Sexual Dysfunction of Patients with Diffuse Low-Grade Glioma: A Qualitative Review of a Neglected Concern
Abstract
:Simple Summary
Abstract
1. Introduction
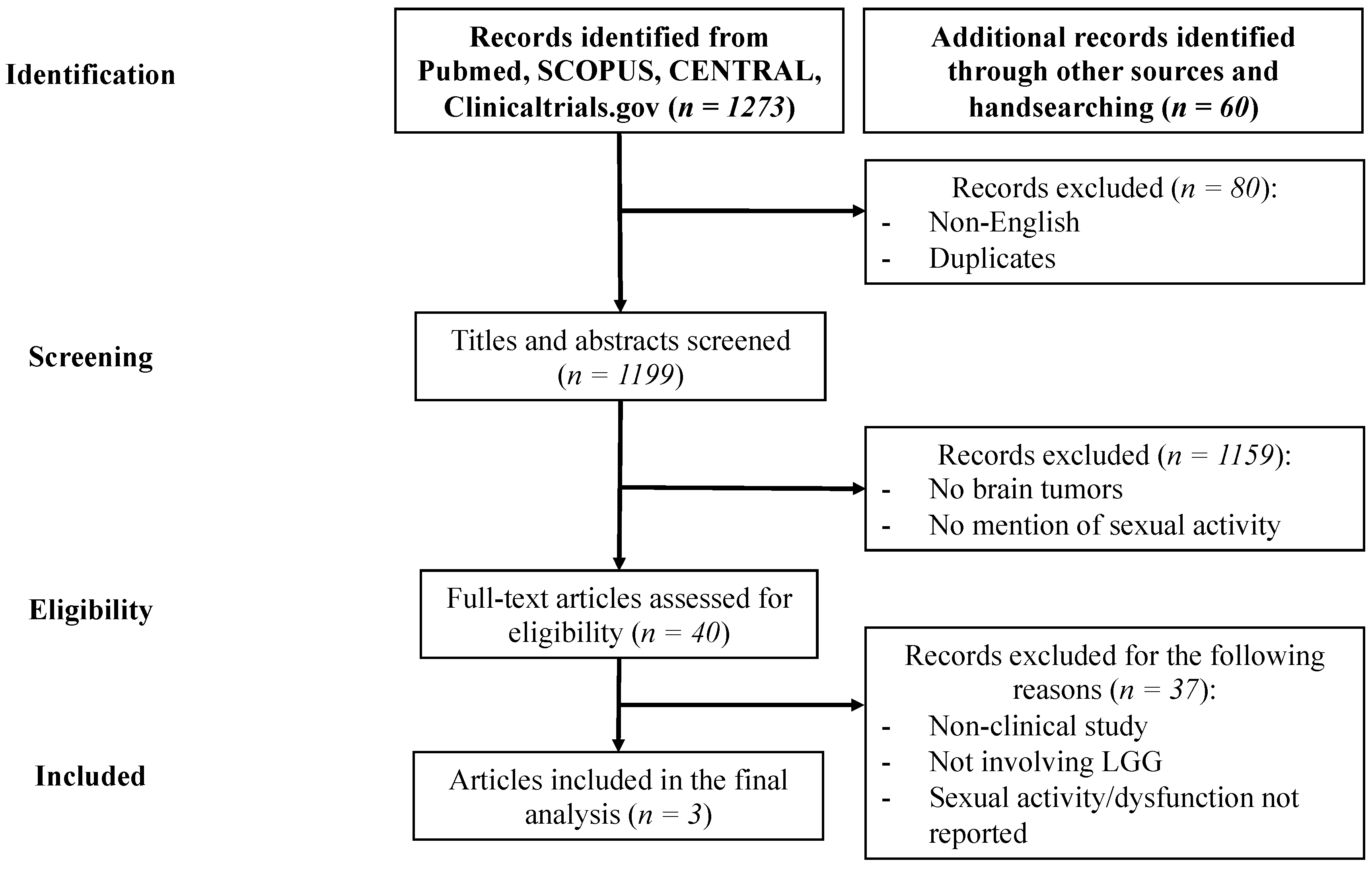
2. Materials and Methods
2.1. Methods of Reviewing
2.2. Methods for Evaluation of Sexual Activity and SD
2.3. Methods for Evaluation of the Repercussions of SD on QoL
3. Results
3.1. Included Studies
3.2. Incidence of SD and Consequences on QoL in LGG Adult Patients
4. Discussion
4.1. Methods for Evaluation of Sexual Activity and SD in LGG Patients
4.2. Incidence of SD and Its Consequences on QoL in LGG Adult Patients: Limitations of Presented Results
4.3. Antiseizure Medications and SD in LGG Patients
4.4. Surgical Treatment and SD in LGG Patients
4.5. Chemotherapy, Radiotherapy and SD in LGG Patients
4.6. Perspectives: Towards a Better Knowledge of Neural Bases of Sexual Activity to Tailor Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. Diffuse Low-Grade Gliomas in Adults; Springer: Berlin, Germany, 2017. [Google Scholar]
- Brown, T.J.; Bota, D.A.; van Den Bent, M.J.; Brown, P.D.; Maher, E.; Aregawi, D.; Liau, L.M.; Buckner, J.C.; Weller, M.; Berger, M.S.; et al. Management of low-grade glioma: A systematic review and meta-analysis. Neuro-Oncol. Pract. 2019, 6, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.M.; et al. Adult diffuse low-grade gliomas: 35-year experience at the Nancy France neurooncology unit. Front. Oncol. 2020, 1873, 574679. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Nazroo, J.; O’Connor, D.B.; Blake, M.; Pendleton, N. Sexual health and well-being among older men and women in England: Findings from the English Longitudinal Study of Ageing. Arch. Sex. Behav. 2016, 45, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Deaton, A.; Stone, A.A. Subjective wellbeing, health, and ageing. Lancet 2015, 385, 640–648. [Google Scholar] [CrossRef] [Green Version]
- Morokqff, P.J.; Gillilland, R. Stress, sexual functioning, and marital satisfaction. J. Sex Res. 1993, 30, 43–53. [Google Scholar] [CrossRef]
- Panjari, M.; Bell, R.J.; Davis, S.R. Sexual function after breast cancer. J. Sex. Med. 2011, 8, 294–302. [Google Scholar] [CrossRef]
- Wilson, C.M.; McGuire, D.B.; Rodgers, B.L.; Elswick, R.K., Jr.; Temkin, S.M. Body Image, Sexuality, and Sexual Functioning in Women With Gynecologic Cancer: An Integrative Review of the Literature and Implications for Research. Cancer Nurs. 2021, 44, E252–E286. [Google Scholar] [CrossRef]
- Surbeck, W.; Herbet, G.; Duffau, H. Sexuality after surgery for diffuse low-grade glioma. Neuro-Oncology 2015, 17, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Shepardson, R.L.; Carey, M.P. Sexual Dysfunctions; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Frankel Kelvin, J.; Steed, R.; Jarrett, J. Discussing safe sexual practices during cancer treatment. Clin. J. Oncol. Nurs. 2014, 18, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Cappelleri, J.C.; Rosen, R.C. The Sexual Health Inventory for Men (SHIM): A 5-year review of research and clinical experience. Int. J. Impot. Res. 2005, 17, 307–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, J.; Heiman, S.; Leiblum, C.; Meston, R.; Shabsigh, D.; Ferguson, R.; D’Agostino, R.C.B. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef]
