Simple Summary
Treatment interruption during concurrent chemoradiotherapy (CCRT) jeopardizes the outcomes of patients with locally advanced head and neck squamous-cell carcinoma (LAHNSCC), which is associated with increased toxicity during CCRT. Nonetheless, we found that under the provision of recommended calories (25 kcal/kg/day) and protein (1 g/kg/day) throughout the treatment course, treatment-interval changes in serum albumin and histidine levels—not treatment toxicity—greatly contributed to the interruption of treatment in patients with LAHNSCC. We believe that serum levels of albumin and histidine should be integrated into the routine nutritional assessment of patients undergoing CCRT.
Abstract
We investigated risk factors for treatment interruption (TI) in patients with locally advanced head and neck squamous-cell carcinoma (LAHNSCC) following concurrent chemoradiotherapy (CCRT), under the provision of recommended calorie and protein intake; we also evaluated the associations between clinicopathological variables, calorie and protein supply, nutrition–inflammation biomarkers (NIBs), total body composition change (TBC), and a four-serum-amino-acid metabolite panel (histidine, leucine, ornithine, and phenylalanine) among these patients. Patients with LAHNSCC who completed the entire planned CCRT course and received at least 25 kcal/kg/day and 1 g of protein/kg/day during CCRT were prospectively recruited. Clinicopathological variables, anthropometric data, blood NIBs, CCRT-related factors, TBC data, and metabolite panels before and after treatment were collected; 44 patients with LAHNSCC were enrolled. Nine patients (20.4%) experienced TIs. Patients with TIs experienced greater reductions in hemoglobin, serum levels of albumin, uric acid, histidine, and appendicular skeletal mass, and suffered from more grade 3/4 toxicities than those with no TI. Neither increased daily calorie supply (≥30 kcal/kg/day) nor feeding tube placement was correlated with TI. Multivariate analysis showed that treatment-interval changes in serum albumin and histidine levels, but not treatment toxicity, were independently associated with TI. Thus, changes in serum levels of albumin and histidine over the treatment course could cause TI in patients with LAHNSCC following CCRT.
1. Introduction
Most patients with head and neck cancer (HNC) present with a locally advanced stage at the time of diagnosis [1]. Patients with locally advanced head and neck squamous-cell carcinoma (LAHNSCC) typically require a multidisciplinary treatment approach, including surgery, chemotherapy, and radiotherapy (RT). To improve treatment outcomes, concurrent chemoradiotherapy (CCRT) is used either as adjuvant therapy following surgery or as curative-intent primary CCRT therapy [2,3]. Nonetheless, CCRT often causes severe toxicity, resulting in treatment interruption and inferior outcomes [4,5,6,7,8,9,10,11]. Only 60% of patients complete the CCRT protocol through the administration of three cycles of 100 mg/m2 cisplatin concurrently with RT [12,13,14,15]; over 50% and approximately 10–20% of patients experience at least one day and one week or more of treatment interruption, respectively [10,16,17,18]. There is a reduction of 0.7–1.4% in local tumor control for one-day treatment interruption, which rises to 14–20% for one week in patients with HNC undergoing RT [19,20]. In these patients, treatment interruption causes a decrease in 5-year survival rates from 61% to 28% [21], and an additional day break lowers the 5-year relapse-free survival rate by 1–2% after an RT interruption duration of over 6 days [22]. Thus, even a short RT break or just a one-day increase in the interruption period may have significant negative consequences. Therefore, it is essential to avoid or minimize interruption events during RT and CCRT courses.
The negative impact of RT interruption on treatment outcomes is attributed to repopulation of residual tumor cells—especially cancer stem cells, which proliferate indefinitely and cause tumor recurrence when RT is delayed or interrupted [9,23]. Accumulating evidence shows that under a standard and conventional fractioning radiation course scheduled for 5 days per week over 6–8 weeks, the major causes of RT interruption events are statutory/regular holidays, machine problems such as maintenance work and unexpected breakdown, waiting time for RT initiation after surgery, and patient-related factors—including non-compliance, psychological stress, insufficient knowledge about cancer and treatment, and socioeconomic problems—among patients with HNC undergoing RT [17,18,24]. To address this issue, several strategies have been successfully implemented over the past three decades to reduce treatment interruptions [17,18,24]. Two audits were conducted in the United Kingdom in 2002 and 2008 [17,18]. In the second audit, it was found that formulating suitable policies and guidelines—including setting up machinery maintenance, provision of an alternative backup linear accelerator machine, and prudent treatment scheduling with a compensation program—shortened the waiting time for patients to start RT after surgery, and increased the percentage of patients completing treatment within 2 days of the scheduled time [18]. Additionally, Chen et al. used pretreatment nursing consultation and adequate support from social and administrative resources to enhance patients’ tolerance to adverse effects and enable compliance with the treatment process, reducing RT interruptions in patients with nasopharyngeal carcinoma [24]. Currently, these planned (e.g., statutory/public holidays, machine maintenance, the time interval between surgery completion and RT initiation) and unplanned interruptions (e.g., machine faults, patient non-compliance) have been decreased in clinical practice due to effective preemptive administrative work and therapeutic schedule arrangement.
