The Prognostic Role of the C-Reactive Protein and Serum Lactate Dehydrogenase in a Pediatric Series of Bone Ewing Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomarkers
2.2. Treatment
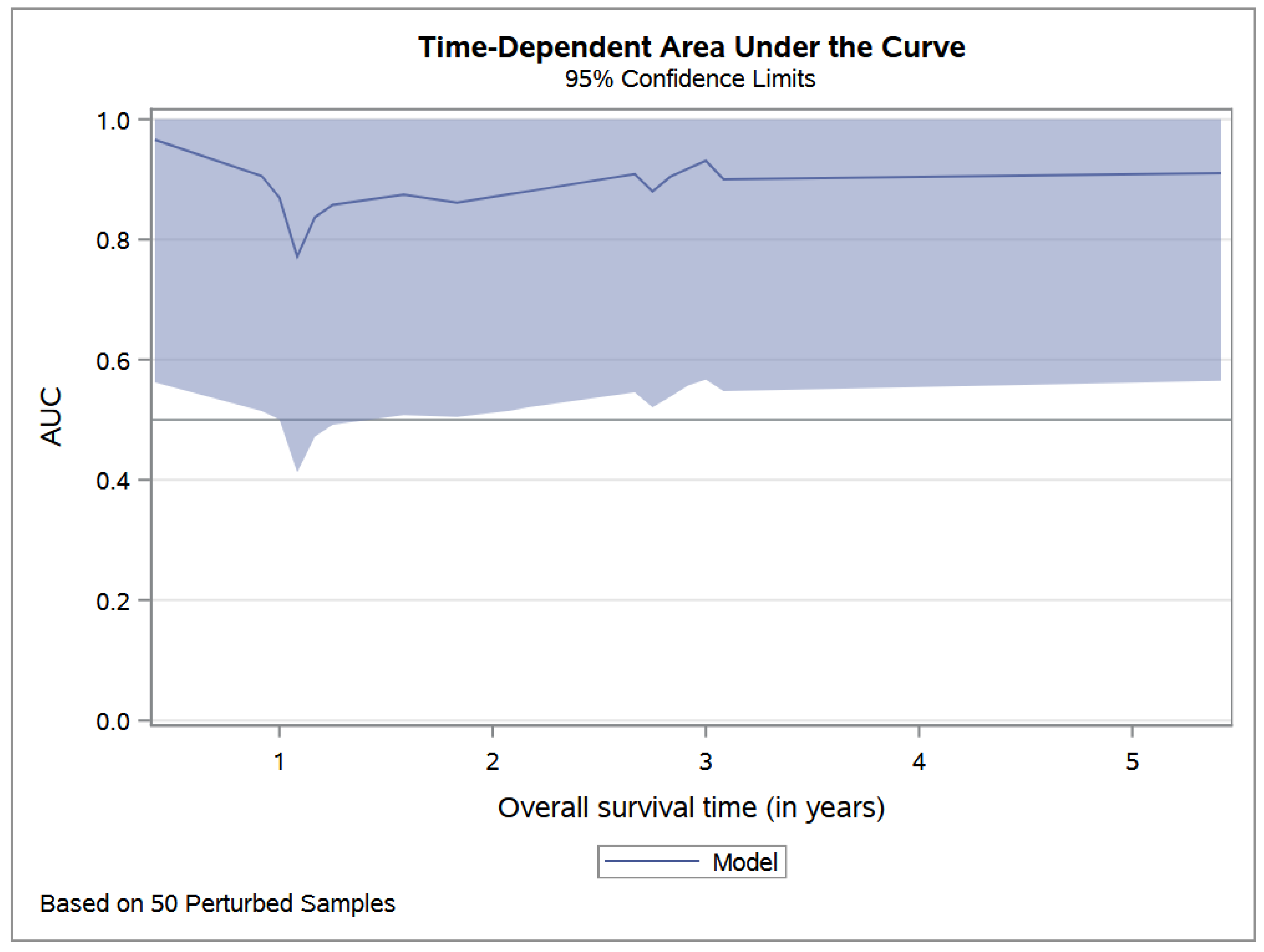
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Features
3.2. Treatment Details
3.3. Evaluation of Prognostic Factors
3.4. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delattre, O.; Zucman, J.; Plougastel, B.; Desmaze, C.; Melot, T.; Peter, M.; Kovar, H.; Joubert, I.; de Jong, P.; Rouleau, G.; et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992, 359, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Sundby Hall, K.; Luksch, R.; Tienghi, A.; Wiebe, T.; Fagioli, F.; Alvegard, T.A.; Brach del Prever, A.; Tamburini, A.; Alberghini, M.; et al. Nonmetastatic Ewing family tumors: High-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann. Oncol. 2011, 22, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized Controlled Trial of Interval-Compressed Chemotherapy for the Treatment of Localized Ewing Sarcoma: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef] [Green Version]
- Le Deley, M.C.; Paulussen, M.; Lewis, I.; Brennan, B.; Ranft, A.; Whelan, J.; Le Teuff, G.; Michon, J.; Ladenstein, R.; Marec-Bérard, P.; et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 2440–2448. [Google Scholar] [CrossRef]
- Hesla, A.C.; Papakonstantinou, A.; Tsagkozis, P. Current Status of Management and Outcome for Patients with Ewing Sarcoma. Cancers 2021, 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Ferrari, S.; Longhi, A.; Donati, D.; De Paolis, M.; Forni, C.; Versari, M.; Setola, E.; Briccoli, A.; Barbieri, E. Therapy and survival after recurrence of Ewing’s tumors: The Rizzoli experience in 195 patients treated with adjuvant and neoadjuvant chemotherapy from 1979 to 1997. Ann. Oncol. 2003, 14, 1654–1659. [Google Scholar] [CrossRef]
- McTiernan, A.; Driver, D.; Michelagnoli, M.P.; Kilby, A.M.; Whelan, J.S. High dose chemotherapy with bone marrow or peripheral stem cell rescue is an effective treatment option for patients with relapsed or progressive Ewing’s sarcoma family of tumours. Ann. Oncol. 2006, 17, 1301–1305. [Google Scholar] [CrossRef]
- Rasper, M.; Jabar, S.; Ranft, A.; Jürgens, H.; Amler, S.; Dirksen, U. The value of high-dose chemotherapy in patients with first relapsed Ewing sarcoma: High-Dose in Relapsed Ewing Sarcoma. Pediatr. Blood Cancer 2014, 61, 1382–1386. [Google Scholar] [CrossRef]
- Ferrari, S.; Luksch, R.; Hall, K.S.; Fagioli, F.; Prete, A.; Tamburini, A.; Tienghi, A.; DiGirolamo, S.; Paioli, A.; Abate, M.E.; et al. Post-relapse survival in patients with Ewing sarcoma. Pediatr. Blood Cancer 2015, 62, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Picci, P.; Rougraff, B.T.; Bacci, G.; Neff, J.R.; Sangiorgi, L.; Cazzola, A.; Baldini, N.; Ferrari, S.; Mercuri, M.; Ruggieri, P.; et al. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing’s sarcoma of the extremities. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 1763–1769. [Google Scholar] [CrossRef]