- Maroufizadeh, S.; Riazi, H.; Lotfollahi, H.; Omani-Samani, R.; Amini, P. The 6-item Female Sexual Function Index (FSFI-6): Factor structure, reliability, and demographic correlates among infertile women in Iran. Middle East Fertil. Soc. J. 2020, 24, 7. [Google Scholar] [CrossRef] [Green Version]
- McGahuey, C.A.; Gelenberg, A.J.; Laukes, C.A.; Moreno, F.A.; Delgado, P.L.; McKnight, K.M.; Manber, R. The Arizona sexual experience scale (ASEX): Reliability and validity. J. Sex Marital Ther. 2000, 26, 25–40. [Google Scholar]
- Costantini, M.; Musso, M.; Viterbori, P.; Bonci, F.; Del Mastro, L.; Garrone, O.; Venturini, M.; Morasso, G. Detecting psychological distress in cancer patients: Validity of the Italian version of the Hospital Anxiety and Depression Scale. Support. Care Cancer 1999, 7, 121–127. [Google Scholar] [CrossRef]
- Morasso, G.; Costantini, M.; Baracco, G.; Borreani, C.; Capelli, M. Assessing psychological distress in cancer patients: Validation of a self-administered questionnaire. Oncology 1996, 53, 295–302. [Google Scholar] [CrossRef]
- Sprangers, M.A.G.; Cull, A.; Bjordal, K.; Groenvold, M.; Aaronson, N.K. The European Organization for Research and Treatment of Cancer approach to quality of life assessment: Guidelines for developing questionnaire modules. Qual. Life Res. 1993, 2, 287–295. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Claassens, L.; Aaronson, N.K.; Coens, C.; Mauer, M.; Osoba, D.; Stupp, R.; Mirimanoff, R.O.; van den Bent, M.J.; Bottomley, A.; et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur. J. Cancer 2010, 46, 1033–1040. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, C.Y.; Petruzzi, A.; Fedeli, G.; Vistalli, M.G.; Innocenti, A.; Silvani, A.; Lamperti, E. Hidden reality: Sexual sphere in brain tumor patients. Psychol. Health Med. 2017, 22, 370–380. [Google Scholar] [CrossRef]
- Boccia, M.L.; Anyanda, E.I.; Fonkem, E. A preliminary report on quality of life and sexual function in brain tumor patients. J. Sex. Med. 2021, 18, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Laldjising, E.; Sekercan, A.; Gadjradj, P.S. Neurosurgeons’ opinions on discussing sexual health among brain tumor patients: Room for improvement? J. Clin. Neurosci. 2021, 94, 292–297. [Google Scholar] [CrossRef]
- Krouwel, E.M.; Albers, L.F.; Nicolai, M.P.J.; Putter, H.; Osanto, S.; Pelger, R.C.M.; Elzevier, H.W. Discussing sexual health in the medical oncologist’s practice: Exploring current practice and challenges. J. Cancer Educ. 2020, 35, 1072–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bräutigam, E.; Schratter-Sehn, A.; Kottmel, A.; Bitzer, J.; Teleky, B.; Ucsnik, L. Do radiation oncologists talk about sexual health and dysfunction with their cancer patients? Results of the igls-vienna-sexmed-survey. Clin. Transl. Radiat. Oncol. 2020, 21, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wincze, J.P.; Weisberg, R.B. Sexual Dysfunction: A Guide for Assessment and Treatment; Guilford Publications: New York, NY, USA, 2015. [Google Scholar]