Although these strategies greatly reduce treatment interruptions, cumulative treatment toxicity from RT and chemotherapy—such as ulcerative oral mucositis and xerostomia—is still an important issue among patients afflicted with unplanned interruptions [9,16,17,18,25]. Various effective strategies—such as radiation course modulation, chemotherapy delivery modification, and diet therapy—reportedly improve these toxicities [9]; however, these results pertaining to the effects of radiation morbidity on treatment interruption should be interpreted with caution. First, most of these studies were conducted retrospectively among heterogeneous patient populations with mixed tumor stages, varying radiotherapy regimens, and different definitions of treatment interruption. These variations cause a large difference in the incidence of treatment toxicity causing interruption, varying from 2% to 20% [17,18,19]. Second, patients’ characteristics, extent of cancer involvement, and status of nutrition and inflammation are associated with the incidence and severity of treatment toxicities [26,27,28,29,30,31,32,33,34,35]. Unfortunately, few studies have investigated the interplay between these variables, treatment toxicity, and interruption. Furthermore, adding concurrent chemotherapy to RT enhances tumor control and survival outcomes, and also increases treatment-related toxicity and treatment interruptions [9]. Moreover, some reports show that treatment delay may not always contribute to worse outcomes in patients with locally advanced head and neck cancers following modifications in the chemotherapy regimen and radiation protocol over the treatment course [36,37], suggesting that certain therapeutic modes delivered during CCRT could affect treatment interruption. Finally, intensive nutritional support is mandatory for patients with LAHNSCC undergoing CCRT [27]. The European Society for Clinical Nutrition and Metabolism (ESPEN) suggests an energy intake of at least 25–30 kcal/kg/day for each patient, and recommends feeding tube usage to maintain calorie supply over the treatment course [38]. However, the effect of the recommended energy intake and feeding tube placement on treatment interruption during CCRT has seldom been addressed.
Alterations in total body composition (TBC), assessed by dual-energy X-ray absorptiometry (DXA), are commonly observed in cancer patients in response to various physiological and pathological stimuli, such as aging, sex, intercurrent illness, metabolic disturbance, and therapy; therefore, they can appropriately reflect the status of nutritional and inflammatory changes caused by cancer or treatment. We observed a significant loss in lean body muscle (LBM) and total fat mass (TFM) in patients with LAHNSCC during CCRT [39,40]. Additionally, the pretreatment level and treatment-interval changes in TBC are associated with increased treatment-related toxicity, early treatment failure, recurrence-free survival, and two-year mortality rate among LAHNSCC patients undergoing CCRT [39,40,41,42]. Therefore, further research is needed to investigate the effect of TBC changes on treatment interruption in patients with LAHNSCC undergoing CCRT.
Metabolomics, which is a useful tool for assessing biochemical changes in tissues and bodily fluids, as well as for understanding complicated disease processes, is used for patients with HNC [43,44,45,46,47]. A comprehensive review conducted by Shin et al. confirmed different metabolite profiles between healthy subjects with no cancer and patients with HNC using various sample types and metabolomic platforms [43]. Although the results of this research improve our knowledge of the possible pathogenesis of HNC, they are not widely applicable in daily practice, probably because of the limited availability of technological platforms such as proton nuclear magnetic resonance and mass spectrometry; moreover, there are complex interpretations of multi-metabolite measurements and inherent inconsistencies of metabolite profiles from different tissue sample collections [44,45,46,47]. Recently, we utilized ultrahigh-performance liquid chromatography (UPLC) to develop a simple and easy-to-read metabolite panel by measuring serum levels of only four amino acids (i.e., histidine, leucine, ornithine, and phenylalanine (HLOP)) [48,49]. This panel represents the nutritional status, muscle synthesis and breakdown, and the amount of nitrogen waste from amino acids via the urea cycle; it has proven its prognostic value in patients with heart failure [48,49], severe infection [50], chronic pulmonary obstructive disease [51], and chronic kidney disease [52]. However, the clinical role of this HLOP-based panel in LAHNSCC patients undergoing CCRT has not yet been addressed.
Therefore, a prospective observational study is required to address these issues. We prospectively enrolled a homogeneous group of patients with LAHNSCC (stage III, IVA, or IVB). They received a standard CCRT protocol that delivered RT at a fraction daily for 5 days per week over 6–8 weeks concurrently with weekly cisplatin infusion chemotherapy. To reduce treatment non-compliance and machine faults, all participants consulted psychiatrists and social workers, and our institution prepared a backup linear accelerator machine for breakdown/maintenance. Following the ESPEN guidelines, each cancer patient was requested to have a calorie intake of at least 25 kcal/kg/day with 1.0 g/kg/day of protein during the CCRT course to avoid malnutrition. Under the provision of recommended daily calories and protein throughout the treatment course, we aimed to identify potential factors contributing to treatment interruption in patients with LAHNSCC who completed the CCRT course, by simultaneously analyzing all covariates, including clinicopathological variables, anthropometric data, blood nutrition–inflammation biomarkers (NIBs), treatment-related profiles, DXA-associated measurements, and serum HLOP metabolic profiles.
2. Materials and Methods
2.1. Patient Recruitment
We prospectively recruited eligible patients with histologically proven LAHNSCC originating in the oral cavity, oropharynx, hypopharynx, and/or larynx between January 2018 and July 2019. The head and neck cancer committee confirmed the tumor stage according to the 8th edition of the American Joint Committee on Cancer (AJCC)’s staging system and criteria for inclusion and exclusion. LAHNSCC includes stages III (T1-2, N1 or T3, N0-1), IVA (T4a, N0-1 or T1-4a, N2), and IVB (any T, N3, or T4b, any N). The inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status score ≤2, age <75 years, sufficient hematopoietic or organ function, and p-16-negative expression in the tumor specimen. Patients who met the following criteria were excluded: (1) one of the following systemic illnesses: heart failure with New York Heart Association Classification IV, severe chronic obstructive pulmonary disease, major gastrointestinal disorders, decompensated liver cirrhosis with intractable ascites or hepatic encephalopathy, end-stage renal disease, uncontrolled diabetes mellitus, enduring infections, or autoimmune diseases; and (2) regular use of medications that could markedly interfere with metabolism or weight, such as steroids or megestrol acetate.