- Picci, P.; Böhling, T.; Bacci, G.; Ferrari, S.; Sangiorgi, L.; Mercuri, M.; Ruggieri, P.; Manfrini, M.; Ferraro, A.; Casadei, R.; et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 1553–1559. [Google Scholar] [CrossRef]
- Rosito, P.; Mancini, A.F.; Rondelli, R.; Abate, M.E.; Pession, A.; Bedei, L.; Bacci, G.; Picci, P.; Mercuri, M.; Ruggieri, P.; et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: A preliminary report of 6 years of experience. Cancer 1999, 86, 421–428. [Google Scholar] [CrossRef]
- Raney, R.B.; Asmar, L.; Newton, W.A.; Bagwell, C.; Breneman, J.C.; Crist, W.; Gehan, E.A.; Webber, B.; Wharam, M.; Wiener, E.S.; et al. Ewing’s sarcoma of soft tissues in childhood: A report from the Intergroup Rhabdomyosarcoma Study, 1972 to 1991. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, M.; Ahrens, S.; Burdach, S.; Craft, A.; Dockhorn-Dworniczak, B.; Dunst, J.; Fröhlich, B.; Winkelmann, W.; Zoubek, A.; Jürgens, H. Primary metastatic (stage IV) Ewing tumor: Survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1998, 9, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.J.; Ahrens, S.; Paulussen, M.; Jürgens, H.F.; Voûte, P.A.; Gadner, H.; Craft, A.W. Prognostic factors in Ewing’s tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Samuel, G.; Crow, J.; Godwin, A.K.; Zeng, Y. Molecular assessment of circulating exosomes toward liquid biopsy diagnosis of Ewing sarcoma family of tumors. Transl. Res. 2018, 201, 136–153. [Google Scholar] [CrossRef]
- Hara, M.; Matsuzaki, Y.; Shimuzu, T.; Tomita, M.; Ayabe, T.; Enomoto, Y.; Onitsuka, T. Preoperative Serum C-reactive Protein Level in Non-small Cell Lung Cancer. Anticancer Res. 2007, 27, 3001–3004. [Google Scholar]
- Shrotriya, S.; Walsh, D.; Nowacki, A.S.; Lorton, C.; Aktas, A.; Hullihen, B.; Benanni-Baiti, N.; Hauser, K.; Ayvaz, S.; Estfan, B. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS ONE 2018, 13, e0202555. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, J.; Meng, Y.; Zhou, Y.; Li, W. Higher pretreatment lactate dehydrogenase concentration predicts worse overall survival in patients with lung cancer. Medicine 2018, 97, e12524. [Google Scholar] [CrossRef]
- Chen, J.; Sun, M.; Hua, Y.; Cai, Z. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: A meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. Stockh. Swed. 2015, 54, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Bien, E.; Rapala, M.; Krawczyk, M.; Balcerska, A. The serum levels of soluble interleukin-2 receptor α and lactate dehydrogenase but not of B2-Microglobulin correlate with selected clinico-pathological prognostic factors and response to therapy in childhood soft tissue sarcomas. J. Cancer Res. Clin. Oncol. 2009, 136, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Moritake, H.; Kamimura, S.; Akiyoshi, K.; Nagatoshi, Y.; Chuman, H.; Okamura, J. Prognostic significance of elevated lactate dehydrogenase and creatine kinase in patients with rhabdomyosarcoma. Med. Pediatr. Oncol. 