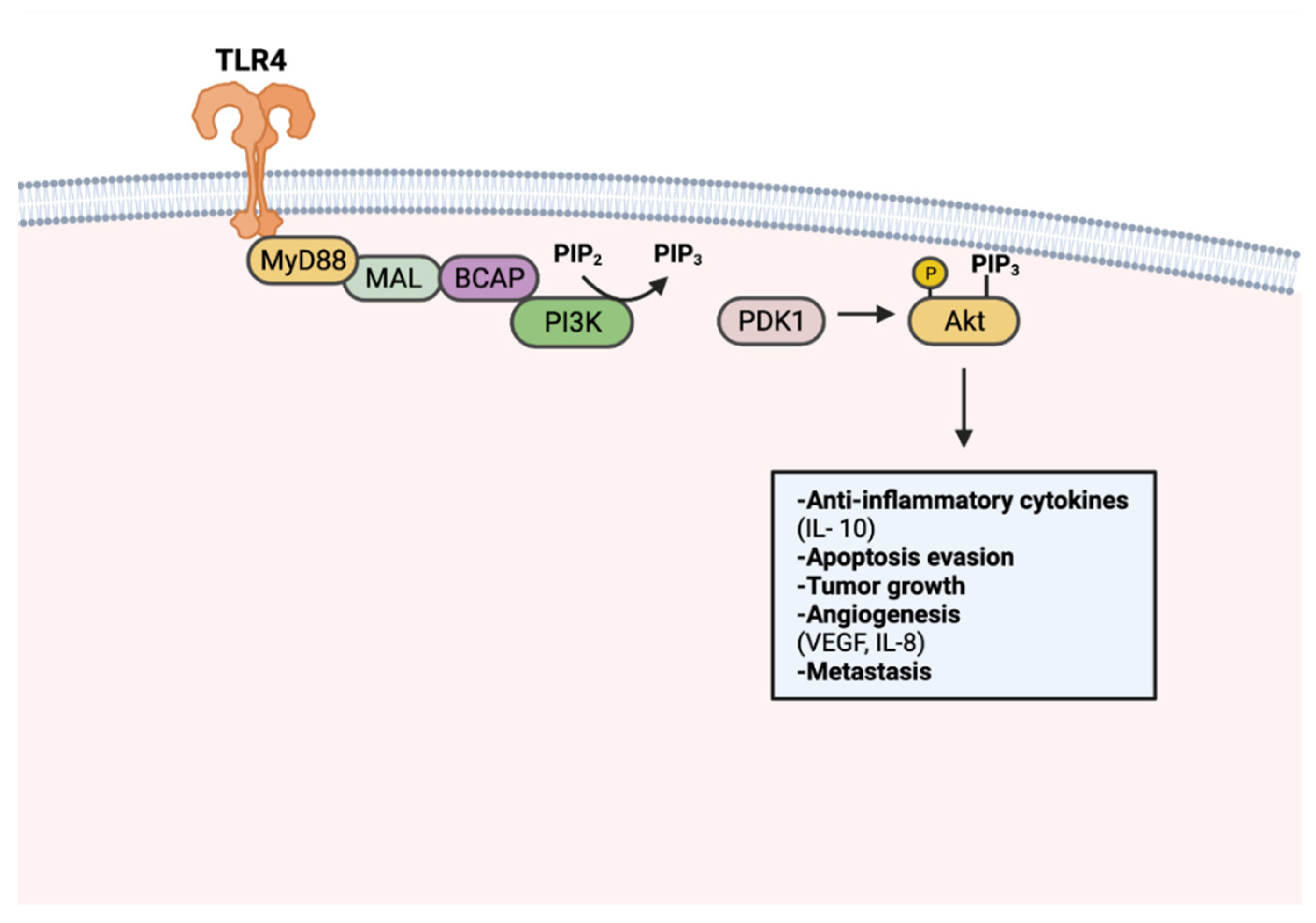
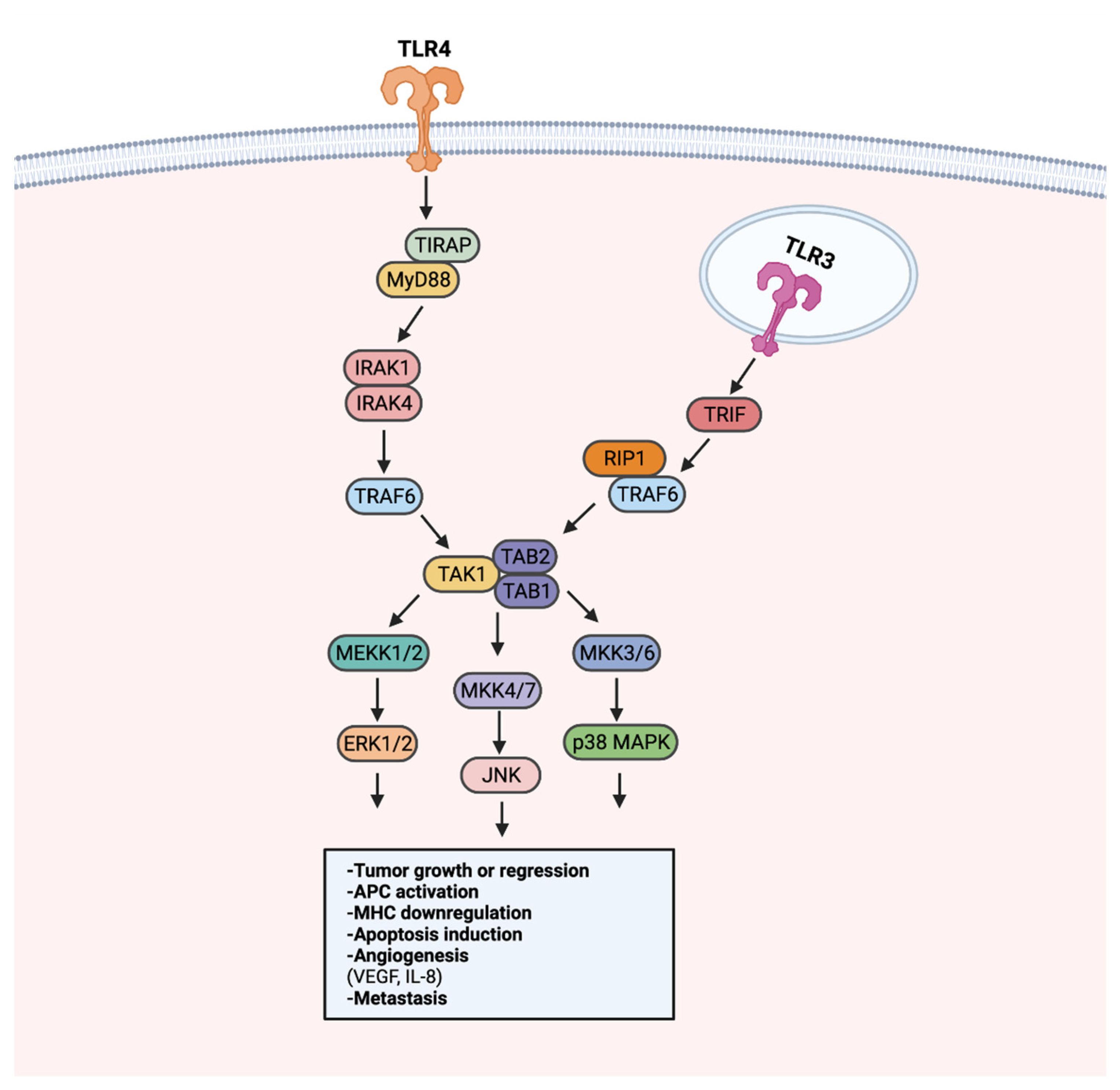
8. TLR Signaling in Cancer
8.1. Effects of Tumor-Promoting TLR Signaling
8.2. Effects of Anti-Tumor TLR Signaling
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Imler, J.L.; Hoffmann, J.A. Toll receptors in Drosophila: A family of molecules regulating development and immunity. Curr. Top. Microbiol. Immunol. 2002, 270, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, C.; Hudson, K.L.; Anderson, K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988, 52, 269–279. [Google Scholar] [CrossRef]
- Vogel, S.N. How Discovery of Toll-Mediated Innate Immunity in Drosophila Impacted Our Understanding of TLR Signaling (and Vice Versa). J. Immunol. 2012, 188, 5207–5209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutler, B. Toll-like receptors: How they work and what they do. Curr. Opin. Hematol. 2002, 9, 2–10. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Kobe, B.; Deisenhofer, J. Proteins with leucine-rich repeats. Curr. Opin. Struct. Biol. 1995, 5, 409–416. [Google Scholar] [CrossRef]
- Rock, F.L.; Hardiman, G.; Timans, J.C.; Kastelein, R.A.; Bazan, J.F. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 1998, 95, 588–593. [Google Scholar] [CrossRef] [Green Version]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Akira, S. Toll-like receptors in innate immunity. Int. Immunol. 2005, 17, 1–14. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Takeuchi, O.; Kawai, T.; Hoshino, K.; Akira, S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 2001, 166, 5688–5694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbibe, L.; Mira, J.P.; Teusch, N.; Kline, L.; Guha, M.; Mackman, N.; Godowski, P.J.; Ulevitch, R.J.; Knaus, U.G. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 2000, 1, 533–540. [Google Scholar] [CrossRef]
- Santoni, M.; Andrikou, K.; Sotte, V.; Bittoni, A.; Lanese, A.; Pellei, C.; Piva, F.; Conti, A.; Nabissi, M.; Santoni, G.; et al. Toll like receptors and pancreatic diseases: From a pathogenetic mechanism to a therapeutic target. Cancer Treat. Rev. 2015, 41, 569–576. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: Impact on the adaptive immune response. Curr. Opin. Immunol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Szekely, B.; Bossuyt, V.; Li, X.; Wali, V.B.; Patwardhan, G.A.; Frederick, C.; Silber, A.; Park, T.; Harigopal, M.; Pelekanou, V.; et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018, 29, 2232–2239. [Google Scholar] [CrossRef]
- Mehmeti, M.; Allaoui, R.; Bergenfelz, C.; Saal, L.H.; Ethier, S.P.; Johansson, M.E.; Jirström, K.; Leandersson, K. Expression of functional toll like receptor 4 in estrogen receptor/progesterone receptor-negative breast cancer. Breast Cancer Res. 2015, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, S.; Zhang, Y.; Yang, J. Dysregulation of TLR2 Serves as a Prognostic Biomarker in Breast Cancer and Predicts Resistance to Endocrine Therapy in the Luminal B Subtype. Front. Oncol. 2020, 10, 547. [Google Scholar] [CrossRef]
- González-Reyes, S.; Marín, L.; González, L.; González, L.O.; del Casar, J.M.; Lamelas, M.L.; González-Quintana, J.M.; Vizoso, F.J. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010, 10, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaun, B.; Coste, I.; Rissoan, M.C.; Lebecque, S.J.; Renno, T. TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 2006, 176, 4894–4901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Reyes, S.; Fernández, J.M.; González, L.O.; Aguirre, A.; Suárez, A.; González, J.M.; Escaff, S.; Vizoso, F.J. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol. Immunother. 2011, 60, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Haricharan, S.; Brown, P. TLR4 has a TP53-dependent dual role in regulating breast cancer cell growth. Proc. Natl. Acad. Sci. USA 2015, 112, E3216–E3225. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Zhao, S.; Jiang, W.; Zhang, C.; Liang, T.; Hou, G. TLR5: A prognostic and monitoring indicator for triple-negative breast cancer. Cell Death Dis. 2019, 10, 954. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like receptor 5 on breast cancer cells by flagellin suppresses cell proliferation and tumor growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Han, Y.; Liang, T.; Zhang, C.; Gao, F.; Hou, G. Down-Regulation of Toll-Like Receptor 5 (TLR5) Increased VEGFR Expression in Triple Negative Breast Cancer (TNBC) Based on Radionuclide Imaging. Front. Oncol. 2021, 11, 708047. [Google Scholar] [CrossRef]
- Diao, Y.; Wang, X.; Wan, Y.; Zhong, J.; Gao, D.; Liu, Y.; Gao, N.; Li, W.; Liu, B.; Huang, X.; et al. Antitumor activity of a novel small molecule TLR7 agonist via immune response induction and tumor microenvironment modulation. Oncol. Rep. 2016, 35, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.F.; Grimm, A.J.; Chiang, C.L.; Mookerjee, A.; Flies, D.; Jean, S.; McCann, G.A.; Michaux, J.; Pak, H.; Huber, F.; et al. Rapid tumor vaccine using Toll-like receptor-activated ovarian cancer ascites monocytes. J. Immunother. Cancer 2020, 8, e000875. [Google Scholar] [CrossRef]