We obtained written informed consent from cancer patients upon enrollment. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan (approval numbers: 101-4047B and 201700158B0), and was conducted in compliance with the Good Clinical Practice Guidelines and the Declaration of Helsinki.
2.2. CCRT Treatment
Patients with LAHNSCC received either postoperative adjuvant CCRT for oral cavity cancer or curative-intent primary CCRT for unresectable non-oral-cavity cancer. Adjuvant CCRT was administered based on the following pathological features: (1) extranodal extension, (2) positive surgical margin, or (3) at least three minor risk factors, including pT4, pN1, poorly differentiated histology, invasion of blood vessels, lymphatic drainage, or perineural space and close margin ≤4 mm. Intensity-modulated RT at a dose of 60–72 Gy in 30–36 fractions—a fraction daily for 5 days per week over 6–8 weeks—with concurrent chemotherapy with weekly cisplatin (40 mg/m2), was administered. Patients with oral cavity cancer were required to initiate the postoperative adjuvant CCRT program within 6 weeks of the completion of surgery. Patients with non-oral-cavity cancer received primary CCRT within two weeks of diagnosis.
RT dose was defined as the total radiation dose administered during CCRT; RT duration was defined as the number of days required to complete the RT dose; cisplatin dose was defined as the cumulative dose of weekly cisplatin administration.
The severity of adverse effects, including hematological and non-hematological toxicities, was graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).
2.3. Definition of Treatment Interruption
Patients who completed the entire planned course of RT and chemotherapy but developed an RT break of over 5 days or failed to receive scheduled weekly cisplatin during the CCRT course were considered to have faced treatment interruptions. An RT break of >5 days was considered as the criterion for treatment interruption, because it was associated with the local relapse-free survival and overall survival of patients with locally advanced head and neck cancer and nasopharyngeal carcinoma [19,53,54]. Nonetheless, interruptions due to public holidays were permitted. The “incomplete CCRT group” included (1) patients who requested drop-out from the study during the treatment course; and (2) incomplete data collection—patients who could not complete the required examinations or missed scheduled tests on the planned timetable.
2.4. Provision of Recommended Calorie Support during CCRT
We reviewed the dietary records of patients at weekly dietitian visits. Patients could obtain oral nutritional supplements of at least 25 kcal/kg/day, with % calories from carbohydrates:lipids of 60:40 and protein of 1.0 g/kg/day during the treatment course, based on the ESPEN recommendation guidelines [38]. Therefore, feeding tube placement was required if the body weight (BW) loss was over 5% or the daily calorie intake was less than 25 kcal/kg/day for three consecutive days during the CCRT course.
2.5. Clinicopathological Data
Clinicopathological data—including sex, age, BW, body height (BH), ECOG performance status, comorbid illness, tumor location, tumor stage, status of tumor size (T), regional lymph node involvement (N), histological differentiation grade, records of cigarette smoking exposure, alcohol consumption, betel nut use, tracheostomy and feeding tube, treatment protocol, and toxicity profiles—were collected. Patients who were current cigarette smokers or were previously exposed to cigarette smoke were considered smokers. Patients who consumed alcohol more than 4 times per week were considered alcohol drinkers. Patients who consumed betel nuts during the previous year were considered betel nut users. We assessed the severity of the comorbidity using the head and neck Charlson Comorbidity Index (HN-CCI) [55]. Body mass index (BMI) was calculated from the BH and BW of each participant (weight in kilograms divided by the square of the height in meters, kg/m2).
2.6. Biochemical Data and Blood NIBs
Blood samples were collected after overnight fasting within 1 week before CCRT and within 3 days after CCRT. Biochemical data and NIBs—including hemoglobin levels (Hb, g/dL), white blood cell counts (WBC, 103/mm3), platelet counts (103/mm3), and albumin (g/dL), prealbumin (g/dL), transferrin (g/dL), creatinine (mg/dL), alanine transaminase (ALT, U/L), total bilirubin (mg/dL), uric acid (mg/dL), fasting glucose (mg/dL), total cholesterol (mg/dL), triglyceride (mg/dL), and C-reactive protein levels (CRP, mg/dL)—were measured using an auto-analyzer (Beckman, CA, USA) at the CGMH central laboratory in Keelung, Taiwan.
The estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) was calculated using the abbreviated Modification of Diet in Renal Disease Study equation, corrected to a body surface area of 1.73 m2 [56]. The total lymphocyte count (TLC) was calculated as the WBC count (/mm3) × the percentage of lymphocytes in the blood. The total neutrophil count (TNC) was calculated as the WBC count (/mm3) × the percentage of neutrophils in the blood. The total monocyte count (TMC) was calculated as the WBC count (/mm3) × the percentage of monocytes in the blood.
Malnutrition status was considered for BMI < 18.5 kg/m2, albumin < 3.5 g/dL, TLC < 1.5 × 103 cells/mm3, or when the patient-generated subjective global assessment (PG-SGA) scores ranged from 0 to 35: no malnourished status with scores of 0–3, moderately malnourished status with scores of 4–8, and severely malnourished status with scores ≥9 [57,58,59].