2003, 40, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Kotoh, K.; Kato, M.; Kohjima, M.; Tanaka, M.; Miyazaki, M.; Nakamura, K.; Enjoji, M.; Nakamuta, M.; Takayanagi, R. Lactate dehydrogenase production in hepatocytes is increased at an early stage of acute liver failure. Exp. Ther. Med. 2011, 2, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Mythili, S.; Malathi, N. Diagnostic markers of acute myocardial infarction. Biomed. Rep. 2015, 3, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Cao, J.; Gao, X.; Zhang, J.; Wang, L.; Wang, B.; Guo, L.; Hu, X.; Wang, Z. Overall survival of cancer patients with serum lactate dehydrogenase greater than 1000 IU/L. Tumor Biol. 2016, 37, 14083–14088. [Google Scholar] [CrossRef] [Green Version]
- Casadei, R.; Magnani, M.; Biagini, R.; Mercuri, M. Prognostic factors in Ewing’s sarcoma of the foot. Clin. Orthop. 2004, 420, 230–238. [Google Scholar] [CrossRef]
- Li, S.; Yang, Q.; Wang, H.; Wang, Z.; Zuo, D.; Cai, Z.; Hua, Y. Prognostic significance of serum lactate dehydrogenase levels in Ewing’s sarcoma: A meta-analysis. Mol. Clin. Oncol. 2016, 5, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Szkandera, J.; Gerger, A.; Liegl-Atzwanger, B.; Absenger, G.; Stotz, M.; Samonigg, H.; Maurer-Ertl, W.; Stojakovic, T.; Ploner, F.; Leithner, A.; et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br. J. Cancer 2013, 109, 2316–2322. [Google Scholar] [CrossRef] [Green Version]
- Milano, G.M.; Cozza, R.; Ilari, I.; De Sio, L.; Boldrini, R.; Jenkner, A.; de Ioris, M.; Inserra, A.; Dominici, C.; Donfrancesco, A. High histologic and overall response to dose intensification of ifosfamide, carboplatin, and etoposide with cyclophosphamide, doxorubicin, and vincristine in patients with high-risk Ewing sarcoma family tumors. Cancer 2006, 106, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Puma, N.; Sebastian, A.D.; Paioli, A.; Bisogno, G.; Rabusin, M.; Coccoli, L.; Tamburini, A.; Milano, G.M.; Mascarin, M.; Bertulli, R.; et al. Maintenance therapy with oral cyclophosphamide plus celecoxib in patients with metastatic Ewing sarcoma: Results of the Italian Sarcoma Group/AIEOP EW-2 study. J. Clin. Oncol. 2020, 38 (Suppl. 15), 10517. [Google Scholar] [CrossRef]
- Tural, D.; Molinas Mandel, N.; Dervisoglu, S.; Dincbas, F.O.; Koca, S.; Oksuz, D.C.; Kantarci, F.; Turna, H.; Selcukbiricik, F.; Hiz, M. Extraskeletal Ewing’s sarcoma family of tumors in adults: Prognostic factors and clinical outcome. J. Clin. Oncol. 2012, 42, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Bertoni, F.; Bacchini, P.; Cesari, M.; Picci, P. Predictive Factors of Histologic Response to Primary Chemotherapy in Patients with Ewing Sarcoma. J. Pediatr. Hematol. Oncol. 2007, 29, 5. [Google Scholar] [CrossRef]
- Bacci, G.; Longhi, A.; Ferrari, S.; Mercuri, M.; Versari, M.; Bertoni, F. Prognostic factors in non-metastatic Ewing’s sarcoma tumor of bone: An analysis of 579 patients treated at a single institution with adjuvant or neoadjuvant chemotherapy between 1972 and 1998. Acta Oncol. 2006, 45, 469–475. [Google Scholar] [CrossRef]
