PET-CT in Clinical Adult Oncology—IV. Gynecologic and Genitourinary Malignancies
Abstract
Simple Summary
Abstract
1. Introduction
2. Gynecological Malignancies
2.1. Normal Physiologic and Benign Patterns
2.2. Ovarian Epithelial, Fallopian Tube and Primary Peritoneal Carcinoma
2.3. Uterine Neoplasms: Endometrial Carcinoma, Uterine Sarcoma and Leiomyoma
2.3.1. Endometrial Carcinoma
2.3.2. Uterine Sarcoma
2.4. Cervical Cancer
2.5. Vaginal Cancer
2.6. Vulvar Carcinoma
2.7. Considerations of Pregnancy
3. Urological Malignancies
3.1. Prostate Cancer
3.2. Urothelial Carcinoma
3.3. Primary Testicular Cancer
3.4. Renal Cell Carcinoma
3.5. Penile Cancer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, Y.J.; Grigsby, P.W. The Role of PET Imaging in Gynecologic Radiation Oncology. PET Clin. 2018, 13, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.W. Role of PET in gynecologic malignancy. Curr. Opin. Oncol. 2009, 21, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Fuser, D.; DeWees, T.A.; Wan, L.; Gang, M.; Hui, C.Y.; Rao, Y.J.; Siegel, B.A.; Dehdashti, F.; Mutch, D.G.; et al. Radiologic Assessment of Groin Lymph Nodes in Pelvic Malignancies. Int. J. Gynecol. Cancer 2020, 30, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, Q.; Dong, L.; Jia, Y.; Hua, C.; Mi, F.; Li, C. Different imaging techniques for the detection of pelvic lymph nodes metastasis from gynecological malignancies: A systematic review and meta-analysis. Oncotarget 2016, 8, 14107–14125. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, Z.; Tang, X.; Li, C.; Wang, Q. Performance of Different Imaging Techniques for Detection of Para-Aortic Lymph Node Metastasis from Gynecological Malignancies: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2019, 85, 53–71. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Grigsby, P.W.; Dehdashti, F.; Delbeke, D. The Role of 18F-FDG PET in Assessing Therapy Response in Cancer of the Cervix and Ovaries. J. Nucl. Med. 2009, 50, 64S–73S. [Google Scholar] [CrossRef]
- Gorospe, L.; Jover-Díaz, R.; Vicente-Bártulos, A. Spectrum of PET–CT pelvic pitfalls in patients with gynecologic malignancies. Gastrointest. Radiol. 2012, 37, 1041–1065. [Google Scholar] [CrossRef]
- Lakhani, A.; Khan, S.R.; Bharwani, N.; Stewart, V.; Rockall, A.; Khan, S.; Barwick, T.D. FDG PET/CT Pitfalls in Gynecologic and Genitourinary Oncologic Imaging. Radiographics 2017, 37, 577–594. [Google Scholar] [CrossRef]
- Esfahani, S.A.; Torrado-Carvajal, A.; Amorim, B.J.; Groshar, D.; Domachevsky, L.; Bernstine, H.; Stein, D.; Gervais, D.; Catalano, O.A. PET/MRI and PET/CT Radiomics in Primary Cervical Cancer: A Pilot Study on the Correlation of Pelvic PET, MRI, and CT Derived Image Features. Mol. Imaging Biol. 2021, 24, 60–69. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z. Radiomics Analysis of PET and CT Components of 18F-FDG PET/CT Imaging for Prediction of Progression-Free Survival in Advanced High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 638124. [Google Scholar] [CrossRef]
- Caobelli, F.; Young AIMN Working Group; Alongi, P.; Evangelista, L.; Picchio, M.; Saladini, G.; Rensi, M.; Geatti, O.; Castello, A.; Laghai, I.; et al. Predictive value of 18F-FDG PET/CT in restaging patients affected by ovarian carcinoma: A multicentre study. Eur. J. Pediatr. 2015, 43, 404–413. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur. J. Pediatr. 2021, 48, 4089–4100. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Murakami, K.; Kaji, Y.; Sugimura, K. Spectrum of FDG PET/CT Findings of Uterine Tumors. Am. J. Roentgenol. 2010, 195, 737–743. [Google Scholar] [CrossRef]
- Yun, M.; Cho, A.; Lee, J.H.; Choi, Y.-J.; Lee, J.D.; Kim, C.K. Physiologic 18F-FDG Uptake in the Fallopian Tubes at Mid Cycle on PET/CT. J. Nucl. Med. 2010, 51, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Khan, S.M.; Rahaman, J.; Cameron, K.L.; Prasad-Hayes, M.; Chuang, L.; Machac, J.; Heiba, S.; Kostakoglu, L. Role of FDG PET/CT in Staging of Recurrent Ovarian Cancer. Radiographics 2011, 31, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer Statistics. World Cancer Research Fund. Available online: https://www.wcrf.org/dietandcancer/cancer-trends/ovarian-cancer-statistics (accessed on 14 January 2022).
- Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Ovary Cancer. National Cancer Institute. Available online: http://seer.cancer.gov/statfacts/html/ovary.html (accessed on 15 January 2022).
- Lacey, J.V.; Sherman, M.E. Ovarian Neoplasia. In Robboy’s Pathology of the Female Reproductive Tract, 2nd ed.; Robboy, S.L., Mutter, G.L., Prat, J., Eds.; Churchill Livingstone Elsevier: Oxford, UK, 2009; p. 601. [Google Scholar]
- Goff, B.A.; Mandel, L.S.; Drescher, C.W.; Urban, N.; Gough, S.; Ba, K.M.S.; Patras, J.; Mahony, B.S.; Andersen, M.R. Development of an ovarian cancer symptom index. Cancer 2007, 109, 221–227. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee on Practice Bulletins—Gynecology Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet. Gynecol. 2016, 128, e210–e226. [Google Scholar] [CrossRef]
- Temkin, S.M.; Bergstrom, J.; Samimi, G.; Minasian, L. Ovarian Cancer Prevention in High-risk Women. Clin. Obstet. Gynecol. 2017, 60, 738–757. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Efficacy of Maintenance Olaparib for Patients With Newly Diagnosed Advanced Ovarian Cancer With a BRCA Mutation: Subgroup Analysis Findings From the SOLO1 Trial. J. Clin. Oncol. 2020, 38, 3528–3537. [Google Scholar] [CrossRef]
- [Guideline] NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer. NCCN.org Version 1.2021—September 9 2021. Available online: https://www.nccn.org/professionals/physician_gls/PDF/ovarian.pdf (accessed on 13 January 2022).