2.7. Body Composition Measurements
Body composition parameters—including LBM, TFM, and appendicular skeletal mass (ASM, including arms and legs)—were obtained by dual-energy fan-beam X-ray absorptiometry (Lunar iDXA, GE Medical Systems, Madison, WI, USA), following the guidelines of the International Society for Clinical Densitometry to accurately place each participant [60]. According to BMI and body size, the scan mode (standard, thin, or thick) was selected using scanner software. Scans were analyzed using the enCORE Software, version 15 (GE Lunar). Data were collected within 1 week before CCRT and within 3 days after CCRT.
2.8. Ultrahigh-Performance Liquid Chromatography (UPLC)-Based Measurements
Serum histidine, leucine, ornithine, and phenylalanine levels were measured as previously described [49,51,61]. Briefly, EDTA-treated plasma samples were collected within 1 week before CCRT and 3 days after CCRT. They were stored at −80 °C until assayed. Plasma samples (100 μL) were precipitated by adding an equal volume of 10% sulfosalicylic acid containing 200 μM norvaline (an internal standard). After protein precipitation and centrifugation at 12,000× g for 10 min at room temperature, derivatization was initiated by adding 10 mM AQC in acetonitrile. Eluent A (20 mM ammonium formate/1.0% acetonitrile) was added to the mixture after 10 min of incubation, and the amino acids were analyzed using the ACQUITY UPLC System [62,63], which comprised a binary solvent manager, sample manager, and tunable UV detector. The system was controlled and data were collected using Empower™ 2 Software. Separations were performed on a 2.1 × 100 mm ACQUITY BEH C18 column at a flow rate of 0.70 mL/min. The average intra-assay coefficients of variation for histidine, ornithine, leucine, and phenylalanine were 4.3%, 4.6%, 4.5%, and 4.6%, respectively. The total coefficients of variation for histidine, ornithine, leucine, and phenylalanine were 3.1%, 3.6%, 4.1%, and 3.7%, respectively. The detection limit was 0.5 μM for histidine, 2.0 μM for ornithine, 0.9 μM for leucine, and 3.3 μM for phenylalanine. The linear range was 25–500 μM for these four amino acids.
∆ indicates the interval changes in the abovementioned biochemical and anthropometric data, blood NIBs, DXA-derived parameters, and four metabolites before and after the CCRT course.
2.9. Statistical Analysis
SPSS (version 22.0; SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Based on a power of 80%, α error of 0.05, and the annual number of patients with LAHNSCC receiving CCRT at our institute, the calculated minimum sample size was 42. Considering the drop-out rate due to CCRT toxicity or poor compliance with the data collection schedule, we assumed that the attrition rate was 30% and the required sample size of patients was 50. All continuous variables were examined for normality before the analysis. Independent t-tests or nonparametric Mann–Whitney tests were used for continuous variables, where appropriate. The chi-squared test was used for categorical variables. Paired t-tests and the Wilcoxon signed-rank test were used to detect differences before and after CCRT.
The association of different clinicopathological variables, biochemical and anthropometric data, NIBs, DXA-derived parameters, nutritional and calorie supply, metabolites, and CCRT factors with treatment interruption events was analyzed using logistic regression analysis. Forward stepwise selection was used in univariate and multivariate analyses. All independent variables that were significantly associated with treatment interruption events (p < 0.05) in the univariate analysis were included in the multivariate analysis.
Receiver operating characteristic (ROC) curves were used to determine the optimal cutoff value of treatment-interval changes in albumin and histidine levels, both of which were statistically significant in multivariate analysis. All differences in mortality rates were considered to be statistically significant (two-tailed p-value < 0.05).
The univariate associations of different clinicopathological variables, biochemical and anthropometric data, NIBs, DXA-derived parameters, nutritional and calorie supply, metabolites, and CCRT factors with treatment-interval changes in albumin and histidine levels were first analyzed using simple linear regression, nonparametric Mann–Whitney tests, or Kruskal–Wallis tests. All independent variables that were significantly associated with treatment-interval changes in albumin and histidine levels (p < 0.05) in the univariate analysis were included in the multivariable linear regression model analysis with forward stepwise selection. Variance inflation factors were used as variables to test collinearity.
Correlation matrices used to visualize correlations in treatment-interval changes among metabolites, biochemical and anthropometric factors, NIBs, and DXA-derived parameters were obtained using the Pearson correlation coefficient between each pair of variables, and were constructed using Statgraphics Centurion version 19 (Statgraphics Technologies, Inc., The Plains, VA, USA).
