Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Features of BWSp
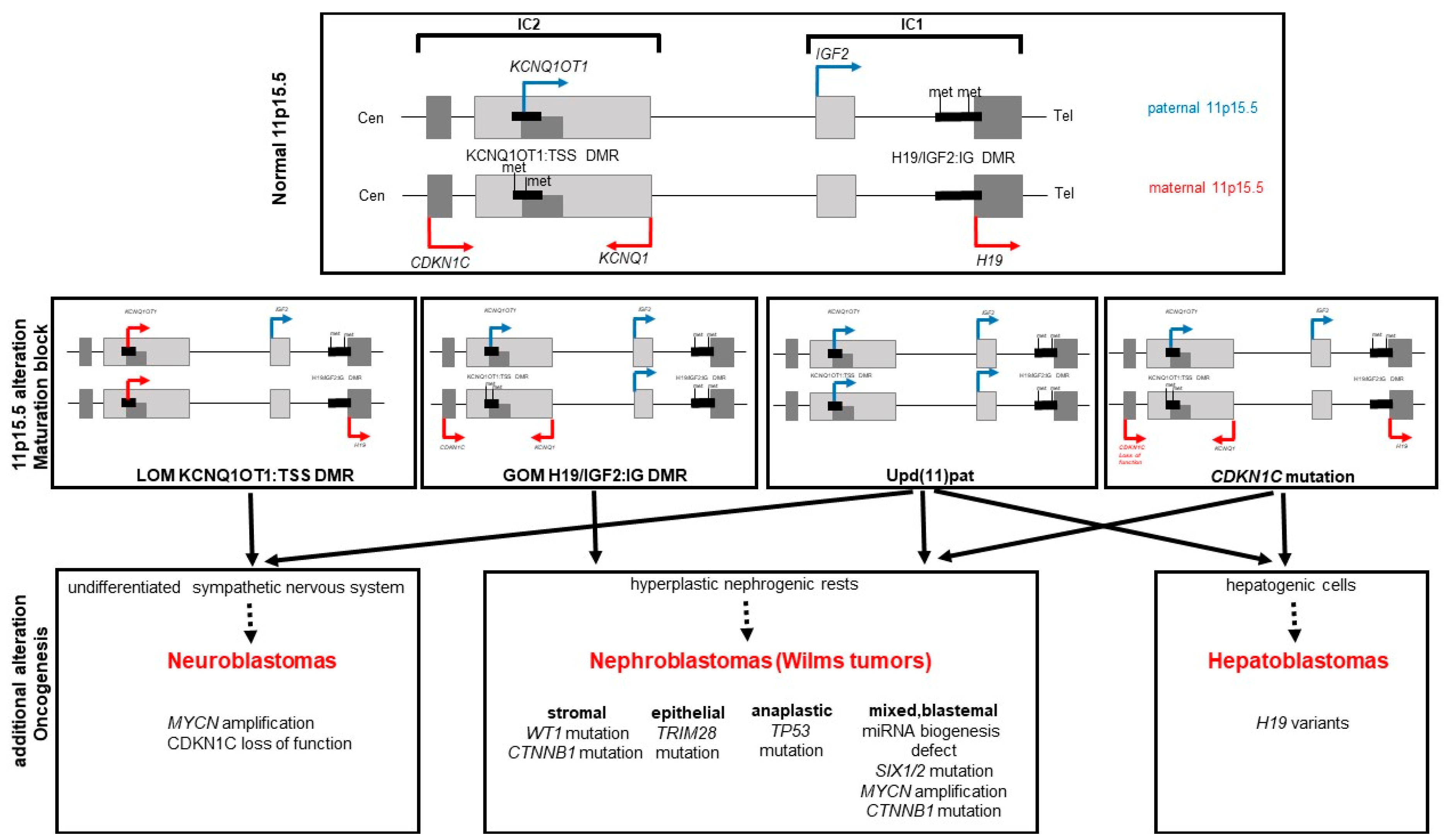
3. The Chromosomal Region 11p15.5
4. Molecular Subgroups and Their Causes
5. (Epi) genotype–Tumor Correlation
6. General Aspects of the Molecular Basics of Tumors in BWS
7. Relevance for Molecular Diagnostic Testing
8. Relevance for Clinical Management
9. Relevance for Follow-Up
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Coktu, S.; Spix, C.; Kaiser, M.; Beygo, J.; Kleinle, S.; Bachmann, N.; Kohlschmidt, N.; Prawitt, D.; Beckmann, A.; Klaes, R.; et al. Cancer incidence and spectrum among children with genetically confirmed Beckwith-Wiedemann spectrum in Germany: A retrospective cohort study. Br. J. Cancer 2020, 123, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Turleau, C.; de Grouchy, J.; Chavin-Colin, F.; Martelli, H.; Voyer, M.; Charlas, R. Trisomy 11p15 and Beckwith-Wiedemann syndrome. A report of two cases. Hum. Genet. 1984, 67, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Drut, R.M.; Drut, R. Nonimmune fetal hydrops and placentomegaly: Diagnosis of familial Wiedemann-Beckwith syndrome with trisomy 11p15 using FISH. Am. J. Med. Genet. 1996, 62, 145–149. [Google Scholar] [CrossRef]
- Waziri, M.; Patil, S.R.; Hanson, J.W.; Bartley, J.A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J. Pediatr. 1983, 102, 873–876. [Google Scholar] [CrossRef]
- Okano, Y.; Osasa, Y.; Yamamoto, H.; Hase, Y.; Tsuruhara, T.; Fujita, H. An infant with Beckwith-Wiedemann syndrome and chromosomal duplication 11p13—Pter: Correlation of symptoms between 11p trisomy and Beckwith-Wiedemann syndrome. Jinrui Idengaku Zasshi 1986, 31, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Pueschel, S.M.; Padre-Mendoza, T. Chromosome 11 and Beckwith-Wiedemann syndrome. J. Pediatr. 1984, 104, 484–485. [Google Scholar] [CrossRef]
- Tommerup, N.; Brandt, C.A.; Pedersen, S.; Bolund, L.; Kamper, J. Sex dependent transmission of Beckwith-Wiedemann syndrome associated with a reciprocal translocation t(9; 11)(p11.2; p15.5). J. Med. Genet. 1993, 30, 958–961. [Google Scholar] [CrossRef] [Green Version]
- Hoovers, J.M.; Kalikin, L.M.; Johnson, L.A.; Alders, M.; Redeker, B.; Law, D.J.; Bliek, J.; Steenman, M.; Benedict, M.; Wiegant, J.; et al. Multiple genetic loci within 11p15 defined by Beckwith-Wiedemann syndrome rearrangement breakpoints and subchromosomal transferable fragments. Proc. Natl. Acad. Sci. USA 1995, 92, 12456–12460. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.W.; Villar, A.J.; Bickmore, W.; Clayton-Smith, J.; Catchpoole, D.; Maher, E.R.; Reik, W. Imprinting mutation in the Beckwith-Wiedemann syndrome leads to biallelic IGF2 expression through an H19-independent pathway. Hum. Mol. Genet. 1996, 5, 2027–2032. [Google Scholar] [CrossRef] [Green Version]