- Tuomela, J.; Sandholm, J.; Karihtala, P.; Ilvesaro, J.; Vuopala, K.S.; Kauppila, J.H.; Kauppila, S.; Chen, D.; Pressey, C.; Härkönen, P.; et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res. Treat. 2012, 135, 481–493. [Google Scholar] [CrossRef]
- Droemann, D.; Albrecht, D.; Gerdes, J.; Ulmer, A.J.; Branscheid, D.; Vollmer, E.; Dalhoff, K.; Zabel, P.; Goldmann, T. Human lung cancer cells express functionally active Toll-like receptor 9. Respir. Res. 2005, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Crozet, L.; Damotte, D.; Iribarren, K.; Schramm, C.; Alifano, M.; Lupo, A.; Cherfils-Vicini, J.; Goc, J.; Katsahian, S.; et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res. 2014, 74, 5008–5018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, F.; Milione, M.; Casalini, P.; Centonze, G.; Le Noci, V.M.; Storti, C.; Alexiadis, S.; Truini, M.; Sozzi, G.; Pastorino, U.; et al. Toll-like receptor 3 as a new marker to detect high risk early stage Non-Small-Cell Lung Cancer patients. Sci. Rep. 2019, 9, 14288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Yang, J.; Qian, J.; Liu, R.; Huang, E.; Wang, Y.; Luo, F.; Chu, Y. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol. Immunol. 2019, 112, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, J.H.; Hu, J.; Luo, Y.Z.; Li, F.; Xiao, L.; Zhong, M.Z. High expression of Toll-like receptor 5 correlates with better prognosis in non-small-cell lung cancer: An anti-tumor effect of TLR5 signaling in non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 633–643. [Google Scholar] [CrossRef]
- Bianchi, F.; Alexiadis, S.; Camisaschi, C.; Truini, M.; Centonze, G.; Milione, M.; Balsari, A.; Tagliabue, E.; Sfondrini, L. TLR3 Expression Induces Apoptosis in Human Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 1440. [Google Scholar] [CrossRef] [Green Version]
- Vinod, N.; Hwang, D.; Azam, S.H.; Van Swearingen, A.E.D.; Wayne, E.; Fussell, S.C.; Sokolsky-Papkov, M.; Pecot, C.V.; Kabanov, A.V. High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma. Sci. Adv. 2020, 6, eaba5542. [Google Scholar] [CrossRef]
- Cherfils-Vicini, J.; Platonova, S.; Gillard, M.; Laurans, L.; Validire, P.; Caliandro, R.; Magdeleinat, P.; Mami-Chouaib, F.; Dieu-Nosjean, M.C.; Fridman, W.H.; et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J. Clin. Investig. 2010, 120, 1285–1297. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Wei, F.; Zhao, N.; Yang, F.; Ren, X. Expression of TLR4 in Non-Small Cell Lung Cancer Is Associated with PD-L1 and Poor Prognosis in Patients Receiving Pulmonectomy. Front. Immunol. 2017, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Waldmannová, E.; Caisová, V.; Fáberová, J.; Sváčková, P.; Kovářová, M.; Sváčková, D.; Kumžáková, Z.; Jačková, A.; Vácová, N.; Nedbalová, P.; et al. The use of Zymosan A and bacteria anchored to tumor cells for effective cancer immunotherapy: B16-F10 murine melanoma model. Int. Immunopharmacol. 2016, 39, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Arigami, T.; Kitago, M.; Nguyen, S.L.; Narita, N.; Ferrone, S.; Morton, D.L.; Irie, R.F.; Hoon, D.S. Activation of Toll-like receptors 2, 3, and 4 on human melanoma cells induces inflammatory factors. Mol. Cancer Ther. 2008, 7, 3642–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Jean, M.; Knol, A.C.; Nguyen, J.M.; Khammari, A.; Dréno, B. TLR expression in human melanoma cells. Eur. J. Dermatol. 2011, 21, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Eiró, N.; Ovies, C.; Fernandez-Garcia, B.; Álvarez-Cuesta, C.C.; González, L.; González, L.O.; Vizoso, F.J. Expression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: Relationship with clinicopathological characteristics and prognosis. Arch. Dermatol. Res. 2013, 305, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Q.; Liu, B.; Wang, Y.P.; Li, J.K.; Zhu, P.L.; Li, T.; Tse, K.W.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; et al. Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression. Cell Death Dis. 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, Z.; Wang, J.; Yao, X. Toll-like receptors 7 and 8 expression correlates with the expression of immune biomarkers and positively predicts the clinical outcome of patients with melanoma. OncoTargets Ther. 2017, 10, 4339–4346. [Google Scholar] [CrossRef] [Green Version]
- Aspord, C.; Tramcourt, L.; Leloup, C.; Molens, J.P.; Leccia, M.T.; Charles, J.; Plumas, J. Imiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularization. J. Investig. Dermatol. 2014, 134, 2551–2561. [Google Scholar] [CrossRef] [Green Version]
- Kapp, K.; Volz, B.; Curran, M.A.; Oswald, D.; Wittig, B.; Schmidt, M. EnanDIM—A novel family of L-nucleotide-protected TLR9 agonists for cancer immunotherapy. J. Immunother. Cancer 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Nihon-Yanagi, Y.; Terai, K.; Murano, T.; Matsumoto, T.; Okazumi, S. Tissue expression of Toll-like receptors 2 and 4 in sporadic human colorectal cancer. Cancer Immunol. Immunother. 2012, 61, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Beilmann-Lehtonen, I.; Hagström, J.; Kaprio, T.; Stenman, U.H.; Strigård, K.; Palmqvist, R.; Gunnarsson, U.; Böckelman, C.; Haglund, C. The Relationship between the Tissue Expression of TLR2, TLR4, TLR5, and TLR7 and Systemic Inflammatory Responses in Colorectal Cancer Patients. Oncology 2021, 99, 790–801. [Google Scholar] [CrossRef]