- Mikkelsen, M.S.; Petersen, L.K.; Blaakaer, J.; Marinovskij, E.; Rosenkilde, M.; Andersen, G.; Bouchelouche, K.; Iversen, L.H. Assessment of peritoneal metastases with DW-MRI, CT, and FDG PET/CT before cytoreductive surgery for advanced stage epithelial ovarian cancer. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 2134–2141. [Google Scholar] [CrossRef]
- Lee, E.Y.P.; An, H.; Tse, K.Y.; Khong, P.-L. Molecular Imaging of Peritoneal Carcinomatosis in Ovarian Carcinoma. Am. J. Roentgenol. 2020, 215, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Cascales-Campos, P.; Gil, J.; Frutos, L.; Andrade, R.; Fuster-Quiñonero, M.; Feliciangeli, E.; Gil, E.; Parrilla, P. Use of 18 F-FDG PET/CT in the preoperative evaluation of patients diagnosed with peritoneal carcinomatosis of ovarian origin, candidates to cytoreduction and hipec. A pending issue. Eur. J. Radiol. 2016, 85, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Stat Fact Sheets: Endometrial Cancer. Surveillance, Epidemiology, and End Results (SEER) Program. Available online: http://seer.cancer.gov/statfacts/html/corp.html (accessed on 15 January 2022).
- Brabaharan, S.; Veettil, S.K.; Kaiser, J.E.; Rao, V.R.R.; Wattanayingcharoenchai, R.; Maharajan, M.; Insin, P.; Talungchit, P.; Anothaisintawee, T.; Thakkinstian, A.; et al. Association of Hormonal Contraceptive Use with Adverse Health Outcomes. JAMA Netw. Open 2022, 5, e2143730. [Google Scholar] [CrossRef]
- De Haydu, C.; Black, J.D.; Schwab, C.L.; English, D.P.; Santin, A.D. An update on the current pharmacotherapy for endometrial cancer. Expert Opin. Pharmacother. 2015, 17, 489–499. [Google Scholar] [CrossRef]
- Papadia, A.; Bellati, F.; Ditto, A.; Bogani, G.; Gasparri, M.L.; Di Donato, V.; Martinelli, F.; Lorusso, D.; Benedetti-Panici, P.; Raspagliesi, F. Surgical Treatment of Recurrent Endometrial Cancer: Time for a Paradigm Shift. Ann. Surg. Oncol. 2015, 22, 4204–4210. [Google Scholar] [CrossRef]
- Fotopoulou, C.; El-Balat, A.; Du Bois, A.; Sehouli, J.; Harter, P.; Muallem, M.Z.; Krätschell, R.W.; Traut, A.; Heitz, F. Systematic pelvic and paraaortic lymphadenectomy in early high-risk or advanced endometrial cancer. Arch. Gynecol. Obstet. 2015, 292, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi33–vi38. [Google Scholar] [CrossRef]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Abu Shahin, F. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014, 134, 393–402. [Google Scholar] [CrossRef]
- Rockall, A.G.; Barwick, T.D.; Wilson, W.; Singh, N.; Bharwani, N.; Sohaib, A.; Nobbenhuis, M.; Warbey, V.; Miquel, M.; Koh, D.-M.; et al. Diagnostic Accuracy of FEC-PET/CT, FDG-PET/CT, and Diffusion-Weighted MRI in Detection of Nodal Metastases in Surgically Treated Endometrial and Cervical Carcinoma. Clin. Cancer Res. 2021, 27, 6457–6466. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Ytre-Hauge, S.; Bollineni-Balabay, O.; Salvesen, H.B.; Haldorsen, I.S. High Diagnostic Value of 18F-FDG PET/CT in Endometrial Cancer: Systematic Review and Meta-Analysis of the Literature. J. Nucl. Med. 2016, 57, 879–885. [Google Scholar] [CrossRef]
- [Guideline] NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. NCCN.org Version 1.2022—November 4 2021. Available online: https://www.nccn.org/professionals/physician_gls/PDF/uterine.pdf (accessed on 13 January 2022).
- Salani, R.; Backes, F.; Fung, M.F.K.; Holschneider, C.H.; Parker, L.P.; Bristow, R.E.; Goff, B. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am. J. Obstet. Gynecol. 2011, 204, 466–478. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Prat, J. Uterine sarcomas: A review. Gynecol. Oncol. 2010, 116, 131–139. [Google Scholar] [CrossRef]
- Albano, D.; Zizioli, V.; Treglia, G.; Odicino, F.; Giubbini, R.; Bertagna, F. 18F-FDG PET/TC en reestadificación y seguimiento de pacientes con sarcomas uterinos. Rev. Española Med. Nucl. E Imagen Mol. 2018, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.F.; Queiroz, M.; Buchpiguel, C.; Carvalho, F.M.; Carvalho, J.P. Aberrant Hypermetabolism of Benign Uterine Leiomyoma on 18F-FDG PET/CT. Clin. Nucl. Med. 2019, 44, e413–e414. [Google Scholar] [CrossRef] [PubMed]
- Sawai, Y.; Shimizu, T.; Yamanaka, Y.; Niki, M.; Nomura, S. Benign metastasizing leiomyoma and 18-FDG-PET/CT: A case report and literature review. Oncol. Lett. 2017, 14, 3641–3646. [Google Scholar] [CrossRef][Green Version]
- Fujiishi, K.; Nagata, S.; Kano, R.; Kubo, C.; Shirayanagi, M.; Ozaki, M.; Yamamoto, T.; Nakanishi, K.; Kamiura, S.; Nakatsuka, S.-I. JAZF1–SUZ12 endometrial stromal sarcoma forming subserosal masses with extraordinary uptake of fluorodeoxyglucose on positron emission tomography: A case report. Diagn. Pathol. 2019, 14, 110. [Google Scholar] [CrossRef]
- Seagle, B.-L.L.; Shilpi, A.; Buchanan, S.; Goodman, C.; Shahabi, S. Low-grade and high-grade endometrial stromal sarcoma: A National Cancer Database study. Gynecol. Oncol. 2017, 146, 254–262. [Google Scholar] [CrossRef]
- Maruyama, S.; Sato, Y.; Satake, Y.; Mise, H.; Kim, T. Diffusion-Weighted MRI and FDG-PET in Diagnosis of Endometrial Stromal Nodule. Case Rep. Obstet. Gynecol. 2015, 2015, 540283. [Google Scholar] [CrossRef][Green Version]
- Sadeghi, R.; Zakavi, S.R.; Hasanzadeh, M.; Treglia, G.; Giovanella, L.; Kadkhodayan, S. Diagnostic Performance of Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography Imaging in Uterine Sarcomas. Int. J. Gynecol. Cancer 2013, 23, 1349–1356. [Google Scholar] [CrossRef]
- NIH Surveillance, Epidemiology and End Results Program. SEER Stat Fact Sheets: Cervical Cancer. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 16 January 2022).
- Vinh-Hung, V.; Bourgain, C.; Vlastos, G.; Cserni, G.; De Ridder, M.; Storme, G.; Vlastos, A.-T. Prognostic value of histopathology and trends in cervical cancer: A SEER population study. BMC Cancer 2007, 7, 164. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Khatri, G.; Rivera-Colón, G.; Albuquerque, K.; Lea, J.; Pinho, D.F. Multimodality Imaging of Uterine Cervical Malignancies. Am. J. Roentgenol. 2020, 215, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Q.; Zhao, L.; Feng, X.; Wang, Y.; Zou, Y.; Li, Q. A Systematic Review and Meta-Analysis of the Prognostic Impact of Pretreatment Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Parameters in Patients with Locally Advanced Cervical Cancer Treated with Concomitant Chemoradiotherapy. Diagnostics 2021, 11, 1258. [Google Scholar] [CrossRef]
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer Version 1.2022. Available online: http://www.nccn.org/professionals/physician_gls/PDF/cervical.pdf (accessed on 16 January 2022).