- Henry, I.; Bonaiti-Pellie, C.; Chehensse, V.; Beldjord, C.; Schwartz, C.; Utermann, G.; Junien, C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature 1991, 351, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Deardorff, M.A. Tumor screening in Beckwith-Wiedemann syndrome-To screen or not to screen? Am. J. Med. Genet. A 2016, 170, 2261–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilinich, N.J.; Day, C.D.; Fitzpatrick, G.V.; Caldwell, G.M.; Lossie, A.C.; Cooper, P.R.; Smallwood, A.C.; Joyce, J.A.; Schofield, P.N.; Reik, W.; et al. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc. Natl. Acad. Sci. USA 1999, 96, 8064–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.P.; DeBaun, M.R.; Mitsuya, K.; Galonek, H.L.; Brandenburg, S.; Oshimura, M.; Feinberg, A.P. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc. Natl. Acad. Sci. USA 1999, 96, 5203–5208. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Meyer, N.; Day, C.D.; Khatod, K.; Maher, E.R.; Cooper, W.; Reik, W.; Junien, C.; Graham, G.; Algar, E.; Der Kaloustian, V.M.; et al. Silencing of CDKN1C (p57KIP2) is associated with hypomethylation at KvDMR1 in Beckwith-Wiedemann syndrome. J. Med. Genet. 2003, 40, 797–801. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Delgado, M.; Riccio, A.; Eggermann, T.; Maher, E.R.; Lapunzina, P.; Mackay, D.; Monk, D. Causes and Consequences of Multi-Locus Imprinting Disturbances in Humans. Trends Genet. 2016, 32, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Reik, W.; Brown, K.W.; Schneid, H.; Le Bouc, Y.; Bickmore, W.; Maher, E.R. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Hum. Mol. Genet. 1995, 4, 2379–2385. [Google Scholar] [CrossRef]
- Reik, W.; Brown, K.W.; Slatter, R.E.; Sartori, P.; Elliott, M.; Maher, E.R. Allelic methylation of H19 and IGF2 in the Beckwith-Wiedemann syndrome. Hum. Mol. Genet. 1994, 3, 1297–1301. [Google Scholar] [CrossRef]
- Kraft, F.; Wesseler, K.; Begemann, M.; Kurth, I.; Elbracht, M.; Eggermann, T. Novel familial distal imprinting centre 1 (11p15.5) deletion provides further insights in imprinting regulation. Clin. Epigenet. 2019, 11, 30. [Google Scholar] [CrossRef]
- Eggermann, T.; Kraft, F.; Kluth, K.; Klopocki, E.; Hüning, I.; Hempel, M.; Kunstmann, E. Heterogeneous phenotypes in families with duplications of the paternal allele within the imprinting center 1 (H19/IGF2:TSS-DMR) in 11p15.5. Clin. Genet. 2020, in press. [CrossRef]
- Monk, D.; Mackay, D.J.G.; Eggermann, T.; Maher, E.R.; Riccio, A. Genomic imprinting disorders: Lessons on how genome, epigenome and environment interact. Nat. Rev. Genet. 2019, 20, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Abi Habib, W.; Azzi, S.; Brioude, F.; Steunou, V.; Thibaud, N.; Das Neves, C.; Le Jule, M.; Chantot-Bastaraud, S.; Keren, B.; Lyonnet, S.; et al. Extensive investigation of the IGF2/H19 imprinting control region reveals novel OCT4/SOX2 binding site defects associated with specific methylation patterns in Beckwith-Wiedemann syndrome. Hum. Mol. Genet. 