- Lu, C.C.; Kuo, H.C.; Wang, F.S.; Jou, M.H.; Lee, K.C.; Chuang, J.H. Upregulation of TLRs and IL-6 as a marker in human colorectal cancer. Int. J. Mol. Sci. 2014, 16, 159–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Miura, T.; Matsumiya, T.; Yoshida, H.; Morohashi, H.; Sakamoto, Y.; Kurose, A.; Imaizumi, T.; Hakamada, K. Toll-Like Receptor 3 as a Recurrence Risk Factor and a Potential Molecular Therapeutic Target in Colorectal Cancer. Clin. Exp. Gastroenterol. 2020, 13, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Bugge, M.; Bergstrom, B.; Eide, O.K.; Solli, H.; Kjønstad, I.F.; Stenvik, J.; Espevik, T.; Nilsen, N.J. Surface Toll-like receptor 3 expression in metastatic intestinal epithelial cells induces inflammatory cytokine production and promotes invasiveness. J. Biol. Chem. 2017, 292, 15408–15425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammarota, R.; Bertolini, V.; Pennesi, G.; Bucci, E.O.; Gottardi, O.; Garlanda, C.; Laghi, L.; Barberis, M.C.; Sessa, F.; Noonan, D.M.; et al. The tumor microenvironment of colorectal cancer: Stromal TLR-4 expression as a potential prognostic marker. J. Transl. Med. 2010, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.H.; Im, E.; Pothoulakis, C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology 2008, 135, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xie, Y.; Xiong, Y.; Liu, S.; Qiu, C.; Zhu, Z.; Mao, H.; Yu, M.; Wang, X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020, 469, 173–185. [Google Scholar] [CrossRef]
- Gao, C.; Qiao, T.; Zhang, B.; Yuan, S.; Zhuang, X.; Luo, Y. TLR9 signaling activation at different stages in colorectal cancer and NF-kappaB expression. OncoTargets Ther. 2018, 11, 5963–5971. [Google Scholar] [CrossRef] [Green Version]
- Lundy, J.; Gearing, L.J.; Gao, H.; West, A.C.; McLeod, L.; Deswaerte, V.; Yu, L.; Porazinski, S.; Pajic, M.; Hertzog, P.J.; et al. TLR2 activation promotes tumour growth and associates with patient survival and chemotherapy response in pancreatic ductal adenocarcinoma. Oncogene 2021, 40, 6007–6022. [Google Scholar] [CrossRef]
- Grimmig, T.; Moench, R.; Kreckel, J.; Haack, S.; Rueckert, F.; Rehder, R.; Tripathi, S.; Ribas, C.; Chandraker, A.; Germer, C.T.; et al. Toll Like Receptor 2, 4, and 9 Signaling Promotes Autoregulative Tumor Cell Growth and VEGF/PDGF Expression in Human Pancreatic Cancer. Int. J. Mol. Sci. 2016, 17, 2060. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.L.; Malgor, R.; Dickerson, E.; Weeraratna, A.T.; Slominski, A.; Wortsman, J.; Harii, N.; Kohn, A.D.; Moon, R.T.; Schwartz, F.L.; et al. Phenylmethimazole decreases Toll-like receptor 3 and noncanonical Wnt5a expression in pancreatic cancer and melanoma together with tumor cell growth and migration. Clin. Cancer Res. 2009, 15, 4114–4122. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, H.; Oberg, H.H.; Juricke, M.; Marischen, L.; Kunz, M.; Mundhenke, C.; Gieseler, F.; Kabelitz, D.; Wesch, D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human gammadelta T cells. Cancer Res. 2009, 69, 8710–8717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochi, A.; Nguyen, A.H.; Bedrosian, A.S.; Mushlin, H.M.; Zarbakhsh, S.; Barilla, R.; Zambirinis, C.P.; Fallon, N.C.; Rehman, A.; Pylayeva-Gupta, Y.; et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med. 2012, 209, 1671–1687. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, T.; Matthes, N.; Hoeland, K.; Tripathi, S.; Chandraker, A.; Grimm, M.; Moench, R.; Moll, E.M.; Friess, H.; Tsaur, I.; et al. TLR7 and TLR8 expression increases tumor cell proliferation and promotes chemoresistance in human pancreatic cancer. Int. J. Oncol. 2015, 47, 857–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef]
- Zambirinis, C.P.; Levie, E.; Nguy, S.; Avanzi, A.; Barilla, R.; Xu, Y.; Seifert, L.; Daley, D.; Greco, S.H.; Deutsch, M.; et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015, 212, 2077–2094. [Google Scholar] [CrossRef]
- Volk-Draper, L.; Hall, K.; Griggs, C.; Rajput, S.; Kohio, P.; DeNardo, D.; Ran, S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014, 74, 5421–5434. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; McFarland-Mancini, M.M.; Funk, H.M.; Husseinzadeh, N.; Mounajjed, T.; Drew, A.F. Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol. Immunother. 2009, 58, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.G.; Alvero, A.B.; Chen, R.; Silasi, D.A.; Abrahams, V.M.; Chan, S.; Visintin, I.; Rutherford, T.; Mor, G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006, 66, 3859–3868. [Google Scholar] [CrossRef] [Green Version]