- Kim, Y.J.; Han, S.; Kim, Y.S.; Nam, J.-H. Prognostic value of post-treatment18F-fluorodeoxyglucose positron emission tomography in uterine cervical cancer patients treated with radiotherapy: A systematic review and meta-analysis. J. Gynecol. Oncol. 2019, 30, e66. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Association of Posttherapy Positron Emission Tomography With Tumor Response and Survival in Cervical Carcinoma. JAMA 2007, 298, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Gandy, N.; Arshad, M.A.; Park, W.-H.E.; Rockall, A.; Barwick, T.D. FDG-PET Imaging in Cervical Cancer. Semin. Nucl. Med. 2019, 49, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.J.; Dehdashti, F.; Grigsby, P.W. Molecular Imaging for Radiotherapy Planning and Response Assessment for Cervical Cancer. Semin. Nucl. Med. 2019, 49, 493–500. [Google Scholar] [CrossRef]
- Robertson, N.; Hricak, H.; Sonoda, Y.; Sosa, R.; Benz, M.; Lyons, G.; Abu-Rustum, N.; Sala, E.; Vargas, H. The impact of FDG-PET/CT in the management of patients with vulvar and vaginal cancer. Gynecol. Oncol. 2016, 140, 420–424. [Google Scholar] [CrossRef]
- Lamoreaux, W.T.; Grigsby, P.W.; Dehdashti, F.; Zoberi, I.; Powell, M.A.; Gibb, R.K.; Rader, J.S.; Mutch, D.G.; Siegel, B.A. FDG-PET evaluation of vaginal carcinoma. Int. J. Radiat. Oncol. 2005, 62, 733–737. [Google Scholar] [CrossRef]
- Kilcoyne, A.; Gottumukkala, R.V.; Kang, S.K.; Akin, E.A.; Hauck, C.; Hindman, N.M.; Huang, C.; Khanna, N.; Paspulati, R.; Rauch, G.M.; et al. ACR Appropriateness Criteria® Staging and Follow-up of Primary Vaginal Cancer. J. Am. Coll. Radiol. 2021, 18, S442–S455. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020. Available online: https://seer.cancer.gov/archive/csr/1975_2017/ (accessed on 13 February 2022).
- Khadraoui, H.; Thappa, S.; Smith, M.; Davidov, A.; Castellanos, M.R. Age-associated trends of vulvar cancer in the US. Menopause 2020, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Management of Advanced Squamous Cell Carcinoma of the Vulva. Cancers 2021, 14, 167. [Google Scholar] [CrossRef]
- Crivellaro, C.; Guglielmo, P.; De Ponti, E.; Elisei, F.; Guerra, L.; Magni, S.; La Manna, M.; Di Martino, G.; Landoni, C.; Buda, A. 18F-FDG PET/CT in preoperative staging of vulvar cancer patients. Medicine 2017, 96, e7943. [Google Scholar] [CrossRef]
- Oldan, J.D.; Sullivan, S.A. Positron emission tomography-computed tomography for inguinal nodes in vulvar cancer. World J. Nucl. Med. 2018, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Triumbari, E.K.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in Vulvar Cancer. Clin. Nucl. Med. 2020, 46, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Akhurst, T.; Larson, S.; Alektiar, K.; Barakat, R.R.; Chi, D.S. A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration. Gynecol. Oncol. 2007, 106, 177–180. [Google Scholar] [CrossRef]
- Despierres, M.; Boudy, A.-S.; Selleret, L.; Gligorov, J.; Richard, S.; Thomassin, I.; Dabi, Y.; Zilberman, S.; Touboul, C.; Montravers, F.; et al. Feasibility, Safety and Impact of (18F)-FDG PET/CT in patients with pregnancy-associated cancer: Experience of the French CALG (Cancer Associé à La Grossesse) network. Acta Oncol. 2021, 61, 302–308. [Google Scholar] [CrossRef]
- Takalkar, A.M.; Khandelwal, A.; Lokitz, S.; Lilien, D.L.; Stabin, M.G. 18F-FDG PET in Pregnancy and Fetal Radiation Dose Estimates. J. Nucl. Med. 2011, 52, 1035–1040. [Google Scholar] [CrossRef]
- Colletti, P.M.; Image Wisely. PET-CT in the Pregnant Patient; American College of Radiology: Reston, VA, USA, 2012; Available online: https://www.imagewisely.org/~/media/ImageWisely-Files/NucMed/PETCT-in-the-Pregnant-Patient.pdf (accessed on 17 January 2022).
- Mapelli, P.; Mangili, G.; Picchio, M.; Gentile, C.; Rabaiotti, E.; Giorgione, V.; Spinapolice, E.G.; Gianolli, L.; Messa, C.; Candiani, M. Role of 18F-FDG PET in the management of gestational trophoblastic neoplasia. Eur. J. Pediatr. 2013, 40, 505–513. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Force, U.P.S.T.; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; et al. Screening for Prostate Cancer. JAMA J. Am. Med. Assoc. 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Broderick, J. Incidence of Metastatic Prostate Cancer On the Rise. Oncology 2020, 34, 460. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Elboga, U.; Kalender, E.; Basıbuyuk, M.; Demir, H.D.; Celen, Y.Z. Clinical significance of incidental FDG uptake in the prostate gland detected by PET/CT. Int. J. Clin. Exp. Med. 2015, 8, 10577–10585. [Google Scholar] [PubMed]
- Chetan, M.R.; Barrett, T.; Gallagher, F.A. Clinical significance of prostate 18F-labelled fluorodeoxyglucose uptake on positron emission tomography/computed tomography: A five-year review. World J. Radiol. 2017, 9, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Akhurst, T.; Larson, S.M.; DiTullio, M.; Chu, E.; Siedlecki, K.; Verbel, D.; Heller, G.; Kelly, W.K.; Slovin, S.; et al. Fluorodeoxyglucose Positron Emission Tomography as an Outcome Measure for Castrate Metastatic Prostate Cancer Treated with Antimicrotubule Chemotherapy. Clin. Cancer Res. 2005, 11, 3210–3216. [Google Scholar] [CrossRef]
- Jadvar, H. The Vision Forward: Recognition and Implication of PSMA-/FDG+ mCRPC. J. Nucl. Med. 2021; ahead of print. [Google Scholar] [CrossRef]
- Package Insert. Axumin (Fluciclovine F 18) Injection, for Intravenous Use; Blue Earth Diagnostics Ltd.: Oxford, UK, 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/208054s034lbl.pdf (accessed on 1 February 2022).