- Elnazer, H.Y.; Baldwin, D.S. Structured review of the use of the Arizona sexual experiences scale in clinical settings. Hum. Psychopharmacol. Clin. Exp. 2020, 35, e2730. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.; Husson, O.; Alberti, P.; Arraras, J.I.; Chinot, O.L.; Costantini, A.; Darlington, A.S.; Dirven, L.; Eichler, M.; Hammerlid, E.B.; et al. Understanding the quality of life (QOL) issues in survivors of cancer: Towards the development of an EORTC QOL cancer survivorship questionnaire. Health Qual. Life Outcomes 2018, 16, 114. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, D.S. Depression and sexual dysfunction. Br. Med. Bull. 2001, 57, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Tierney, D.K. Sexuality: A quality-of-life issue for cancer survivors. Semin. Oncol. Nurs. 2008, 24, 71–79. [Google Scholar] [CrossRef]
- Lindau, S.T.; Abramsohn, E.M.; Matthews, A.C. A manifesto on the preservation of sexual function in women and girls with cancer. Am. J. Obstet. Gynecol. 2015, 213, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Krychman, M.L.; Amsterdam, A.; Carter, J.; Castiel, M.; DeAngelis, L. Brain cancer and sexual health: A case report. Palliat. Support. Care 2004, 2, 315–318. [Google Scholar] [CrossRef]
- Barton, D.L.; Pugh, S.L.; Ganz, P.A.; Plaxe, S.C.; Koontz, B.F.; Carter, J.; Greyz-Yusupov, N.; Page, S.J.; Rowland, K.M.; Balcueva, E.P.; et al. Randomized controlled phase II evaluation of two dose levels of bupropion versus placebo for sexual desire in female cancer survivors: NRG-CC004. J. Clin. Oncol. 2022, 40, 324–334. [Google Scholar] [CrossRef]
- Rathore, C.; Henning, O.J.; Luef, G.; Radhakrishnan, K. Sexual dysfunction in people with epilepsy. Epilepsy Behav. 2019, 100, 106495. [Google Scholar] [CrossRef]
- Herzog, A.G.; Drislane, F.W.; Schomer, D.L.; Pennell, P.B.; Bromfield, E.B.; Dworetzky, B.A.; Farina, E.L.; Frye, C.A. Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 2005, 65, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Choi, H.; Hirsch, L.J.; Katz, A.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2017, 76, 24–31. [Google Scholar] [CrossRef]
- Gil-Nagel, A.; Lopez-Munoz, F.; Serratosa, J.M.; Moncada, I.; Garcia-Garcia, P.; Alamo, C. Effect of lamotrigine on sexual function in patients with epilepsy. Seizure 2006, 15, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Maschio, M.; Saveriano, F.; Dinapoli, L.; Jandolo, B. Reversible erectile dysfunction in a patient with brain tumor-related epilepsy in therapy with zonisamide in add-on. J. Sex. Med. 2011, 8, 3515–3517. [Google Scholar] [CrossRef]
- Johnson, J. Sexual impotence and the limbic system. Br. J. Psychiatry 1965, 111, 300–303. [Google Scholar] [CrossRef]
- Erickson, T.C. Erotomania (nymphomania) as an expression of cortical epileptiform discharge. Arch. Neurol. Psychiatry 1945, 53, 226–231. [Google Scholar] [CrossRef]