- Bacci, G.; Ferrari, S.; Longhi, A.; Rimondini, S.; Versari, M.; Zanone, A.; Forni, C. Prognostic significance of serum LDH in Ewing’s sarcoma of bone. Oncol. Rep. 1999, 6, 807–811. [Google Scholar] [CrossRef]
- Bacci, G.; Avella, M.; McDonald, D.; Toni, A.; Orlandi, M.; Campanacci, M. Serum lactate dehydrogenase (LDH) as a tumor marker in Ewing’s sarcoma. Tumori 1988, 74, 649–655. [Google Scholar] [CrossRef]
- Biswas, B.; Shukla, N.K.; Deo, S.V.; Agarwala, S.; Sharma, D.N.; Vishnubhatla, S.; Bakhshi, S. Evaluation of outcome and prognostic factors in extraosseous Ewing sarcoma. Pediatr. Blood Cancer 2014, 61, 1925–1931. [Google Scholar] [CrossRef]
- Riley, R.D.; Burchill, S.A.; Abrams, K.R.; Heney, D.; Sutton, A.J.; Jones, D.R.; Lambert, P.C.; Young, B.; Wailoo, A.J.; Lewiset, I.J. A systematic review of molecular and biological markers in tumours of the Ewing’s sarcoma family. Eur. J. Cancer 2003, 39, 19–30. [Google Scholar] [CrossRef]
- Nakamura, T.; Asanuma, K.; Hagi, T.; Sudo, A. Is Serum Lactate Dehydrogenase Useful for Predicting Oncological Outcome in Patients with Soft Tissue Sarcoma? Anticancer Res. 2019, 39, 6871–6875. [Google Scholar] [CrossRef]
- Cao, L.L.; Yang, L.; Chen, Z.; Yue, Z.; Pei, L.; Jia, M.; Wang, H.; Li, T. A New Classifier Based on Laboratory Indicators for Early Diagnosis and Prognosis Prediction of Ewing’s Sarcoma. Clin. Lab. 2020, 66, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zhao, X.; Fang, E.; Zhao, X. The value of C-reactive protein as an independent prognostic indicator for disease-specific survival in patients with soft tissue sarcoma: A meta-analysis. PLoS ONE 2019, 14, e0219215. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Grimer, R.; Gaston, C.; Francis, M.; Charman, J.; Graunt, P.; Uchida, A.; Sudo, A.; Jeys, L. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur. J. Cancer 2013, 49, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Gibson, A.E.; Issaq, S.H.; Oshima, N.; Baumgart, J.T.; Edessa, L.D.; Rai, G.; Urban, D.J.; Johnson, M.S.; Benavides, G.A.; et al. Targeting Glycolysis through Inhibition of Lactate Dehydrogenase Impairs Tumor Growth in Preclinical Models of Ewing Sarcoma. Cancer Res. 2019, 79, 5060–5073. [Google Scholar] [CrossRef] [Green Version]


| Characteristics at Diagnostic | Overall Population | n = 89 |
|---|---|---|
| Age at diagnosis (years) | Mean (sd) | 10.7 (4.3) |
| Median [Q1–Q3] | 10 [8–14] | |
| Minimum/Maximum | 1/24 | |
| Gender | Male, n (%) | 49 (55%) |
| Female, n (%) | 40 (45%) | |
| Tumor localization | Peripheral site n (%) | 53 (60%) |
| Lower limb, n (% of peripheral) | 21 (40%) | |
| Upper limb, n (% of peripheral) | 13 (24%) | |
| Rib bone, n (% of peripheral) | 19 (36%) | |
| Axial site, n (%) | 36 (40%) | |
| Vertebra and spinal column, n (% of axial) | 13 (36%) | |
| Skull, n (% of axial) | 9 (25%) | |
| Pelvis, n (% of axial) | 13 (36%) | |