- Morris, M.J.; Rowe, S.P.; Gorin, M.A.; Saperstein, L.; Pouliot, F.; Josephson, D.Y.; Wong, J.Y.; Pantel, A.R.; Cho, S.Y.; Gage, K.L.; et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the Condor Phase III, Multicenter Study. Clin. Cancer Res. 2021, 27, 3674–3682. [Google Scholar] [CrossRef]
- Package Insert. Pylarify® (Piflufolastat F 18) Injection, for Intravenous Use; Progenics Pharmaceuticals, Inc.: Billerica, MA, USA, 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf (accessed on 1 February 2022).
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019, 5, 856–863. [Google Scholar] [CrossRef]
- Package Insert. Gallium Ga 68PSMA-11 Injection, for Intravenous Use; University of California, Los Angeles: Los Angeles, CA, USA, 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212642s000lbl.pdf (accessed on 1 February 2022).
- Package Insert. Choline C 11 Injection, for Intravenous Use; Mayo Clinic PET Radiochemistry Facility: Rochester, MI, USA, 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203155s000lbl.pdf (accessed on 1 February 2022).
- Zacho, H.D.; Fonager, R.F.; Nielsen, J.B.; Haarmark, C.; Hendel, H.W.; Johansen, M.B.; Mortensen, J.C.; Petersen, L.J. Observer Agreement and Accuracy of 18F-Sodium Fluoride PET/CT in the Diagnosis of Bone Metastases in Prostate Cancer. J. Nucl. Med. 2019, 61, 344–349. [Google Scholar] [CrossRef]
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Version 3.2022. 10 January 2022. Available online: http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf (accessed on 16 January 2022).
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Klassen, Z. EMUC 2020: PSMA PET for Upfront Staging in High-Risk Prostate Cancer. Urotoday.com. Available online: https://www.urotoday.com/conference-highlights/emuc-2020/emuc-2020-gu-malignancies-prostate/125874-emuc-2020-psma-pet-for-upfront-staging-in-high-risk-disease.html (accessed on 29 January 2022).
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. [18F]Fluciclovine PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging—Version 1.0. Eur. J. Pediatr. 2019, 47, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2017, 59, 479–485. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.A.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Chiu, L.W.; Lawhn-Heath, C.; Behr, S.C.; Juarez, R.; Perez, P.M.; Lobach, I.; Bucknor, M.D.; Hope, T.A.; Flavell, R.R. Factors Predicting Metastatic Disease in 68Ga-PSMA-11 PET–Positive Osseous Lesions in Prostate Cancer. J. Nucl. Med. 2020, 61, 1779–1785. [Google Scholar] [CrossRef]
- Pernthaler, B.; Kulnik, R.; Gstettner, C.; Salamon, S.; Aigner, R.M.; Kvaternik, H. A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin. Nucl. Med. 2019, 44, e566–e573. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, J.; Shen, Y.; Yun, C.; Wang, R.; Wang, G.; Tan, J.; Wang, T.; Yao, Q.; Wang, B.; et al. Comparison of PET/CT and MRI in the Diagnosis of Bone Metastasis in Prostate Cancer Patients: A Network Analysis of Diagnostic Studies. Front. Oncol. 2021, 11, 736654. [Google Scholar] [CrossRef]
- Reale, M.L.; Buttigliero, C.; Tucci, M.; Giardino, R.; Poti, C. 68Ga-PSMA Uptake in Fibrous Dysplasia. Clin. Nucl. Med. 2019, 44, e396–e397. [Google Scholar] [CrossRef]
- Artigas, C.; Otte, F.-X.; Lemort, M.; van Velthoven, R.; Flamen, P. Vertebral Hemangioma Mimicking Bone Metastasis in 68Ga—PSMA Ligand PET/CT. Clin. Nucl. Med. 2017, 42, 368–370. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-Amino-3-18F-Fluorocyclobutane-1-Carboxylic Acid: Physiologic Uptake Patterns, Incidental Findings, and Variants That May Simulate Disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef]
- Dietlein, M.; Kobe, C.; Kuhnert, G.; Stockter, S.; Fischer, T.; Schomäcker, K.; Schmidt, M.; Dietlein, F.; Zlatopolskiy, B.; Krapf, P.; et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015, 17, 575–584. [Google Scholar] [CrossRef]
- Carter, L.M.; Kesner, A.L.; Pratt, E.C.; Sanders, V.A.; Massicano, A.V.F.; Cutler, C.S.; Lapi, S.E.; Lewis, J.S. The Impact of Positron Range on PET Resolution, Evaluated with Phantoms and PHITS Monte Carlo Simulations for Conventional and Non-conventional Radionuclides. Mol. Imaging Biol. 2019, 22, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Draulans, C.; Pos, F.; Smeenk, R.J.; Kerkmeijer, L.; Vogel, W.V.; Nagarajah, J.; Janssen, M.; Mai, C.; Heijmink, S.; van der Leest, M.; et al. 68Ga-PSMA-11 PET, 18F-PSMA-1007 PET, and MRI for Gross Tumor Volume Delineation in Primary Prostate Cancer: Intermodality and Intertracer Variability. Prac. Radiat. Oncol. 2021, 11, 202–211. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Q.; Zhang, C.; Zhou, Y.-H.; Zhao, X.; Fu, Y.; Gao, J.; Wang, F.; Qiu, X.; Guo, H. Comparison of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and multi-parametric magnetic resonance imaging (MRI) in the evaluation of tumor extension of primary prostate cancer. Transl. Androl. Urol. 2020, 9, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Bettermann, A.S.; Zamboglou, C.; Kiefer, S.; Jilg, C.A.; Spohn, S.; Kranz-Rudolph, J.; Fassbender, T.F.; Bronsert, P.; Nicolay, N.H.; Gratzke, C.; et al. [68Ga-]PSMA-11 PET/CT and multiparametric MRI for gross tumor volume delineation in a slice by slice analysis with whole mount histopathology as a reference standard—Implications for focal radiotherapy planning in primary prostate cancer. Radiother. Oncol. 2019, 141, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Basso Dias, A.; Finelli, A.; Bauman, G.; Veit-Haibach, P.; Berlin, A.; Ortega, C.; Avery, L.; Metser, U. Impact of 18F-DCFPyL PET on Staging and Treatment of Unfavorable Intermediate or High-Risk Prostate Cancer. Radiology 2022, 24, 211836. [Google Scholar] [CrossRef] [PubMed]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Köber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef]
- Alongi, P.; Stefano, A.; Comelli, A.; Laudicella, R.; Scalisi, S.; Arnone, G.; Barone, S.; Spada, M.; Purpura, P.; Bartolotta, T.V.; et al. Radiomics analysis of 18F-Choline PET/CT in the prediction of disease outcome in high-risk prostate cancer: An explorative study on machine learning feature classification in 94 patients. Eur. Radiol. 2021, 31, 4595–4605. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gage, K.L.; Faraj, S.F.; Macura, K.J.; Cornish, T.C.; Gonzalez-Roibon, N.; Guner, G.; Munari, E.; Partin, A.W.; Pavlovich, C.P.; et al. 18F-DCFBC PET/CT for PSMA-Based Detection and Characterization of Primary Prostate Cancer. J. Nucl. Med. 2015, 56, 1003–1010. [Google Scholar] [CrossRef]