- Szajnik, M.; Szczepanski, M.J.; Czystowska, M.; Elishaev, E.; Mandapathil, M.; Nowak-Markwitz, E.; Spaczynski, M.; Whiteside, T.L. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene 2009, 28, 4353–4363. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.C.; Su, Q.B.; Wu, F.X.; Zhang, X.L.; Liu, P.S. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur. J. Clin. Investig. 2009, 39, 157–164. [Google Scholar] [CrossRef]
- Block, M.S.; Vierkant, R.A.; Rambau, P.F.; Winham, S.J.; Wagner, P.; Traficante, N.; Tołoczko, A.; Tiezzi, D.G.; Taran, F.A.; Sinn, P.; et al. MyD88 and TLR4 Expression in Epithelial Ovarian Cancer. Mayo Clin. Proc. 2018, 93, 307–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.B.; Kim, D. TLR5/7-mediated PI3K activation triggers epithelial-mesenchymal transition of ovarian cancer cells through WAVE3-dependent mesothelin or OCT4/SOX2 expression. Oncol. Rep. 2017, 38, 3167–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Feng, D.; Yao, Y.; Li, P.; Sun, H.; Yang, H.; Li, C.; Jiang, R.; Sun, B.; Chen, Y. Listeria-based hepatocellular carcinoma vaccine facilitates anti-PD-1 therapy by regulating macrophage polarization. Oncogene 2020, 39, 1429–1444. [Google Scholar] [CrossRef]
- Berger, R.; Fiegl, H.; Goebel, G.; Obexer, P.; Ausserlechner, M.; Doppler, W.; Hauser-Kronberger, C.; Reitsamer, R.; Egle, D.; Reimer, D.; et al. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci. 2010, 101, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Galli, R.; Starace, D.; Busà, R.; Angelini, D.F.; Paone, A.; De Cesaris, P.; Filippini, A.; Sette, C.; Battistini, L.; Ziparo, E.; et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J. Immunol. 2010, 184, 6658–6669. [Google Scholar] [CrossRef]
- Rezania, S.; Amirmozaffari, N.; Rashidi, N.; Mirzadegan, E.; Zarei, S.; Ghasemi, J.; Zarei, O.; Katouzian, L.; Zarnani, A.H. The same and not the same: Heterogeneous functional activation of prostate tumor cells by TLR ligation. Cancer Cell Int. 2014, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Paone, A.; Starace, D.; Galli, R.; Padula, F.; De Cesaris, P.; Filippini, A.; Ziparo, E.; Riccioli, A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis 2008, 29, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Hua, D.; Liu, M.Y.; Cheng, Z.D.; Qin, X.J.; Zhang, H.M.; Chen, Y.; Qin, G.J.; Liang, G.; Li, J.N.; Han, X.F.; et al. Small interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Mol. Immunol. 2009, 46, 2876–2884. [Google Scholar] [CrossRef]
- Väisänen, M.R.; Väisänen, T.; Jukkola-Vuorinen, A.; Vuopala, K.S.; Desmond, R.; Selander, K.S.; Vaarala, M.H. Expression of toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate 2010, 70, 817–824. [Google Scholar] [CrossRef]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487. [Google Scholar] [CrossRef]
- Gupta, K.H.; Nowicki, C.; Giurini, E.F.; Marzo, A.L.; Zloza, A. Bacterial-Based Cancer Therapy (BBCT): Recent Advances, Current Challenges, and Future Prospects for Cancer Immunotherapy. Vaccines 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Orange, M.; Reuter, U.; Hobohm, U. Coley’s Lessons Remembered: Augmenting Mistletoe Therapy. Integr. Cancer Ther. 2016, 15, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Hobohm, U.; Stanford, J.L.; Grange, J.M. Pathogen-associated molecular pattern in cancer immunotherapy. Crit. Rev. Immunol. 2008, 28, 95–107. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Bendelac, A.; Medzhitov, R. Adjuvants of immunity: Harnessing innate immunity to promote adaptive immunity. J. Exp. Med. 2002, 195, F19–F23. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, N.; Bettegowda, C.; Cheong, I.; Geschwind, J.F.; Drake, C.G.; Hipkiss, E.L.; Tatsumi, M.; Dang, L.H.; Diaz, L.A., Jr.; Pomper, M.; et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 15172–15177. [Google Scholar] [CrossRef] [Green Version]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Martínez-Vélez, N.; Garcia-Moure, M.; Marigil, M.; González-Huarriz, M.; Puigdelloses, M.; Gallego Pérez-Larraya, J.; Zalacaín, M.; Marrodán, L.; Varela-Guruceaga, M.; Laspidea, V.; et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models. Nat. Commun. 2019, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene 2008, 27, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, A.H.; Taylor, D.K.; Turka, L.A. The contribution of direct TLR signaling to T cell responses. Immunol. Res. 2009, 45, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabelitz, D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007, 19, 39–45. [Google Scholar] [CrossRef]
- Cerullo, V.; Diaconu, I.; Romano, V.; Hirvinen, M.; Ugolini, M.; Escutenaire, S.; Holm, S.L.; Kipar, A.; Kanerva, A.; Hemminki, A. An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol. Ther. 2012, 20, 2076–2086. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.Y.; Yoon, S.R.; Kim, T.D.; Choi, I.; Jung, H. Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy. J. Immunol. Res. 2020, 2020, 2045860. [Google Scholar] [CrossRef]