2014, 23, 5763–5773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, F.M.; Sparago, A.; Freschi, A.; Hill-Harfe, K.; Maas, S.M.; Frints, S.G.M.; Alders, M.; Pignata, L.; Franzese, M.; Angelini, C.; et al. Transcription alterations of KCNQ1 associated with imprinted methylation defects in the Beckwith-Wiedemann locus. Genet. Med. 2019, 21, 1808–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteagudo-Sanchez, A.; Hernandez Mora, J.R.; Simon, C.; Burton, A.; Tenorio, J.; Lapunzina, P.; Clark, S.; Esteller, M.; Kelsey, G.; Lopez-Siguero, J.P.; et al. The role of ZFP57 and additional KRAB-zinc finger proteins in the maintenance of human imprinted methylation and multi-locus imprinting disturbances. Nucleic Acids. Res. 2020, 48, 11394–11407. [Google Scholar] [CrossRef]
- Elbracht, M.; Mackay, D.; Begemann, M.; Kagan, K.O.; Eggermann, T. Disturbed genomic imprinting and its relevance for human reproduction: Causes and clinical consequences. Hum. Reprod. Update 2020, 26, 197–213. [Google Scholar] [CrossRef]
- Hennekam, R.C.; Biesecker, L.G.; Allanson, J.E.; Hall, J.G.; Opitz, J.M.; Temple, I.K.; Carey, J.C.; Elements of Morphology, C. Elements of morphology: General terms for congenital anomalies. Am. J. Med. Genet. A 2013, 161A, 2726–2733. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Seaver, L.H.; Jones, K.L.; Procopio, F.; Crooks, W.; Feingold, M. Isolated hemihyperplasia (hemihypertrophy): Report of a prospective multicenter study of the incidence of neoplasia and review. Am. J. Med. Genet. 1998, 79, 274–278. [Google Scholar] [CrossRef]
- Clericuzio, C.L.; Chen, E.; McNeil, D.E.; O’Connor, T.; Zackai, E.H.; Medne, L.; Tomlinson, G.; DeBaun, M. Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J. Pediatr. 2003, 143, 270–272. [Google Scholar] [CrossRef]
- Brioude, F.; Hennekam, R.; Bliek, J.; Coze, C.; Eggermann, T.; Ferrero, G.B.; Kratz, C.; Bouc, Y.L.; Maas, S.M.; Mackay, D.J.G.; et al. Revisiting Wilms tumour surveillance in Beckwith-Wiedemann syndrome with IC2 methylation loss, reply. Eur. J. Hum. Genet. 2018, 26, 471–472. [Google Scholar] [CrossRef]
- Maas, S.M.; Vansenne, F.; Kadouch, D.J.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.; Maher, E.R.; Hennekam, R.C. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am. J. Med. Genet. A 2016, 170, 2248–2260. [Google Scholar] [CrossRef] [Green Version]
- Duffy, K.A.; Cielo, C.M.; Cohen, J.L.; Gonzalez-Gandolfi, C.X.; Griff, J.R.; Hathaway, E.R.; Kupa, J.; Taylor, J.A.; Wang, K.H.; Ganguly, A.; et al. Characterization of the Beckwith-Wiedemann spectrum: Diagnosis and management. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Griff, J.R.; Duffy, K.A.; Kalish, J.M. Characterization and Childhood Tumor Risk Assessment of Genetic and Epigenetic Syndromes Associated With Lateralized Overgrowth. Front. Pediatr. 2020, 8, 613260. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xiao, Y.; Li, D.; Hu, H.; Li, X.; Ge, T.; Yu, R.; Wang, Y.; Zhang, T. Clinical and molecular features of children with Beckwith-Wiedemann syndrome in China: A single-center retrospective cohort study. Ital. J. Pediatr. 2020, 46, 55. [Google Scholar] [CrossRef] [PubMed]