- Akin-Akintayo, O.; Tade, F.; Mittal, P.; Moreno, C.; Nieh, P.T.; Rossi, P.; Patil, D.; Halkar, R.; Fei, B.; Master, V.; et al. Prospective evaluation of fluciclovine (18F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur. J. Radiol. 2018, 102, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.T.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, J.; Tade, F.I.; Odewole, O.; et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017, 197, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Buchholz, H.-G.; Wieler, H.J.; Miederer, M.; Rosar, F.; Fischer, N.; Müller-Hübenthal, J.; Trampert, L.; Pektor, S.; Schreckenberger, M. PSA and PSA Kinetics Thresholds for the Presence of 68Ga-PSMA-11 PET/CT-Detectable Lesions in Patients with Biochemical Recurrent Prostate Cancer. Cancers 2020, 12, 398. [Google Scholar] [CrossRef]
- Perry, E.; Talwar, A.; Taubman, K.; Ng, M.; Wong, L.M.; Booth, R.; Sutherland, T.R. [18F]DCFPyL PET/CT in detection and localization of recurrent prostate cancer following prostatectomy including low PSA <0.5 ng/mL. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Brumberg, J.; Beckl, M.; Dierks, A.; Schirbel, A.; Krebs, M.; Buck, A.; Kübler, H.; Lapa, C.; Seitz, A.K. Detection Rate of 68Ga-PSMA Ligand PET/CT in Patients with Recurrent Prostate Cancer and Androgen Deprivation Therapy. Biomedicines 2020, 8, 511. [Google Scholar] [CrossRef]
- Hoberück, S.; Löck, S.; Winzer, R.; Zöphel, K.; Froehner, M.; Fedders, D.; Kotzerke, J.; Hölscher, T. [68Ga]Ga-PSMA-11 PET before and after initial long-term androgen deprivation in patients with newly diagnosed prostate cancer: A retrospective single-center study. EJNMMI Res. 2020, 10, 135. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Debus, N.; Uhrig, M.; Hope, T.A.; Evans, M.J.; Holland-Letz, T.; Giesel, F.L.; Kopka, K.; Hadaschik, B.; Kratochwil, C.; et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Pediatr. 2018, 45, 2045–2054. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Torun, N.; Reyhan, M.; Yapar, A.F. The effect of androgen deprivation therapy on 68Ga-PSMA tracer uptake in non-metastatic prostate cancer patients. Eur. J. Pediatr. 2019, 47, 632–641. [Google Scholar] [CrossRef]
- Leitsmann, C.; Thelen, P.; Schmid, M.; Meller, J.; Sahlmann, C.-O.; Meller, B.; Trojan, L.; Strauss, A. Enhancing PSMA-uptake with androgen deprivation therapy—A new way to detect prostate cancer metastases? Int. Braz. J. Urol. 2019, 45, 459–467. [Google Scholar] [CrossRef]
- Hope, T.A.; Truillet, C.C.; Ehman, E.C.; Afshar-Oromieh, A.; Aggarwal, R.; Ryan, C.J.; Carroll, P.R.; Small, E.J.; Evans, M.J. 68Ga-PSMA-11 PET Imaging of Response to Androgen Receptor Inhibition: First Human Experience. J. Nucl. Med. 2016, 58, 81–84. [Google Scholar] [CrossRef]
- Akin-Akintayo, O.O.; Jani, A.B.; Odewole, O.; Tade, F.I.; Nieh, P.T.; Master, V.A.; Bellamy, L.M.; Halkar, R.K.; Zhang, C.; Chen, Z.; et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin. Nucl. Med. 2017, 42, e22–e28. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6 (Suppl. 10), S13. [Google Scholar]
- Robertson, M.S.; Sakellis, C.G.; Hyun, H.; Jacene, H.A. Extraprostatic Uptake of 18F-Fluciclovine: Differentiation of Nonprostatic Neoplasms From Metastatic Prostate Cancer. Am. J. Roentgenol. 2020, 214, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef]
- Zattoni, F.; Incerti, E.; Moro, F.D.; Moschini, M.; Castellucci, P.; Panareo, S.; Picchio, M.; Fallanca, F.; Briganti, A.; Gallina, A.; et al. 18F-FDG PET/CT and Urothelial Carcinoma: Impact on Management and Prognosis—A Multicenter Retrospective Study. Cancers 2019, 11, 700. [Google Scholar] [CrossRef]
- Girard, A.; Reyes, H.V.; Shaish, H.; Grellier, J.-F.; Dercle, L.; Salaün, P.-Y.; Delcroix, O.; Rouanne, M. The Role of 18F-FDG PET/CT in Guiding Precision Medicine for Invasive Bladder Carcinoma. Front. Oncol. 2020, 10, 565086. [Google Scholar] [CrossRef]
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. NCCNVersion 6.2021—6 December 2021. Available online: http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 2 May 2022).
- Tanaka, H.; Yoshida, S.; Komai, Y.; Sakai, Y.; Urakami, S.; Yuasa, T.; Yamamoto, S.; Masuda, H.; Koizumi, M.; Kohno, A.; et al. Clinical Value of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Upper Tract Urothelial Carcinoma: Impact on Detection of Metastases and Patient Management. Urol. Int. 2015, 96, 65–72. [Google Scholar] [CrossRef]
- Asai, S.; Fukumoto, T.; Tanji, N.; Miura, N.; Miyagawa, M.; Nishimura, K.; Yanagihara, Y.; Shirato, A.; Miyauchi, Y.; Kikugawa, T.; et al. Fluorodeoxyglucose positron emission tomography/computed tomography for diagnosis of upper urinary tract urothelial carcinoma. Int. J. Clin. Oncol. 2015, 20, 1042–1047. [Google Scholar] [CrossRef]
- Hanash, K.A. Metastatic tumors to the testicles. Prog. Clin. Biol. Res. 1985, 203, 61–67. [Google Scholar]
- Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Testis Cancer. National Cancer Institute. Available online: http://seer.cancer.gov/statfacts/html/testis.html (accessed on 6 February 2022).
- Schriefer, P.; Hartmann, M.; Oechsle, K.; Meyer, C.P.; Klutmann, S.; Fisch, M.; Bokemeyer, C.; Oing, C. Positronenemissionstomographie bei Keimzelltumoren des Mannes. Der Urol. 2018, 58, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Annunziata, S.; Caldarella, C.; Bertagna, F.; Giovanella, L. Diagnostic Performance of Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography in the Postchemotherapy Management of Patients with Seminoma: Systematic Review and Meta-Analysis. BioMed Res. Int. 2014, 2014, 852681. [Google Scholar] [CrossRef] [PubMed]
- Nauman, M.; Leslie, S.W. Nonseminomatous Testicular Tumors. [Updated 12 August 2021]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568754/ (accessed on 8 February 2021).
- Kidney Cancer: Introduction. Cancer.net. 2019. Available online: https://www.cancer.net/cancer-types/kidney-cancer/introduction (accessed on 8 February 2022).
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Alt, A.L.; Boorjian, S.A.; Ms, C.M.L.; Costello, B.A.; Leibovich, B.C.; Blute, M.L. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011, 117, 2873–2882. [Google Scholar] [CrossRef]
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer. NCCNVersion 4.2022—21 December 2021. Available online: http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (accessed on 5 February 2022).