- Adib-Conquy, M.; Scott-Algara, D.; Cavaillon, J.M.; Souza-Fonseca-Guimaraes, F. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol. Cell Biol. 2014, 92, 256–262. [Google Scholar] [CrossRef]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.K.; Redecke, V.; Prilliman, K.R.; Takabayashi, K.; Corr, M.; Tallant, T.; DiDonato, J.; Dziarski, R.; Akira, S.; Schoenberger, S.P.; et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J. Immunol. 2003, 170, 4102–4110. [Google Scholar] [CrossRef] [Green Version]
- Crespo, M.I.; Zacca, E.R.; Núñez, N.G.; Ranocchia, R.P.; Maccioni, M.; Maletto, B.A.; Pistoresi-Palencia, M.C.; Morón, G. TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8α+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface. J. Immunol. 2013, 190, 948–960. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Z.; Kurche, J.S.; Burchill, M.A.; Kedl, R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 2011, 118, 3028–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebel, M.; Chartrand, K.; Tarrab, E.; Savard, P.; Leclerc, D.; Lamarre, A. Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016, 16, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Shukla, S.; Wang, C.; Masarapu, H.; Steinmetz, N.F. Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine. J. Am. Chem. Soc. 2019, 141, 6509–6518. [Google Scholar] [CrossRef] [PubMed]
- Kines, R.C.; Thompson, C.D.; Spring, S.; Li, Z.; de Los Pinos, E.; Monks, S.; Schiller, J.T. Virus-Like Particle-Drug Conjugates Induce Protective, Long-lasting Adaptive Antitumor Immunity in the Absence of Specifically Targeted Tumor Antigens. Cancer Immunol. Res. 2021, 9, 693–706. [Google Scholar] [CrossRef]
- Shukla, S.; Wang, C.; Beiss, V.; Steinmetz, N.F. Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer. ACS Nano 2020, 14, 2994–3003. [Google Scholar] [CrossRef]
- De Nardo, D.; De Nardo, C.M.; Nguyen, T.; Hamilton, J.A.; Scholz, G.M. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J. Immunol. 2009, 183, 8110–8118. [Google Scholar] [CrossRef]
- Sørensen, L.N.; Reinert, L.S.; Malmgaard, L.; Bartholdy, C.; Thomsen, A.R.; Paludan, S.R. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 2008, 181, 8604–8612. [Google Scholar] [CrossRef]
- Sieben, M.; Schäfer, P.; Dinsart, C.; Galle, P.R.; Moehler, M. Activation of the human immune system via toll-like receptors by the oncolytic parvovirus H-1. Int. J. Cancer 2013, 132, 2548–2556. [Google Scholar] [CrossRef]
- Zhu, J.; Martinez, J.; Huang, X.; Yang, Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 2007, 109, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Samuelsson, C.; Hausmann, J.; Lauterbach, H.; Schmidt, M.; Akira, S.; Wagner, H.; Chaplin, P.; Suter, M.; O’Keeffe, M.; Hochrein, H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 2008, 118, 1776–1784. [Google Scholar] [CrossRef]
- Whitmore, M.M.; DeVeer, M.J.; Edling, A.; Oates, R.K.; Simons, B.; Lindner, D.; Williams, B.R. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004, 64, 5850–5860. [Google Scholar] [CrossRef] [Green Version]
- Madan-Lala, R.; Pradhan, P.; Roy, K. Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep. 2017, 7, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Anfray, C.; Mainini, F.; Digifico, E.; Maeda, A.; Sironi, M.; Erreni, M.; Anselmo, A.; Ummarino, A.; Gandoy, S.; Expósito, F.; et al. Intratumoral combination therapy with poly(I:C) and resiquimod synergistically triggers tumor-associated macrophages for effective systemic antitumoral immunity. J. Immunother. Cancer 2021, 9, e002408. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-S.; Leng, C.-H.; Yeh, Y.-C.; Wu, C.-C.; Chen, H.-W.; Huang, H.-M.; Liu, S.-J. Toll-like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol. Cancer 2014, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Bocanegra Gondan, A.I.; Ruiz-de-Angulo, A.; Zabaleta, A.; Gómez Blanco, N.; Cobaleda-Siles, B.M.; García-Granda, M.J.; Padro, D.; Llop, J.; Arnaiz, B.; Gato, M.; et al. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials 2018, 170, 95–115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; He, B. Recognition of herpes simplex viruses: Toll-like receptors and beyond. J. Mol. Biol. 2014, 426, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Uyangaa, E.; Choi, J.Y.; Patil, A.M.; Hossain, F.M.A.; Park, S.O.; Kim, B.; Kim, K.; Eo, S.K. Dual TLR2/9 Recognition of Herpes Simplex Virus Infection Is Required for Recruitment and Activation of Monocytes and NK Cells and Restriction of Viral Dissemination to the Central Nervous System. Front. Immunol. 2018, 9, 905. [Google Scholar] [CrossRef]
- Bafica, A.; Scanga, C.A.; Feng, C.G.; Leifer, C.; Cheever, A.; Sher, A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 2005, 202, 1715–1724. [Google Scholar] [CrossRef]