- Azzi, S.; Rossignol, S.; Steunou, V.; Sas, T.; Thibaud, N.; Danton, F.; Le Jule, M.; Heinrichs, C.; Cabrol, S.; Gicquel, C.; et al. Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum. Mol. Genet. 2009, 18, 4724–4733. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Bedeschi, M.F.; Cagnoli, G.A.; Costanza, J.; Persico, N.; Gangi, S.; Porro, M.; Ajmone, P.F.; Colapietro, P.; Santaniello, C.; et al. (Epi)genetic profiling of extraembryonic and postnatal tissues from female monozygotic twins discordant for Beckwith-Wiedemann syndrome. Mol. Genet. Genom. Med. 2020, 8, e1386. [Google Scholar] [CrossRef]
- Behjati, S.; Gilbertson, R.J.; Pfister, S.M. Maturation Block in Childhood Cancer. Cancer Discov. 2021, 11, 542–544. [Google Scholar] [CrossRef]
- McMahon, A.P. Development of the Mammalian Kidney. Curr. Top. Dev. Biol. 2016, 117, 31–64. [Google Scholar] [CrossRef] [Green Version]
- Fukuzawa, R.; Heathcott, R.W.; More, H.E.; Reeve, A.E. Sequential WT1 and CTNNB1 mutations and alterations of beta-catenin localisation in intralobar nephrogenic rests and associated Wilms tumours: Two case studies. J. Clin. Pathol. 2007, 60, 1013–1016. [Google Scholar] [CrossRef] [Green Version]
- Porteus, M.H.; Narkool, P.; Neuberg, D.; Guthrie, K.; Breslow, N.; Green, D.M.; Diller, L. Characteristics and outcome of children with Beckwith-Wiedemann syndrome and Wilms’ tumor: A report from the National Wilms Tumor Study Group. J. Clin. Oncol. 2000, 18, 2026–2031. [Google Scholar] [CrossRef]
- Hol, J.A.; Kuiper, R.P.; van Dijk, F.; Waanders, E.; van Peer, S.E.; Koudijs, M.J.; Bladergroen, R.; van Reijmersdal, S.V.; Morgado, L.M.; Bliek, J.; et al. Prevalence of (Epi)genetic Predisposing Factors in a 5-Year Unselected National Wilms Tumor Cohort: A Comprehensive Clinical and Genomic Characterization. J. Clin. Oncol. 2022, 105, JCO2102510. [Google Scholar] [CrossRef]
- Spreafico, F.; Fernandez, C.V.; Brok, J.; Nakata, K.; Vujanic, G.; Geller, J.I.; Gessler, M.; Maschietto, M.; Behjati, S.; Polanco, A.; et al. Wilms tumour. Nat. Rev. Dis. Primers 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guan, Q.; Guo, H.; Miao, L.; Zhuo, Z. The Genetic Changes of Hepatoblastoma. Front. Oncol. 2021, 11, 690641. [Google Scholar] [CrossRef] [PubMed]
- Corvetta, D.; Chayka, O.; Gherardi, S.; D’Acunto, C.W.; Cantilena, S.; Valli, E.; Piotrowska, I.; Perini, G.; Sala, A. Physical interaction between MYCN oncogene and polycomb repressive complex 2 (PRC2) in neuroblastoma: Functional and therapeutic implications. J. Biol. Chem. 2013, 288, 8332–8341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaccio, R.; De Rosa, P.; Aloisi, S.; Viggiano, M.; Cimadom, L.; Zadran, S.K.; Perini, G.; Milazzo, G. Targeting Oncogenic Transcriptional Networks in Neuroblastoma: From N-Myc to Epigenetic Drugs. Int. J. Mol. Sci. 2021, 22, 2883. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Lunec, J.; Tweddle, D.A. Cell cycle regulation targets of MYCN identified by gene expression microarrays. Cell Cycle 2007, 6, 1249–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, R.H.; Douglas, J.; Baskcomb, L.; Huxter, N.; Barker, K.; Hanks, S.; Craft, A.; Gerrard, M.; Kohler, J.A.; Levitt, G.A.; et al. Constitutional 11p15 abnormalities, including heritable imprinting center mutations, cause nonsyndromic Wilms tumor. Nat. Genet. 2008, 40, 1329–1334. [Google Scholar] [CrossRef]