- Verhoeff, S.R.; van Es, S.C.; Boon, E.; van Helden, E.; Angus, L.; Elias, S.G.; Oosting, S.F.; Aarntzen, E.H.; Brouwers, A.H.; Kwee, T.C.; et al. Lesion detection by [89Zr]Zr-DFO-girentuximab and [18F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur. J. Pediatr. 2019, 46, 1931–1939. [Google Scholar] [CrossRef]
- Wilson, M.P.; Katlariwala, P.; Murad, M.H.; Abele, J.; McInnes, M.D.F.; Low, G. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: A systematic review and meta-analysis. Abdom. Radiol. 2020, 45, 2532–2541. [Google Scholar] [CrossRef]
- Roussel, E.; Capitanio, U.; Kutikov, A.; Oosterwijk, E.; Pedrosa, I.; Rowe, S.P.; Gorin, M.A. Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review. Eur. Urol. 2022, 81, 476–488. [Google Scholar] [CrossRef]
- Ahn, T.; Roberts, M.J.; Abduljabar, A.; Joshi, A.; Perera, M.; Rhee, H.; Wood, S.; Vela, I. A Review of Prostate-Specific Membrane Antigen (PSMA) Positron Emission Tomography (PET) in Renal Cell Carcinoma (RCC). Mol. Imaging Biol. 2019, 21, 799–807. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nye, J.A.; Nieh, P.T.; Votaw, J.R.; Halkar, R.K.; Issa, M.M.; Yu, W.; Sepulveda, J.; Zeng, W.; Young, A.; et al. Initial Experience with the Radiotracer Anti-1-amino-3-[18F]Fluorocyclobutane-1-Carboxylic Acid (Anti-[18F]FACBC) with PET in Renal Carcinoma. Mol. Imaging Biol. 2009, 11, 434–438. [Google Scholar] [CrossRef]
- Park, J.W.; Jo, M.K.; Lee, H.M. Significance of18F-fluorodeoxyglucose positron-emission tomography/computed tomography for the postoperative surveillance of advanced renal cell carcinoma. Br. J. Urol. 2009, 103, 615–619. [Google Scholar] [CrossRef]
- Alongi, P.; Picchio, M.; Zattoni, F.; Spallino, M.; Gianolli, L.; Saladini, G.; Evangelista, L. Recurrent renal cell carcinoma: Clinical and prognostic value of FDG PET/CT. Eur. J. Pediatr. 2015, 43, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Karivedu, V.; Jain, A.L.; Eluvathingal, T.J.; Sidana, A. Role of Positron Emission Tomography Imaging in Metabolically Active Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Fuser, D.; Hedberg, M.L.; Dehner, L.P.; Dehdashti, F.; Siegel, B.A. Extensive Metastatic Sarcomatoid Renal Cell Carcinoma Evaluated by 18F-FDG PET/CT: A Case Report and Review of Literature. J. Kidney Cancer VHL 2018, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guan, Z.; Liang, H.; Cao, H. Predicting the WHO/ISUP Grade of Clear Cell Renal Cell Carcinoma Through CT-Based Tumoral and Peritumoral Radiomics. Front. Oncol. 2022, 12, 831112. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, C.; Li, W.; Chen, X.; Li, Z.; Liao, X.; Cui, Y.; Zhao, G.; Liu, M.; Fu, Z. 2-[18F]FDG PET/CT parameters associated with WHO/ISUP grade in clear cell renal cell carcinoma. Eur. J. Pediatr. 2020, 48, 570–579. [Google Scholar] [CrossRef]
- Argentiero, A.; Solimando, A.G.; Krebs, M.; Leone, P.; Susca, N.; Brunetti, O.; Racanelli, V.; Vacca, A.; Silvestris, N. Anti-angiogenesis and Immunotherapy: Novel Paradigms to Envision Tailored Approaches in Renal Cell-Carcinoma. J. Clin. Med. 2020, 9, 1594. [Google Scholar] [CrossRef]
- Scher, B.; Seitz, M.; Reiser, M.; Hungerhuber, E.; Hahn, K.; Tiling, R.; Herzog, P.; Reiser, M.; Schneede, P.; Dresel, S. 18F-FDG PET/CT for staging of penile cancer. J. Nucl. Med. 2005, 46, 1460–1465. [Google Scholar]
- Ottenhof, S.R.; Vegt, E. The role of PET/CT imaging in penile cancer. Transl. Androl. Urol. 2017, 6, 833–838. [Google Scholar] [CrossRef]
- Sadeghi, R.; Gholami, H.; Zakavi, S.R.; Kakhki, V.R.D.; Horenblas, S. Accuracy of 18F-FDG PET/CT for Diagnosing Inguinal Lymph Node Involvement in Penile Squamous Cell Carcinoma. Clin. Nucl. Med. 2012, 37, 436–441. [Google Scholar] [CrossRef]
- [Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Penile Cancer. NCCNVersion 2.2022—January 26, 2022. Available online: http://www.nccn.org/professionals/physician_gls/pdf/penile.pdf (accessed on 8 February 2022).