- Kumar, P.; John, V.; Marathe, S.; Das, G.; Bhaskar, S. Mycobacterium indicus pranii induces dendritic cell activation, survival, and Th1/Th17 polarization potential in a TLR-dependent manner. J. Leukoc. Biol. 2015, 97, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Sfondrini, L.; Rossini, A.; Besusso, D.; Merlo, A.; Tagliabue, E.; Mènard, S.; Balsari, A. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 2006, 176, 6624–6630. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhan, U.; Ballinger, M.N.; Zeng, X.; Newstead, M.J.; Cornicelli, M.D.; Standiford, T.J. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS ONE 2010, 5, e9896. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; De May, H.; Franco, S.; Noureddine, A.; Tang, L.; Brinker, C.J.; Kusewitt, D.F.; Adams, S.F.; Serda, R.E. Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nat. Biomed. Eng. 2022, 6, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Oshikawa, T.; Okamoto, M.; Tano, T.; Sasai, A.; Kan, S.; Moriya, Y.; Ryoma, Y.; Saito, M.; Akira, S.; Sato, M. Antitumor effect of OK-432-derived DNA: One of the active constituents of OK-432, a streptococcal immunotherapeutic agent. J. Immunother. 2006, 29, 143–150. [Google Scholar] [CrossRef]
- Koya, T.; Yanagisawa, R.; Higuchi, Y.; Sano, K.; Shimodaira, S. Interferon-α-inducible Dendritic Cells Matured with OK-432 Exhibit TRAIL and Fas Ligand Pathway-mediated Killer Activity. Sci. Rep. 2017, 7, 42145. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.D.; Lendvai, N.; Nataraj, S.; Imai, N.; Jungbluth, A.A.; Tsakos, I.; Rahman, A.; Mei, A.H.; Singh, H.; Zarychta, K.; et al. Autologous Lymphocyte Infusion Supports Tumor Antigen Vaccine-Induced Immunity in Autologous Stem Cell Transplant for Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 658–669. [Google Scholar] [CrossRef]
- Hamilton, E.; Blackwell, K.; Hobeika, A.C.; Clay, T.M.; Broadwater, G.; Ren, X.R.; Chen, W.; Castro, H.; Lehmann, F.; Spector, N.; et al. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J. Transl. Med. 2012, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Limentani, S.A.; Campone, M.; Dorval, T.; Curigliano, G.; de Boer, R.; Vogel, C.; White, S.; Bachelot, T.; Canon, J.L.; Disis, M.; et al. A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res. Treat. 2016, 156, 319–330. [Google Scholar] [CrossRef]
- McQuade, J.L.; Homsi, J.; Torres-Cabala, C.A.; Bassett, R.; Popuri, R.M.; James, M.L.; Vence, L.M.; Hwu, W.J. A phase II trial of recombinant MAGE-A3 protein with immunostimulant AS15 in combination with high-dose Interleukin-2 (HDIL2) induction therapy in metastatic melanoma. BMC Cancer 2018, 18, 1274. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, Y.; Wang, W.; Li, J.; Xie, G.; Zhao, Y.; Xu, D.; Shen, L. Activation of TLR4 signaling promotes gastric cancer progression by inducing mitochondrial ROS production. Cell Death Dis. 2013, 4, e794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. Corrigendum to “TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance” [Mol. Immunol. 44 (2007) 2850–2859]. Mol. Immunol. 2020, 122, 232–234. [Google Scholar] [CrossRef]
- Szczepanski, M.J.; Czystowska, M.; Szajnik, M.; Harasymczuk, M.; Boyiadzis, M.; Kruk-Zagajewska, A.; Szyfter, W.; Zeromski, J.; Whiteside, T.L. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009, 69, 3105–3113. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Lucas, R.M.; Liu, L.; Stow, J.L. Signalling, sorting and scaffolding adaptors for Toll-like receptors. J. Cell Sci. 2019, 133, 239194. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, C.; Ma, J.; Yang, Y.; Man, X.; Wu, H.; Li, S. Toll-like receptor 4 promotes angiogenesis in pancreatic cancer via PI3K/AKT signaling. Exp. Cell Res. 2016, 347, 274–282. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [Green Version]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol. Immunol. 2007, 44, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yan, X.; Zhu, Y.; Zhu, H.; Luo, Y.; Liu, P.; Ferrandon, S.; Kalady, M.F.; Gao, R.; He, J.; et al. Fusobacterium Nucleatum Promotes the Development of Colorectal Cancer by Activating a Cytochrome P450/Epoxyoctadecenoic Acid Axis via TLR4/Keap1/NRF2 Signaling. Cancer Res. 2021, 81, 4485–4498. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Luo, F.; Yang, J.; Liu, J.; Liu, R.; Wang, L.; Wang, C.; Deng, Y.; Lu, Z.; Wang, Y.; et al. TLR2 Promotes Glioma Immune Evasion by Downregulating MHC Class II Molecules in Microglia. Cancer Immunol. Res. 2018, 6, 1220–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, P.V.; Seiva, F.R.; Carniato, A.P.; de Mello Júnior, W.; Duran, N.; Macedo, A.M.; de Oliveira, A.G.; Romih, R.; Nunes Ida, S.; Nunes Oda, S.; et al. Increased toll-like receptors and p53 levels regulate apoptosis and angiogenesis in non-muscle invasive bladder cancer: Mechanism of action of P-MAPA biological response modifier. BMC Cancer 2016, 16, 422. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Treinies, M.; Mottas, I.; Rötzer, L.C.; Oberson, A.; Spagnuolo, L.; Perdicchio, M.; Spinetti, T.; Herbst, T.; Bourquin, C. Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses. Oncoimmunology 2016, 5, e1232219. [Google Scholar] [CrossRef] [Green Version]