- Coorens, T.H.H.; Treger, T.D.; Al-Saadi, R.; Moore, L.; Tran, M.G.B.; Mitchell, T.J.; Tugnait, S.; Thevanesan, C.; Young, M.D.; Oliver, T.R.W.; et al. Embryonal precursors of Wilms tumor. Science 2019, 366, 1247–1251. [Google Scholar] [CrossRef]
- Ito, Y.; Koessler, T.; Ibrahim, A.E.; Rai, S.; Vowler, S.L.; Abu-Amero, S.; Silva, A.L.; Maia, A.T.; Huddleston, J.E.; Uribe-Lewis, S.; et al. Somatically acquired hypomethylation of IGF2 in breast and colorectal cancer. Hum. Mol. Genet. 2008, 17, 2633–2643. [Google Scholar] [CrossRef]
- Ribarska, T.; Goering, W.; Droop, J.; Bastian, K.M.; Ingenwerth, M.; Schulz, W.A. Deregulation of an imprinted gene network in prostate cancer. Epigenetics 2014, 9, 704–717. [Google Scholar] [CrossRef] [Green Version]
- Varrault, A.; Gueydan, C.; Delalbre, A.; Bellmann, A.; Houssami, S.; Aknin, C.; Severac, D.; Chotard, L.; Kahli, M.; Le Digarcher, A.; et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev. Cell 2006, 11, 711–722. [Google Scholar] [CrossRef]
- Monnier, P.; Martinet, C.; Pontis, J.; Stancheva, I.; Ait-Si-Ali, S.; Dandolo, L. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc. Natl. Acad. Sci. USA 2013, 110, 20693–20698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ager, E.I.; Pask, A.J.; Gehring, H.M.; Shaw, G.; Renfree, M.B. Evolution of the CDKN1C-KCNQ1 imprinted domain. BMC Evol. Biol. 2008, 8, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopyan, N.M.; Ehrlich, P.F. Surgical Management of Wilms Tumor (Nephroblastoma) and Renal Cell Carcinoma in Children and Young Adults. Surg. Oncol. Clin. N. Am. 2021, 30, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Dome, J.S.; Graf, N.; Geller, J.I.; Fernandez, C.V.; Mullen, E.A.; Spreafico, F.; Van den Heuvel-Eibrink, M.; Pritchard-Jones, K. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol. 2015, 33, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, V.; Magro, G.; Broggi, G.; Luchini, C.; Cappello, F.; Caporalini, C.; Buccoliero, A.M.; Santoro, L. New insights in gastrointestinal “pediatric” neoplasms in adult patients: Pancreatoblastoma, hepatoblastoma and embryonal sarcoma of the liver. A practical approach by GIPPI-GIPAD Groups. Pathologica 2022, 114, 64–78. [Google Scholar] [CrossRef]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Haberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Whitlock, R.S.; Vasudevan, S.A. Surgical Management of Hepatoblastoma and Recent Advances. Cancers 2019, 11, 1944. [Google Scholar] [CrossRef] [Green Version]
- Tomolonis, J.A.; Agarwal, S.; Shohet, J.M. Neuroblastoma pathogenesis: Deregulation of embryonic neural crest development. Cell Tissue Res. 2018, 372, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Berbegall, A.P.; Bogen, D.; Potschger, U.; Beiske, K.; Bown, N.; Combaret, V.; Defferrari, R.; Jeison, M.; Mazzocco, K.; Varesio, L.; et al. Heterogeneous MYCN amplification in neuroblastoma: A SIOP Europe Neuroblastoma Study. Br. J. Cancer 2018, 118, 1502–1512. [Google Scholar] [CrossRef]