3. Results
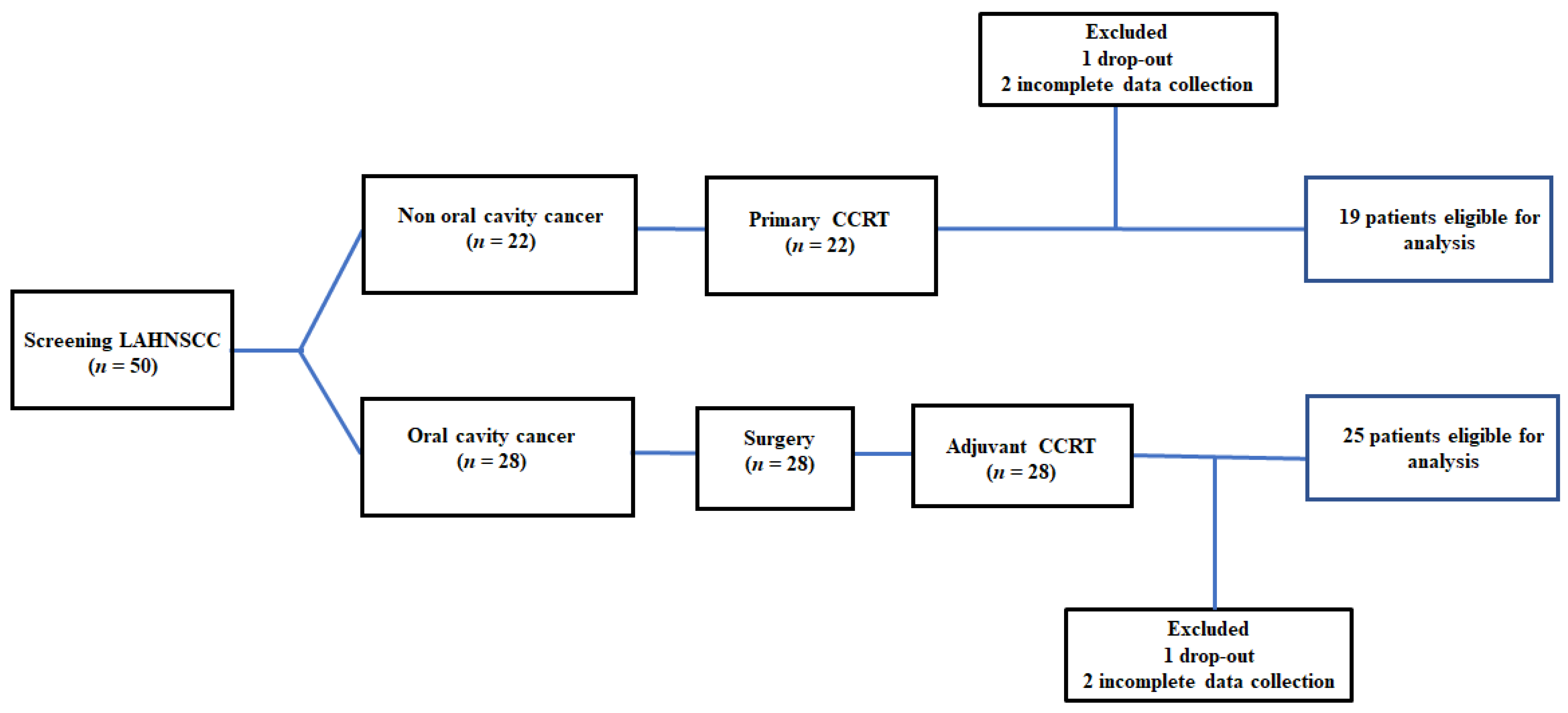
Fifty patients were included in this study. Two patients requested to drop out because their families moved to other counties. Four patients completed the CCRT course with no treatment interruption events but were classified into the “incomplete CCRT group” because they completed the required tests until one week after CCRT. Forty-four patients were eligible for analysis at the end of the study. The recruitment, allocation, treatment modalities, and data collection schedule are shown in Figure 1. The clinical features of patients with LAHNSCC who completed CCRT are presented in Table 1.
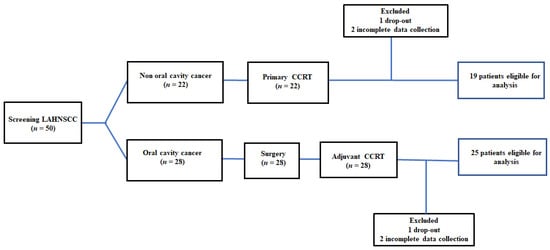
Figure 1.
The flow diagram of recruitment: The incomplete CCRT group was defined as (1) patients who dropped out during the treatment course; and (2) incomplete data collection—patients who did not complete the required examinations or missed scheduled tests. LAHNC, locally advanced head and neck cancer; CCRT, concurrent chemoradiotherapy.

Table 1.
Clinicopathological variables, biochemical and nutrition–inflammation data, anthropometric and body composition characteristics, serum HLOP metabolites, and treatment-related factors of 44 patients with LAHNSCC completing the CCRT course, stratified by treatment interruption.
3.1. Treatment-Interval Changes of the Anthropometric Data, NIBs, DXA-Related Measurements, and Serum Metabolites in Patients with LAHNSCC Completing CCRT
Of the 44 patients with LAHNSCC, 25 with oral cavity cancer received postoperative adjuvant CCRT, and 19 with non-oral-cavity cancer received primary CCRT. The study participants were predominantly men (95.5%), and had an average age of 54.7 years. The tongue (27.2%) was the most common tumor site, followed by the hypopharynx (22.7%) and the gingiva (11.4%). Nearly 90% of the tumors belonged to non-metastatic TNM stage IV, and all had at least one comorbid illness. Most patients were exposed to smoking (84.1%), alcohol consumption (72.7%), and betel nut usage (63.6%); they had an advanced tumor size (T3 + T4:75.0%), extended regional lymph node invasion (N2 + N3:65.9%), good histological differentiation (well + moderate: 86.4%), and optimal ECOG performance status (0 + 1:95.5%); 40% of the patients with cancer underwent tracheostomy before CCRT. Malnutrition rates were assessed using different malnutrition criteria before CCRT: PG-SGA-defined severe malnourished status (20.5%), BMI <18.5 kg/m2 (22.7%), albumin levels <3.5 g/dL (11.4%), and TLC <1.5 × 103 cells/mm3 (31.8%) (Table 1).