| Sacrum, n (% of axial) | 1 (3%) | |
| Tumor stage at diagnosis | Metastatic, n (%) | 24 (27%) |
| Tumor necrosis > 90% | Presence, n (%) | 51 (57%) |
| Local treatment option | Surgery, n (%) | 48 (54%) |
| Radiotherapy, n (%) | 10 (11%) | |
| Radiotherapy and surgery | 21 (24%) | |
| No local treatment | 10 (11%) | |
| Systemic treatment | ICE plus CAV | 16 (18%) |
| AIEOP/ISG EW 1 | 50 (56%) | |
| AIEOP/ISG EW 2 | 23 (26%) | |
| LDH (UI/L) | Median [Q1–Q3] | 443 [322–550] |
| LDH (UI/L) in M+ (n = 24) | Median [Q1–Q3] | 455 [341–651] |
| LDH (UI/L) in localized (n = 65) | Median [Q1–Q3] | 423 [312–528] |
| CRP (mg/dL) | Median [Q1–Q3] | 0.8 [0.14–2.88] |
| CRP (UI/L) in M+ (n = 24) | Median [Q1–Q3] | 2.70 [0.54–5.82] |
| CRP (UI/L) in localized (n = 65) | Median [Q1–Q3] | 0.50 [0.14–2.07] |
| Univariate Analysis | Multivariate Analysis * | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| LDH (log scale) | 4.52 (2.08–9.83) | <0.0001 | 1.58 (1.02–2.45) | 0.04 |
| Age at diagnosis | 1.01 (1.00–1.02) | 0.01 | 1.01 (1.00–1.02) | 0.01 |
| Necrosis | 3.39 (1.24–9.31) | 0.02 | 1.56 (0.49–4.95) | 0.45 |
| Axial tumor | 1.56 (0.66–3.69) | 0.31 | 1.35 (0.50–3.68) | 0.56 |
| TOTAL (n = 89) | LDH Low * (n = 27) | LDH High * (n = 62) | p-Value | ||
|---|---|---|---|---|---|
| Age at diagnosis (in years) | Median [Q1–Q3] | 10 [8–14] | 12 [10–14] | 9.5 [8–13] | 0.13 |
| Min/Max | 12/288 | 24/288 | 12/228 | ||
| Sex | Male, n (%) | 49 (55.1%) | 15 (55.6%) | 34 (54.8%) | 0.95 |
| Status at diagnosis | Peripheral localization, n (%) | 53 (59.6%) | 14 (51.9%) | 39 (62.9%) | 0.36 |
| Metastases, n (%) | 24 (27.0%) | 6 (22.2%) | 18 (29.0%) | 0.51 | |
| Necrosis | n (%) | 51 (57.3%) | 17 (63.0%) | 34 (54.8%) | 0.48 |
| Local treatment | Surgery, n (%) | 48 (53.9%) | 15 (55.6%) | 33 (53.2%) | 0.84 |
| Radiotherapy, n (%) | 32 (36.0%) | 11 (40.7%) | 21 (33.9%) | 0.54 | |
| CRP | Median [Q1–Q3] | 0.8 [0.1–2.9] | 0.5 [0.1–1.8] | 1.0 [0.2–3.5] | 0.25 |
| Min/Max | 0.1/23.4 | 0.1/13.1 | 0.1/23.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Baldo, G.; Abbas, R.; De Ioris, M.A.; Di Ruscio, V.; Alessi, I.; Miele, E.; Mastronuzzi, A.; Milano, G.M. The Prognostic Role of the C-Reactive Protein and Serum Lactate Dehydrogenase in a Pediatric Series of Bone Ewing Sarcoma. Cancers 2022, 14, 3064. https://doi.org/10.3390/cancers14133064
Del Baldo G, Abbas R, De Ioris MA, Di Ruscio V, Alessi I, Miele E, Mastronuzzi A, Milano GM. The Prognostic Role of the C-Reactive Protein and Serum Lactate Dehydrogenase in a Pediatric Series of Bone Ewing Sarcoma. Cancers. 2022; 14(13):3064. https://doi.org/10.3390/cancers14133064
Chicago/Turabian StyleDel Baldo, Giada, Rachid Abbas, Maria Antonietta De Ioris, Valentina Di Ruscio, Iside Alessi, Evelina Miele, Angela Mastronuzzi, and Giuseppe Maria Milano. 2022. "The Prognostic Role of the C-Reactive Protein and Serum Lactate Dehydrogenase in a Pediatric Series of Bone Ewing Sarcoma" Cancers 14, no. 13: 3064. https://doi.org/10.3390/cancers14133064