- Van Reeth, P.C.; Dierkens, J.; Luminet, D. L’hypersexualité dans l’épilepsie et les tumeurs du lobe temporal. Acta Neurol. Belg. 1958, 58, 194–218. [Google Scholar]
- Angelini, L.; Mazzucchi, A.; Picciotto, F.; Nardocci, N.; Broggi, G. Focal lesion of the right cingulum: A case report in a child. J. Neurol. Neurosurg. Psychiatry 1981, 44, 355–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitoh, Y.; Arita, N.; Hayakawa, T.; Nakao, K.; Mogami, H. Recovery of gonadal function after resection of an oligodendroglioma localized in the anterior horn of the lateral ventricle: Relation to pulsatile secretion of luteinizing hormone. Neurosurgery 1989, 25, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Ghika-Schmid, F.; Assal, G.; De Tribolet, N.; Regli, F. Klüver-Bucy syndrome after left anterior temporal resection. Neuropsychologia 1995, 33, 101–113. [Google Scholar] [CrossRef]
- Van Westrhenen, A.; Senders, J.T.; Martin, E.; DiRisio, A.C.; Broekman, M.L.D. Clinical challenges of glioma and pregnancy: A systematic review. J. Neuro-Oncol. 2018, 139, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Peeters, S.; Pagès, M.; Gauchotte, G.; Miquel, C.; Cartalat-Carel, S.; Guillamo, J.S.; Capelle, L.; Delattre, J.Y.; Beauchesne, P.; Debouverie, M.; et al. Interactions between glioma and pregnancy: Insight from a 52-case multicenter series. J. Neurosurg. 2017, 128, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; Duffau, H. Factors Associated With Long-term Survival in Women Who Get Pregnant After Surgery for WHO Grade II Glioma. Neurology 2022. [Google Scholar] [CrossRef]
- Strowd, R.E.; Blackwood, R.; Brown, M.; Harmon, M.; Lovato, J.; Yalcinkaya, T.; Lesser, G. Impact of temozolomide on gonadal function in patients with primary malignant brain tumors. J. Oncol. Pharm. Pract. 2013, 19, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Tosoni, A.; Balestrini, D.; Brandes, A.A. Fertility preservation in women with CNS tumors. Expert Rev. Anticancer Ther. 2017, 17, 439–445. [Google Scholar] [CrossRef]
- Lehmann, V.; Nahata, L.; Ferrante, A.C.; Hansen-Moore, J.A.; Yeager, N.D.; Klosky, J.L.; Gerhardt, C.A. Fertility-related perceptions and impact on romantic relationships among adult survivors of childhood cancer. J. Adolesc. Young Adult Oncol. 2018, 7, 409–414. [Google Scholar] [CrossRef]
- Stiner, R.K.; Clarke, J.L.; Sinha, N.; Chan, J.; Letourneau, J.M.; Niemasik, E.E.; Rabbitt, J.E.; Chang, S.M.; Butowski, N.A.; Prados, M.D. Attitudes toward fertility and fertility preservation in women with glioma. Neuro-Oncol. Pract. 2019, 6, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Wide, A.; Wettergren, L.; Ahlgren, J.; Smedby, K.E.; Hellman, K.; Henriksson, R.; Rodriguez-Wallberg, K.; Ståhl, O.; Lampic, C. Fertility-related information received by young women and men with cancer–a population-based survey. Acta Oncol. 2021, 60, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.B.; Kelvin, J.F.; DeAngelis, L.M. Fertility preservation in primary brain tumor patients. Neuro-Oncol. Pract. 2017, 4, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordan, T.; Thomas, A.M.; Ginsburg, E.S.; Wen, P.Y.; Dolinko, A.V.; Bortoletto, P. Fertility preservation outcomes in women with gliomas: A retrospective case–control study. J. Neuro-Oncol. 2020, 147, 371–376. [Google Scholar] [CrossRef]