Throughout the treatment course. patients consumed 26.9 ± 6.6 kcal/kg/day, which included intake of 3.7 ± 0.9 g of carbohydrates/kg/day, 1.0 ± 0.3 g of protein/kg/day, and 0.8 ± 0.2 g of lipids/kg/day. Nearly 30% of the patients had a calorie intake of ≥30 kcal/kg/day. Half of the patients underwent feeding tube placement, with a mean duration of 20.3 days, for calorie and protein supply (Table 1). Under the recommended calorie and protein support, patients showed significant reductions in BW, BMI, eGFR, fasting sugar, Hb levels, WBC count, TLC, triglyceride levels, all DXA-derived measurements, and the levels of two amino acid metabolites (leucine and ornithine), along with increased TNC and CRP levels. There were no interval changes in hepatic function, TMC, or levels of cholesterol, uric acid, albumin, prealbumin, transferrin, histidine, and phenylalanine at the end of CCRT. Eighteen patients (40.9%) experienced grade 3/4 non-hematologic toxicity, the most common form being infection (31.8%), followed by mucositis (25.0%). Seventeen patients (38.6%) developed grade 3/4 hematological toxicity, with the most common form being neutropenia (34.1%), followed by thrombocytopenia (13.6%) (Table 2).

Table 2.
Treatment-interval changes of anthropometric data, NIBs, serum HLOP metabolites, and DXA-derived body composition measurements in 44 patients with LAHNSCC following CCRT completion.
3.2. Association of Treatment Interruption and Interval Changes in Serum Albumin and Histidine Concentrations of Patients with LAHNSCC during CCRT
No patient in this cohort experienced interruptions from machine faults or maintenance during the treatment course.
Nine patients (20.4%) experienced treatment interruption during CCRT: four patients with RT break > 5 days alone, and five with both RT and chemotherapy breaks. The interruptions were due to grade 3/4 treatment-related toxicities (Table 3). Among these nine patients, the mean interruption duration was 9.3 ± 3.3 days (range, 6–16 days); the interruptions happened in the second half of the treatment course.

Table 3.
Clinical features of nine LAHNSCC patients with treatment interruptions during CCRT.
Considering patients with treatment interruption and those with no treatment interruption, there were no differences in the clinicopathological variables (i.e., age, sex, tumor location, tumor stage, status of T and N, histological differentiation, exposure to smoking, alcohol and betel nut usage, performance status, presence of tracheostomy, and PG-SGA score), mean daily intake (calories; consumption of carbohydrates, protein, and fat), the percentage and mean days of feeding tube placement during CCRT, pretreatment levels of variables including biochemistry and anthropometry, NIBs, DXA-related measurements (LBM, TFM, and ASM), and three individual metabolites (leucine, ornithine, and phenylalanine). Compared to the subgroup with no treatment interruption, the subgroup with treatment interruption presented lower CRP levels but higher histidine concentrations before CCRT, experienced longer RT duration, developed more treatment-interval decreases in uric acid, Hb, albumin, ASM, and histidine, and had a higher proportion of patients with grade 3/4 mucositis toxicity. Patients with treatment interruption experienced more grade 3/4 toxicities than those with no interruption (Table 1).