- Cohn, S.L.; Pearson, A.D.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Pritchard, J.; Berthold, F.; Carlsen, N.L.; Castel, V.; Castelberry, R.P.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Hedborg, F.; et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J. Clin. Oncol. 1993, 11, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.H.; Federico, S.M.; London, W.B.; Naranjo, A.; Irwin, M.S.; Volchenboum, S.L.; Cohn, S.L. Tailoring Therapy for Children With Neuroblastoma on the Basis of Risk Group Classification: Past, Present, and Future. JCO Clin. Cancer Inform. 2020, 4, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.I.; Treis, D.; Johnsen, J.I. Neuroblastoma Heterogeneity, Plasticity, and Emerging Therapies. Curr. Oncol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
| Molecular Subtype | Total Number (n = 1301) | Ratio among Solved Cases | |
|---|---|---|---|
| expected molecular diagnoses | IC1 GOM | 153 | 11.8% |
| IC2 LOM | 833 | 64.0% | |
| of these MLID: 12.8% | 107 | ||
| CNVs 11p | 32 | 2.5% | |
| upd(11)pat | 254 | 19.5% | |
| of these uniparental diploid: 4.3% | 11 | ||
| unexpected molecular diagnoses | IC1 LOM | 27 | 2.1% |
| PHP | 2 | 0.2% |
| Molecular Defect | Frequency of Molecular Defect | Tumor Risk (% of Patients) |
|---|---|---|
| IC1 GOM | 5% | Overall risk (28.1%) |
| Wilms tumor (24%) | ||
| Neuroblastoma (0.7%) | ||
| Pancreatoblastoma (0.7%) | ||
| IC2 LOM | 50% | Overall risk (2.6%) |
| Hepatoblastoma (0.7%) | ||
| Rhabdomyosarcoma (0.5%) | ||
| Neuroblastoma (0.5%) | ||
| Thyroid cancer (0.3%) | ||
| Wilms tumor (0.2%) | ||
| Melanoma (0.1%) | ||
| upd(11)pat | 20% (see also paternal uniploidy) | Overall risk (16%) |
| Wilms tumor (7.9%) | ||
| Hepatoblastoma (3.5%) | ||
| Neuroblastoma (1.4%) | ||
| Adrenocortical carcinoma (1.1%) | ||
| Pheochromocytoma (0.8%) | ||
| Lymphoblastic leukemia (0.5%) | ||
| Pancreatoblastoma (0.3%) | ||
| Hemangiotheloma (0.3%) | ||
| Rhabdomyosarcoma (0.3%) | ||
| Loss-of-function CDKN1C variants | 5% (40% in familial cases) | Overall risk (6.9%) |
| Wilms tumor (1.4%) | ||
| Neuroblastoma (4.2%) | ||
| Acute lymphatic leukemia (1.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggermann, T.; Maher, E.R.; Kratz, C.P.; Prawitt, D. Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice. Cancers 2022, 14, 3083. https://doi.org/10.3390/cancers14133083
Eggermann T, Maher ER, Kratz CP, Prawitt D. Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice. Cancers. 2022; 14(13):3083. https://doi.org/10.3390/cancers14133083
Chicago/Turabian StyleEggermann, Thomas, Eamonn R. Maher, Christian P. Kratz, and Dirk Prawitt. 2022. "Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice" Cancers 14, no. 13: 3083. https://doi.org/10.3390/cancers14133083
APA StyleEggermann, T., Maher, E. R., Kratz, C. P., & Prawitt, D. (2022). Molecular Basis of Beckwith–Wiedemann Syndrome Spectrum with Associated Tumors and Consequences for Clinical Practice. Cancers, 14(13), 3083. https://doi.org/10.3390/cancers14133083