- Umemura, N.; Zhu, J.; Mburu, Y.K.; Forero, A.; Hsieh, P.N.; Muthuswamy, R.; Kalinski, P.; Ferris, R.L.; Sarkar, S.N. Defective NF-κB signaling in metastatic head and neck cancer cells leads to enhanced apoptosis by double-stranded RNA. Cancer Res. 2012, 72, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Gambara, G.; Desideri, M.; Stoppacciaro, A.; Padula, F.; De Cesaris, P.; Starace, D.; Tubaro, A.; Del Bufalo, D.; Filippini, A.; Ziparo, E.; et al. TLR3 engagement induces IRF-3-dependent apoptosis in androgen-sensitive prostate cancer cells and inhibits tumour growth in vivo. J. Cell. Mol. Med. 2015, 19, 327–339. [Google Scholar] [CrossRef]
- Umansky, V.; Schirrmacher, V. Nitric oxide-induced apoptosis in tumor cells. Adv. Cancer Res. 2001, 82, 107–131. [Google Scholar] [CrossRef]
- Yadav, J.; Dikshit, N.; Ismaeel, S.; Qadri, A. Innate Activation of IFN-γ-iNOS Axis During Infection with Salmonella Represses the Ability of T Cells to Produce IL-2. Front. Immunol. 2020, 11, 514. [Google Scholar] [CrossRef]
| Cancer Type | TLR-Cell Line Characterization | Pre-Clinical Findings | Tumor Profile and Patient Outcomes | References | |
|---|---|---|---|---|---|
| Breast Cancer | TLR2 | MDA-MB-231, SUM-149, SUM-159 | - | Expression observed in primary tumors and metastatic tissue; high expression associated with shorter overall survival | [18,19,20] |
| TLR3 | MDA-MB-231, MDA-MB-468, SUM-149, SUM-159 | Poly(I:C) stimulation reduces breast cancer cell proliferation and induces apoptosis | Upregulated in recurring tumors; associated with lower relapse-free survival | [19,21,22] | |
| TLR4 | MDA-MB-231, SUM-149, SUM-159 | LPS stimulation induces IL-6 and IL-9 production; activation promotes chemoresistance and apoptosis evasion; downregulation enhances paclitaxel sensitivity; upregulation promotes paclitaxel resistance | Expression observed in primary tumors and metastatic tissue; upregulation associated with tumor recurrence and poor survival in TP53 mutant tumors | [18,19,21,23,24] | |
| TLR5 | 4T1 | Downregulation upregulates VEGFR and cell proliferation; upregulation and downregulation of receptor increases lung metastases; flagellin treatment reduces tumor growth | Highly expressed in metastatic cancer | [18,25,26,27] | |
| TLR7/8 | - | - | Low expression observed in metastases; imiquimod promotes immune cell infiltration in skin-residing metastases | [18,28,29] | |
| TLR9 | MCF-7, T47D, CAMA, MDA-MB-231, MDA-MB-468, SUM-149, SUM-159 | Receptor knockdown promotes MDA-MB-231 tumor growth | Expression observed on tumors; low expression in metastases; downregulation associated with poor disease-specific survival | [18,19,21,30] | |
| Lung Cancer | TLR2 | - | Treatment with lipoprotein reduces Lewis lung carcinoma tumor growth | High tumoral TLR2 expression is positively correlated with prolonged overall survival and progression-free survival | |
| TLR3 | Calu-3; H460 | Lewis lung carcinoma tumors in TLR3-deficient mice had fewer metastases compared to TLR3 competent mice; stimulation with Poly(I:C) induces apoptosis | TLR3 positive tumors have greater overall survival and slower disease progression in early-stage NSCLC | [26,31,32,33] | |
| TLR4 | A549; H1299 | Stimulation with LPS induces production of TGF-β, VEGF, and IL-8 | High expression associated with decreased overall survival; expression correlated with tumoral PD-L1 expression | [28,34,35] | |
| TLR5 | SPC-A1; A549; H1975; H1299 | Stimulation with flagellin induces IL-6 and CXCL5 production | High expression associated with improved disease-free survival | [36] | |
| TLR7/8 | A549, H1355, SK-MES; LL/2 | Stimulation promotes survival and chemotherapy resistance, CL264 treatment enhances Lewis lung carcinoma tumor growth; resiquimod formulation improves overall survival and reduced 344SQ tumor progression | High expression associated with poor overall survival in stage I-III NSCLC patients | [27,37,38] | |
| TLR9 | A549, NCI-H727 | Expressed on human NSCLC cell line A549; synthetic oligonucleotide treatment reduces tumor growth in H520, H358, A549, and H1299 xenografts | Higher expression in tumors compared to non-cancerous tissue | [39,40] | |
| Melanoma | TLR2 | ME5, ME9, ME16, ME17, ME19 | Stimulation promotes cell migration; treatment with Zymosan-A and bacteria reduces B16-F10 tumor growth | Expression observed on tumors | [41,42,43] |
| TLR3 | ME2, ME9, ME16, ME17, ME19, M288, M301, M305, M299, M342 | Stimulation promotes cell migration | Expression observed on tumors | [42,43,44] | |
| TLR4 | ME2, ME9, ME16, ME17, ME19 | Stimulation promotes cell migration | Highly expressed on primary and metastatic tumors; expression associated with shortened relapse-free survival | [42,43,44,45] | |
| TLR7/8 | M288, M301, M305, M284, M379, M299, M342, M383, M350, M383, M387 | Imiquimod stimulation inhibits tumoral angiogenesis in a melanoma-bearing humanized mouse model | Upregulated expression in stage III melanoma patients; high expression associated with longer overall survival time; expression correlated with CD8+ T-cell infiltration; treatment with imiquimod inhibits metastasis | [43,44,46,47] | |
| TLR9 | M288, M301, M305, M350, M387 | Treatment with L-nucleotide-protected TLR agonists reduce B16-F10 tumor growth | Expression observed on tumors | [43,44,48] | |