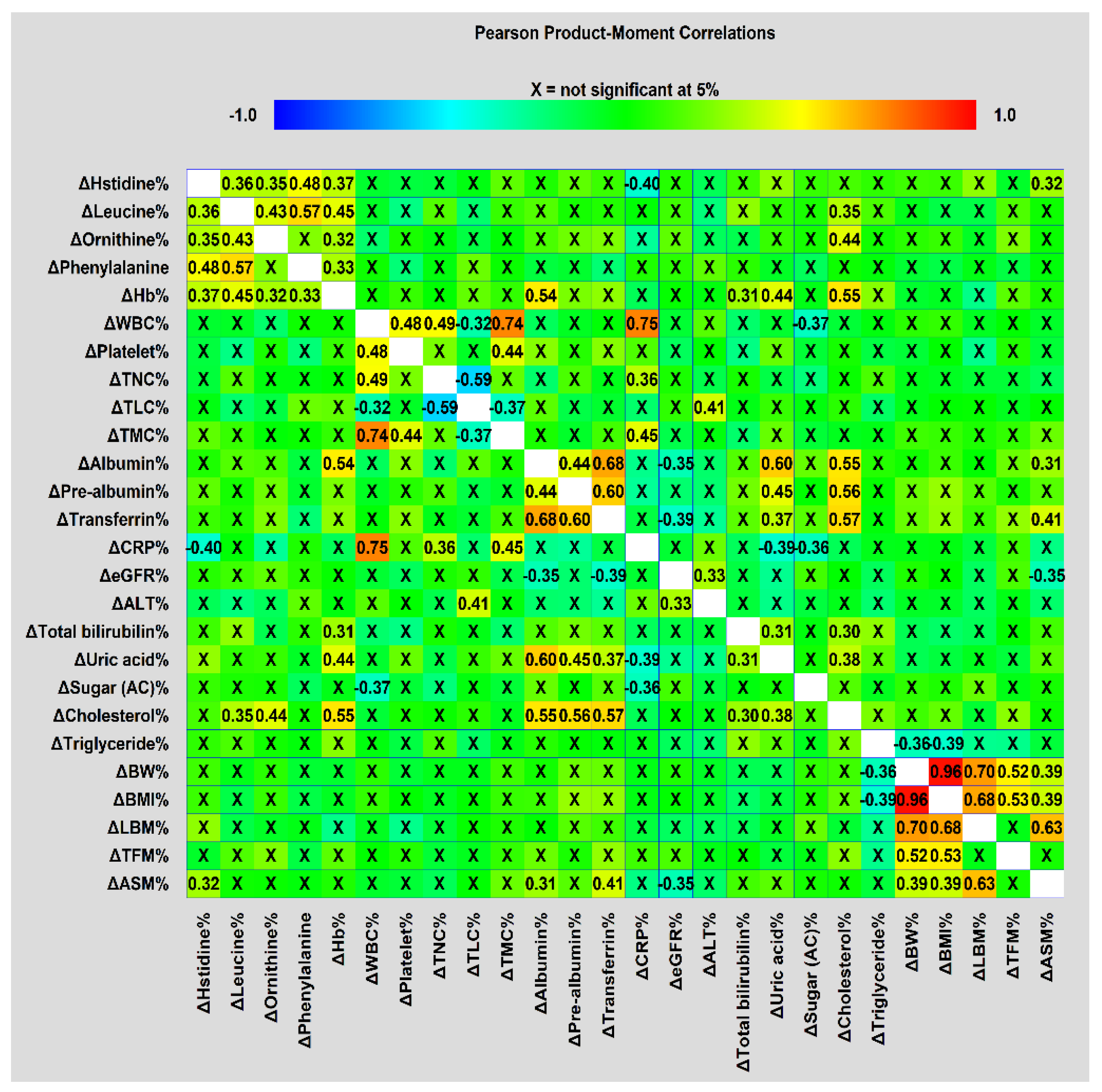
Furthermore, there were significant correlations between treatment-interval levels of biochemical and anthropometric variables, NIBs, DXA-related measurements, and serum metabolites (Figure 2). The four amino acid metabolites and Hb were positively correlated, except for ornithine and phenylalanine. All treatment-interval changes in BW, BMI, and DXA-associated measurements were also positively correlated with one another, except for TFM with LBM and ASM. ASM changes were positively correlated with changes in transferrin, albumin, and histidine levels. The changes in albumin levels were positively correlated with those in prealbumin, transferrin, uric acid, and cholesterol levels, as well as ASM. CRP changes were negatively correlated with changes in histidine and uric acid levels. This correlation analysis suggested that intricate relationships between treatment-interval changes in these blood variables, anthropometric data, and body composition parameters were present, and could cause the occurrence of treatment interruption.
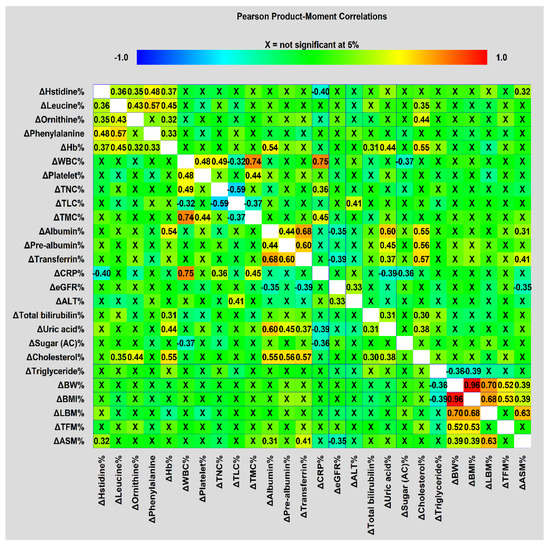
Figure 2.
Correlation matrices used to visualize correlations in treatment-interval changes among metabolites, biochemical and anthropometric factors, NIBs, and DXA-derived parameters were obtained using Pearson’s correlation coefficient.
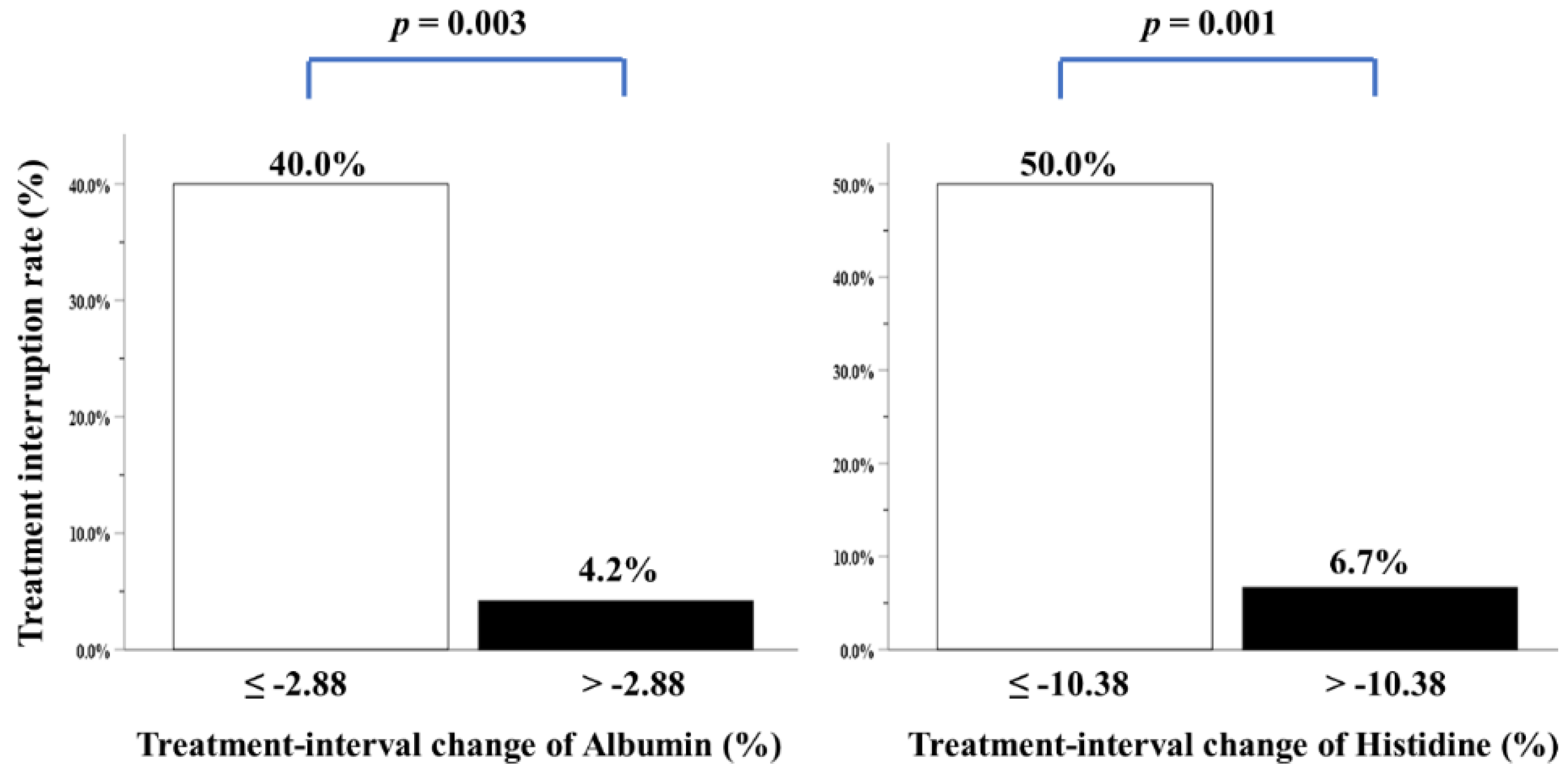
We performed multivariate analysis after adjustment for all covariates, including clinicopathological variables, biochemical and anthropometric data, mean daily intake, feeding tube placement, CCRT-related factors, blood NIBs, body composition parameters, and four individual amino acid metabolites. Only two variables—i.e., treatment-interval changes in albumin and histidine levels—were independently correlated with treatment interruption over the CCRT course (Table 4). Patients with higher albumin and histidine loss during CCRT had a greater treatment interruption rate (Figure 3). Nonetheless, energy consumption of ≥30 kcal/kg/day, duration and percentage of feeding tube placement, grade 3/4 mucositis toxicity, and more grade 3/4 toxicities were not correlated with treatment interruption (Table 4).

Table 4.
Univariate and multivariate logistic regression analyses of risk factors associated with treatment interruption of 44 patients with LAHNSCC completing CCRT.
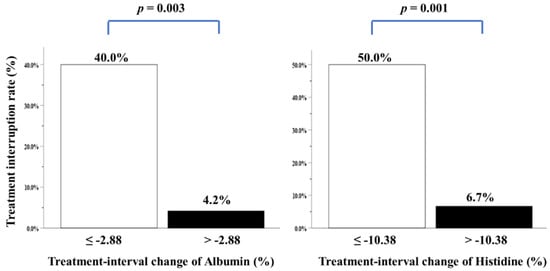
Figure 3.