- Zhang, K.; Da, J.; Yao, H.J.; Zheng, D.C.; Cai, Z.K.; Jiang, Y.Q.; Xu, M.X.; Wang, Z. Metastatic tumors of the penis: A report of 8 cases and review of the literature. Medicine 2015, 94, e132. [Google Scholar] [CrossRef]




























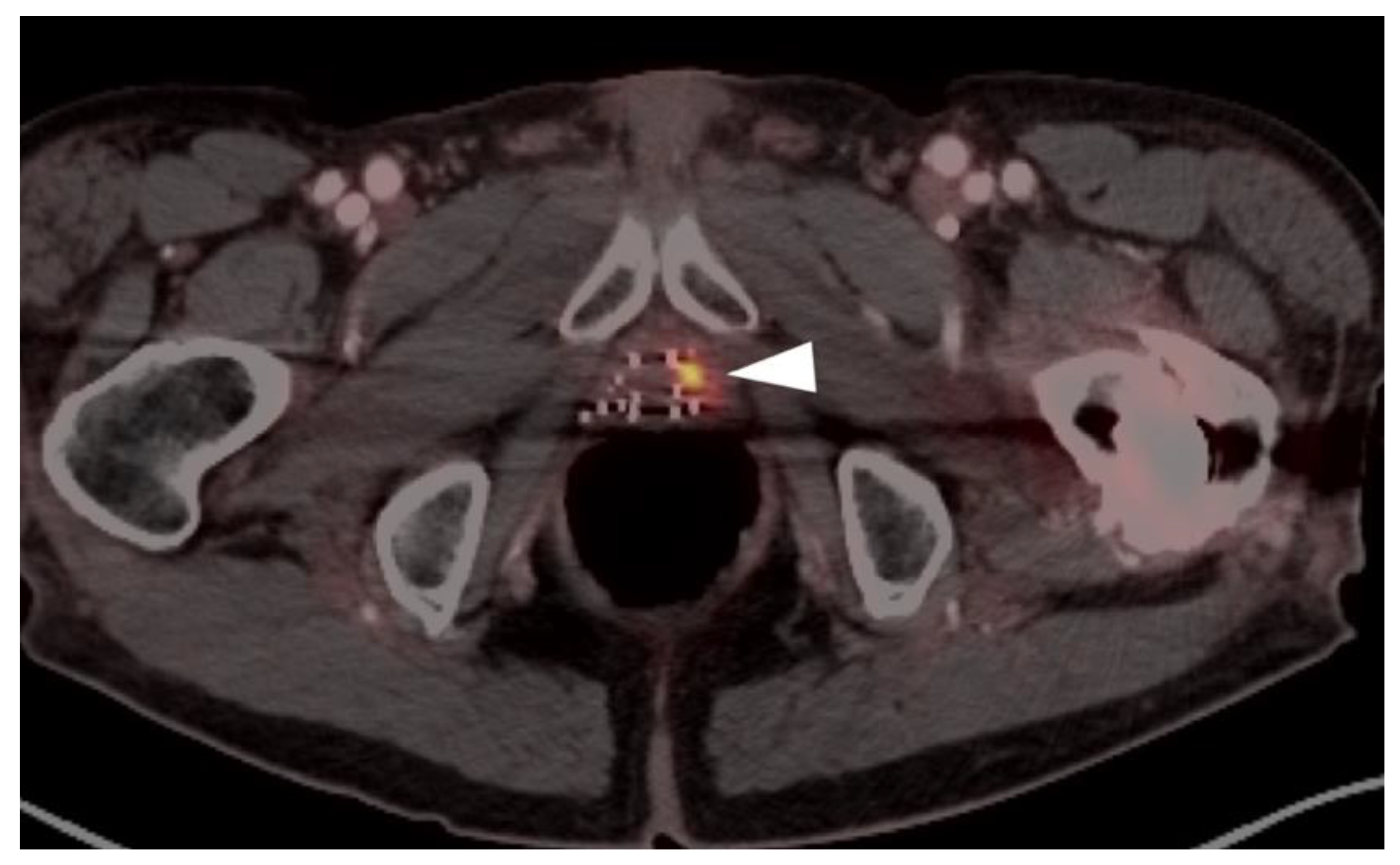
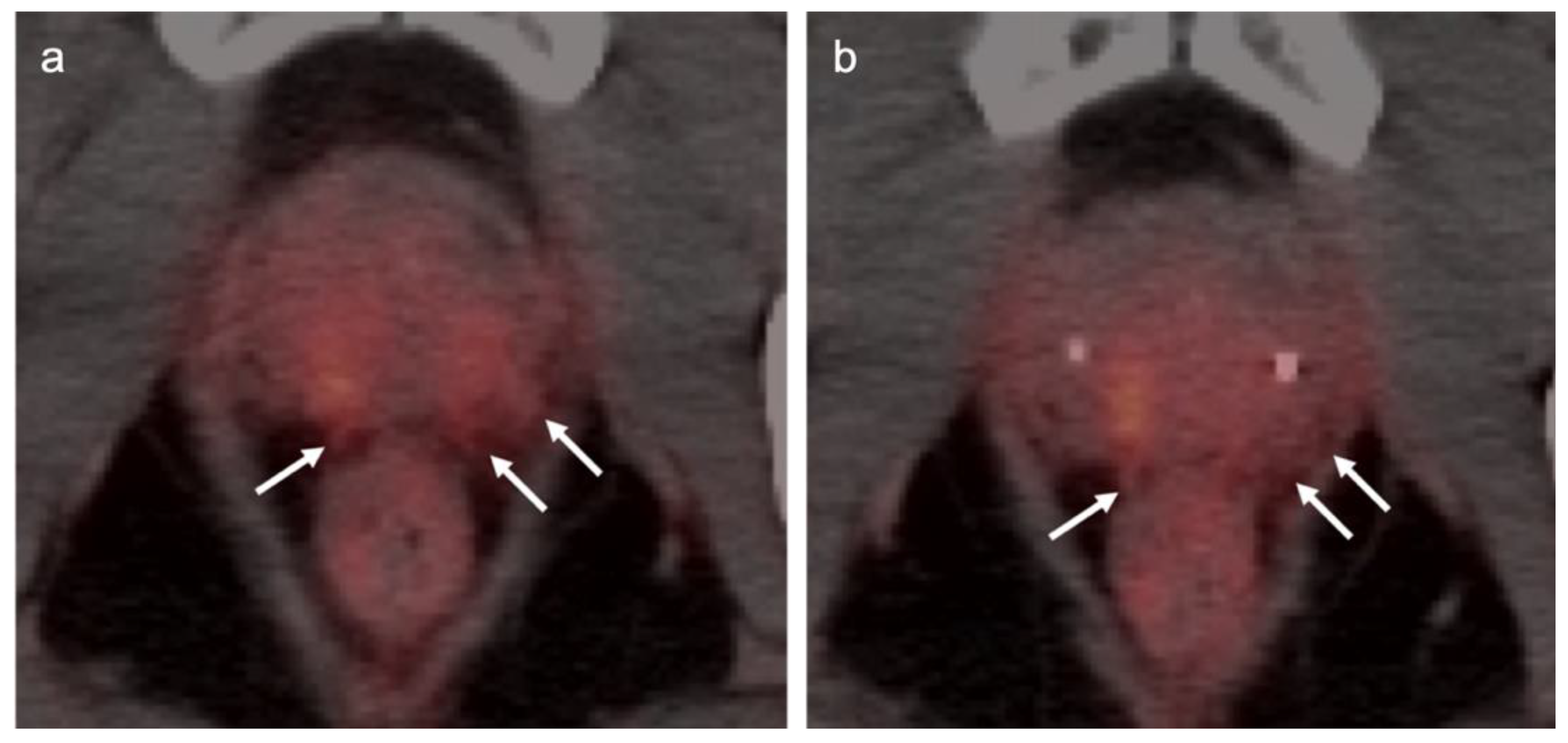



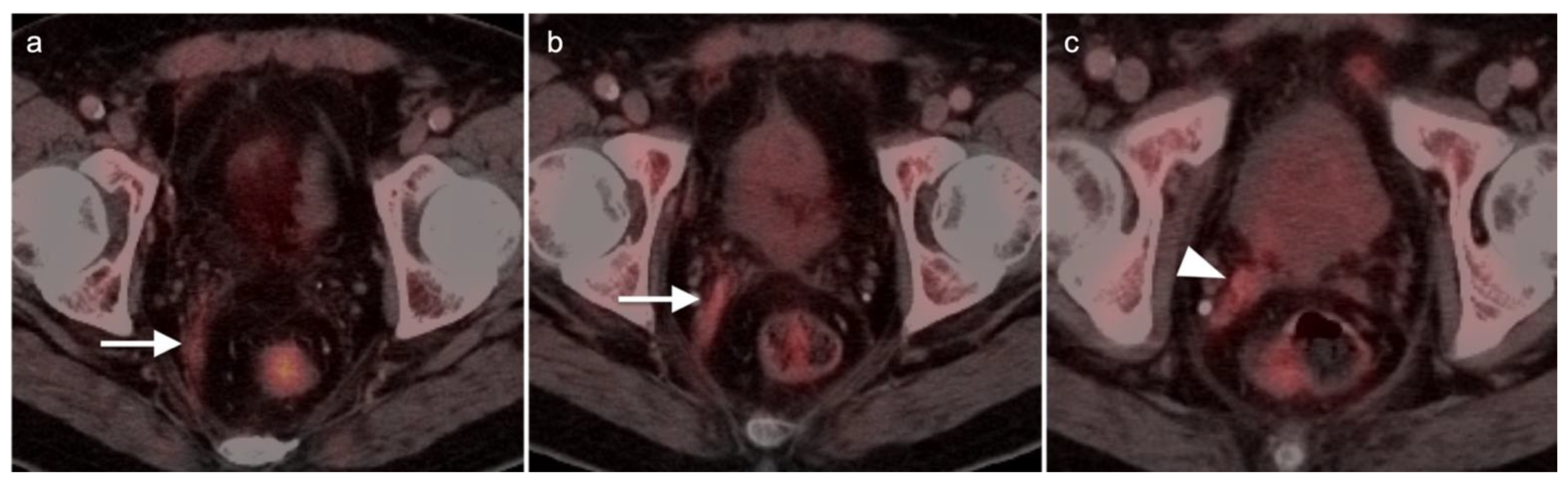





















| Risk Group | Criteria |
|---|---|
| Unfavorable Intermediate risk | Grade Group 3 and cT2b, cT2c or PSA 10–20 ng.mL No high risk features >50% biopsy cores (+) |
| High risk | Has at least one of the following: cT3a Grade Group 4 or Grade Group 5 PSA > 20 ng/mL |
| Very high risk | Has at least one of the following: cT3b–T4 Primary Gleason pattern 5 2–3 high risk features >4 cores with Grade Group 4 or 5 |
| Grade Group | Gleason Score | Gleason Pattern |
|---|---|---|
| Grade 1 | ≤6 | ≤3 + 3 |
| Grade 2 | 7 | 3 + 4 |
| Grade 3 | 7 | 4 + 3 |
| Grade 4 | 8 | 4 + 4, 3 + 5, 5 + 3 |
| Grade 5 | 9–10 | 4 + 5, 5 + 4, 5 + 5 |
| Prostate versus Reference Region | Interpretation | Note |
|---|---|---|
| Uptake between blood pool and bone marrow | Equivocal | Poor specificity, tissue confirmation recommended |
| Diffuse, focal or multifocal uptake > bone marrow | Suspicious for malignancy | Poor specificity, tissue confirmation recommended |
| <1 cm focus, uptake > blood pool | Suspicious for malignancy | Poor specificity, tissue confirmation recommended |
| <blood pool | Likely benign | data |
| Seminal Vesicles versus Reference Region | Interpretation | Note |
|---|---|---|
| Symmetrical, <blood pool | Most likely physiologic | Seminal vesicles are typically removed with prostatectomy, so suspicious uptake in this region may be in the neurovascular pedicle |
| Focal uptake or >blood pool | May be malignant | Recommend MRI |
| Fossa versus Reference Region | Interpretation | Note |
|---|---|---|
| Uptake > bone marrow | Suspicious for malignancy |
|
| Uptake > blood pool if focus < 1 cm | Suspicious for malignancy | |
| Uptake between blood pool and bone marrow | Does not meet criteria for malignancy but requires close follow-up | |
| <blood pool | Most likely benign |
| Lymph Node Region | Uptake Relative to Reference Region | Interpretation | Additional Notes |
|---|---|---|---|
| Typical location | >1 cm long axis >bone marrow | Equivocal |
|
| Typical location | >1 cm long axis between blood pool and bone marrow | Does not meet criteria for malignancy but requires close follow-up | |