- Rees, P.M.; Fowler, C.J.; Maas, C.P. Sexual function in men and women with neurological disorders. Lancet 2007, 369, 512–525. [Google Scholar] [CrossRef]
- Georgiadis, J.R.; Kringelbach, M.L.; Pfaus, J.G. Sex for fun: A synthesis of human and animal neurobiology. Nat. Rev. Urol. 2012, 9, 486–498. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bruschetta, D.; Milardi, D.; Quattrini, F.; Sciarrone, F.; la Rosa, G.; Bramanti, P.; Anastasi, G. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. 2019, 9, e01389. [Google Scholar] [CrossRef]
- Georgiadis, J.R.; Reinders, A.A.T.S.; Paans, A.M.J.; Renken, R.; Kortekaas, R. Men versus women on sexual brain function: Prominent differences during tactile genital stimulation, but not during orgasm. Hum. Brain Mapp. 2009, 30, 3089–3101. [Google Scholar] [CrossRef]
- Duffau, H.; Capelle, L. Preferential brain locations of low-grade gliomas: Comparison with glioblastomas and review of hypothesis. Cancer 2004, 100, 2622–2626. [Google Scholar] [CrossRef]
- Michaud, K.; Duffau, H. Surgery of insular and paralimbic diffuse low-grade gliomas: Technical considerations. J. Neurooncol. 2016, 130, 289–298. [Google Scholar] [CrossRef]
- Lemaitre, A.-L.; Herbet, G.; Duffau, H.; Lafargue, G. Personality and behavioral changes after brain tumor resection: A lesion mapping study. Acta Neurochir. 2021, 163, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Surbeck, 2015 [10] | Finocchiaro, 2017 [24] | Boccia, 2021 [25] |
| Study type, country | Transversal, France | Transversal, Italy | Transversal, USA |
| Sample size (M/F) & Number of LGGs (M/F) | 32 (17/15), 32 (17/15) | 46 (28/18), 21 (NR) | 46 (11/35), 9 (3/6) |
| Mean age (years) | 38.6 | 45 | 53.5 |
| Tumor Location (number) | Frontal (11) Parietal (6) Occipital (2) Temporo-insular (6) Fronto-temporo-insular (7) | NR | Frontal (16) Temporal (8) Other locations (22) |
| Tumor side (Number, %) | Right (18, 56%) Left (14, 44%) | NR | NR |
| Methods of SD evaluation | ASEX | Ad hoc questionnaire | IIEF (M) and FSFI (F) |
| SD general incidence (Number (M/F), % (M/F)) | 14 (5/9), 44% (29%/60%) | 27 (12/13), 58% (43%/72%) | 29 (6/23), 63% (60%/67%) |
| SD incidence in LGG (Number (M/F), % (M/F) | 14 (5/9), 44% (29%/60%) | NR | NR |
| SD Symptoms (number, %) | Sex drive (34%) Arousal (34%) Penile erection/vaginal lubrication (25%) Orgasm (37.5%) Satisfaction (28%) | Sex drive (56%) Arousal (41.5%) Pain (32%) Orgasm (37.5%) Satisfaction (32%) | Sex drive (M:62%, F:38%) Arousal (M: 54%, F:46%) Pain (M:NR, F:42%) Orgasm (M:54%, F:38%) Satisfaction (M:62%, F:50%) |
| SD reported onset after diagnosis (%) | 47% | 34% | NR |
| Association between SD and tumor location | - Right-sided resections and difficulties in reaching orgasm (p < 0.02) - Temporal lobe resection and reduction in sexual drive/arousal in men (p < 0.004) - ASMs, right-sided resection and higher ASEX scores in men (p = 0.031) | NR | - No association between tumor side and FSFI scores in women |