| Colorectal Cancer | TLR1/2 | - | - | Upregulated in cancerous tissue; high expression associated with improved disease-specific survival | [49,50,51] |
| TLR3 | HCT116, HT29, SW620 | Poly(I:C) stimulation induces CCL2, CCL5, and IL-8 production; CXCL8 production, and invasiveness in CRC cell lines | Low expression associated with lymph node metastasis and tumor recurrence | [52,53] | |
| TLR4 | - | Upregulated in chemically induced CRC in Tir8 −/− mice | Expression upregulated in cancerous tissue; high expression associated with poor disease-free survival | [54] | |
| TLR5 | DLD-1 | Knockdown promotes DLD-1 tumor growth and inhibits immune cell infiltration | Low expression associated with advanced cancer stage; high expression associated with improved disease-specific survival | [50,55] | |
| TLR7/8 | - | R848 treatment of CT26 tumors reverses chemoresistance to oxaliplatin | Upregulation observed in tumors, associated with lower cancer stage; high expression associated with improved disease-specific survival | [50,51,56] | |
| TLR9 | - | Stimulation reduces CT26 tumor growth, increases CD8+ T-cell infiltration in the tumor | High expression correlated with invasiveness, metastasis, and advanced-stage CRC | [48,57] | |
| Pancreatic Cancer | TLR2 | HPAC, MIA PaCa-2, PANC-1, BXPC-3, PaCaDD135 | Stimulation promotes cell proliferation, VEGF expression, and colony formation | Highly expressed in all stages of PDAC; upregulation correlated with poor patient survival | [58,59] |
| TLR3 | PANC-1, BXPC-3 | Activation promotes cell proliferation | - | [60,61] | |
| TLR4 | MIA PaCa-2, SW1990 | LPS stimulation mediates tumorigenesis in p48Cre;KrasG12D mice, and promotes cell proliferation and VEGF expression | Upregulated in cancerous tissue | [59,62] | |
| TLR7/8 | PANC-2 | Stimulation promotes cell proliferation, chemoresistance, and tumorigenesis in p48Cre;KrasG12D mice; inhibition prevents tumor progression | Expression upregulated in early and advanced stages of PDAC | [63,64] | |
| TLR9 | PANC-1, SW1990, PaCaDD185, PAN02 | Stimulation promotes cell proliferation, VEGF expression, and tumorigenesis in p48Cre;LsL-KrasG12D mice; inhibition improves survival and prevents tumor progression | Upregulated in cancerous tissue | [59,65] | |
| Ovarian Cancer | TLR2 | SKOV3, CAOV3 | Expression upregulated upon tumor injury in xenografted mice; activation promotes tumoral repair and persistence | Upregulated in cancerous tissue | [66,67] |
| TLR3 | ES2, OVCAR3, SKOV3, CAOV3 | Stimulation induces CCL2 and IL-6 production | Upregulated in cancerous tissue | [67] | |
| TLR4 | R182, CP70, A2780, R179, OVCAR3, SKOV3, AD-10, ES2 | Stimulation promotes cancer cell viability and cell proliferation and induces CCL2, IL-6, and CXCL1 production; knockdown enhances sensitivity to paclitaxel | High expression in cancerous tissue; high expression associated with improved survival | [67,68,69,70,71] | |
| TLR5 | OVCAR3 | TLR5-deficiency reduces tumor growth; stimulation promotes invasion | Polymorphism diminishing TLR5 signaling improves long-term survival | [72,73] | |
| TLR7/8 | CaOV3, OVCAR3, OV90, SKOV3 | Stimulation promotes invasion | - | [72,74] | |
| TLR9 | - | - | Increased expression associated with rising tumor grade | [75] | |
| Prostate Cancer | TLR2/6 | PC3 | Stimulation promotes cell proliferation and invasiveness and induces IL-6 and IL-8 production | - | [76,77] |
| TLR3 | LNCaP, DU145, PC3 | Stimulation inhibits cell proliferation and promotes apoptosis and induces IL-8, CCL3, CCL5, and CXCL10 production | Upregulated in cancerous tissue; high expression associated with poor patient survival | [23,76,77,78] | |
| TLR4 | PC3, DU145 | Stimulation promotes cell proliferation and induces IL-6 and IL-8 production; knockdown diminishes tumorigenesis, reduces cell invasiveness and proliferation, and induces apoptosis | Upregulated in cancerous tissue | [23,76,77,79] | |
| TLR5 | DU145, PC3, LNCaP | Stimulation induces IL-8 and CCL5 production | - | [76] | |
| TLR9 | - | - | Upregulated in cancerous tissue; high expression associated with poor patient survival | [79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurini, E.F.; Madonna, M.B.; Zloza, A.; Gupta, K.H. Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression. Cancers 2022, 14, 2923. https://doi.org/10.3390/cancers14122923
Giurini EF, Madonna MB, Zloza A, Gupta KH. Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression. Cancers. 2022; 14(12):2923. https://doi.org/10.3390/cancers14122923
Chicago/Turabian StyleGiurini, Eileena F., Mary Beth Madonna, Andrew Zloza, and Kajal H. Gupta. 2022. "Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression" Cancers 14, no. 12: 2923. https://doi.org/10.3390/cancers14122923
APA StyleGiurini, E. F., Madonna, M. B., Zloza, A., & Gupta, K. H. (2022). Microbial-Derived Toll-like Receptor Agonism in Cancer Treatment and Progression. Cancers, 14(12), 2923. https://doi.org/10.3390/cancers14122923