| Typical location | <1 cm long axis, >blood pool and approaching bone marrow | Suspicious for malignancy | |
| Atypical location | Mild symmetric activity | Likely benign | |
| Atypical location | Asymmetric activity (>bone marrow) | Suspicious for malignancy |
| PSMA-RADS Number | Interpretation | Description |
|---|---|---|
| PSMA-RADS 1A | Benign | Benign lesion by CT and PET |
| PSMA-RADS 1B | Benign | Lesion characterized by biopsy or addition imaging to be benign |
| PSMA-RADS 2 | Likely benign | Low level uptake in site not typical for prostate cancer metastasis, or uptake in bone lesion more likely to be alternative diagnosis. |
| PSMA-RADS 3A | Equivocal | Borderline soft tissue site of uptake soft tissue, typical location for PCa involvement |
| PSMA-RADS 3B | Equivocal | Equivocal bone lesion |
| PSMA-RADS 3C | Equivocal | Marked uptake at soft tissue or osseous site atypical of prostate cancer |
| PSMA-RADS 3D | Equivocal | Lesion suspicious on anatomic imaging but lacking uptake on PET |
| PSMA-RADS 4 | PCa highly likely | Marked uptake but lacking confirmation on anatomic imaging (includes small nodes) |
| PSMA-RADS 5 | PCa almost certainly present | Marked uptake with anatomic imaging confirmatory |
| Score | Reported PSMA Expression | Uptake in Lesion Relative to that in Normal Reference Tissues |
|---|---|---|
| 0 | None | Uptake < blood pool |
| 1 | Low | >Blood pool but <liver |
| 2 | Intermediate | >liver but <parotid |
| 3 | High | >parotid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, A.E.; Fine, G.C.; Covington, M.F.; Koppula, B.R.; Wiggins, R.H.; Hoffman, J.M.; Morton, K.A. PET-CT in Clinical Adult Oncology—IV. Gynecologic and Genitourinary Malignancies. Cancers 2022, 14, 3000. https://doi.org/10.3390/cancers14123000
Salem AE, Fine GC, Covington MF, Koppula BR, Wiggins RH, Hoffman JM, Morton KA. PET-CT in Clinical Adult Oncology—IV. Gynecologic and Genitourinary Malignancies. Cancers. 2022; 14(12):3000. https://doi.org/10.3390/cancers14123000
Chicago/Turabian StyleSalem, Ahmed Ebada, Gabriel C. Fine, Matthew F. Covington, Bhasker R. Koppula, Richard H. Wiggins, John M. Hoffman, and Kathryn A. Morton. 2022. "PET-CT in Clinical Adult Oncology—IV. Gynecologic and Genitourinary Malignancies" Cancers 14, no. 12: 3000. https://doi.org/10.3390/cancers14123000
APA StyleSalem, A. E., Fine, G. C., Covington, M. F., Koppula, B. R., Wiggins, R. H., Hoffman, J. M., & Morton, K. A. (2022). PET-CT in Clinical Adult Oncology—IV. Gynecologic and Genitourinary Malignancies. Cancers, 14(12), 3000. https://doi.org/10.3390/cancers14123000