| Methods of QoL evaluation | NE | HADS, PDI, EORTC QLQ-C30, EORTC QLQ-BN20 | FACT-Br |
| Association between SD and QoL | NE | - Significant higher levels of anxiety and depression (p < 0.01) and significant worse levels of QoL in patients with SD (p < 0.01) - No association between SD and distress | - In women, correlation of desire with functional wellbeing and total QoL scores, correlation of social wellbeing with desire, arousal and satisfaction. - In men, no correlation between SD and QoL |
| Timing of Evaluation | - At home - Return to normal social and professional life - No RT - No CT for at least 1 year - Not diagnosed or treated for depression | - During hospitalization or day treatments - No data about treatment timing | - Scheduled appointment - No data about treatment timing |
| Adjuvant Treatments | Surgery (32) RT (0) (No CT for at least 1 year) | NR | None (7) Surgery (8) Surgery + CT (1) Surgery + RT (2) All 3 (24) Missing data (8) |
| First Author, Year | Lehman, 2018 [53] | Stiner, 2019 [54] | Wide, 2021 [55] | Stone, 2017 [56] | Nordan, 2020 [57] |
| Number of Cancer Patients (M/F) | 92 (NR) | 69 (0/69) | 1010 (316/694) | 70 (38/32) | 10 (0/10) |
| Number of Brain Tumors (LGG) | 25 (NR) | 69 (35) | 123 (NR) | 70 (12) | 10 (5) |
| Number of Brain Tumor Patients that Had FP Discussion Prior to Treatment | 12 | 21 | 23 | 70 (47 at diagnosis, 23 at progression) | 10 |
| % of Brain Tumor Patients that Had FP Discussion Prior to Treatment | 50% | 30% | 19% | 100% (67% at diagnosis, 33% at progression) | 100% |
| Other results | - While fertility preservation was not important at the time of diagnosis, it was a priority for them at the time of survey completion | - 51 patients (73%) accepted referral to specific unit - Patients were more likely to accept referral if they had no prior children | - The total number of follicles, of oocytes retrieved and the percentage of mature oocytes were similar between cases and controls. - One patient delivered a healthy child. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombard, A.; Duffau, H. Sexual Dysfunction of Patients with Diffuse Low-Grade Glioma: A Qualitative Review of a Neglected Concern. Cancers 2022, 14, 3025. https://doi.org/10.3390/cancers14123025
Lombard A, Duffau H. Sexual Dysfunction of Patients with Diffuse Low-Grade Glioma: A Qualitative Review of a Neglected Concern. Cancers. 2022; 14(12):3025. https://doi.org/10.3390/cancers14123025
Chicago/Turabian StyleLombard, Arnaud, and Hugues Duffau. 2022. "Sexual Dysfunction of Patients with Diffuse Low-Grade Glioma: A Qualitative Review of a Neglected Concern" Cancers 14, no. 12: 3025. https://doi.org/10.3390/cancers14123025
APA StyleLombard, A., & Duffau, H. (2022). Sexual Dysfunction of Patients with Diffuse Low-Grade Glioma: A Qualitative Review of a Neglected Concern. Cancers, 14(12), 3025. https://doi.org/10.3390/cancers14123025