Treatment interruption rate of patients with LAHNSCC completing CCRT, stratified by treatment-interval changes in albumin or histidine levels. The cutoff value analyzed by ROC curves for treatment-interval changes in albumin levels was −2.88% (area under the curve (AUC): 0.762, p = 0.016), while that of treatment-interval changes in histidine levels was −10.38% (AUC: 0.768, p = 0.014).
3.3. Factors Associated with Treatment-Interval Changes in Albumin and Histidine Levels of Patients with LAHNSCC Completing CCRT
We further investigated whether clinicopathological status, pretreatment nutritional and inflammatory conditions, treatment modalities, and relevant adverse effects of CCRT could affect the treatment-interval changes in albumin and histidine levels in patients with LAHNSCC completing CCRT. In multivariate analysis, the interval change in albumin levels was positively correlated with those in transferrin and uric acid levels; the treatment-interval change in histidine levels was positively correlated with phenylalanine level changes, but negatively correlated with CRP level changes (Table 5). Nonetheless, all variables regarding clinicopathological status, pretreatment nutritional and inflammatory conditions, and treatment modalities failed to show independent relationships with treatment-interval changes in serum albumin and histidine levels (Table 5).

Table 5.
Univariate and multivariate associations of risk factors with interval changes in albumin and histidine levels over the CCRT course in 44 patients with LAHNSCC completing CCRT.
4. Discussion
To overcome the confounding effects from mixed stages, varied treatment plans, and inadequate energy and protein supply during the treatment course (which possibly caused treatment interruptions), we prospectively analyzed a homogeneous group of patients with LAHNSCC undergoing the same standard CCRT under the provision of recommended calories of at least ≥25 kcal/kg/day and protein of 1.0 g/kg/day. Compared with treatment completion and toxicity profiles reported from previous studies [9,10,12,13,14,15,16,17,18], our results showed that 44 patients (88.0%) completed the current CCRT schedule and all required examinations with no delay; <40% patients experienced grade 3/4 treatment-related toxicities, and approximately one-fifth of patients experienced treatment interruptions, indicating that following the current study protocol, nearly 90% of patients with LAHNSCC were able to complete the CCRT course within the planned treatment duration, and fewer patients succumbed to treatment-related toxicities and interruptions. Therefore, comprehensive and multidisciplinary teamwork—including administrative work coordination, suitable delivery schedule of CCRT, psychological and social support, and adequate supply of energy and protein—could prevent unnecessary drop-outs and non-compliance events, improve treatment completion rates, and reduce treatment-induced adverse effects and interruptions.
Acute toxicity from CCRT needs further research, and remains a major limitation of continuous and uninterrupted CCRT courses [16,17,18]. In particular, grade 3/4 mucositis was reported as the root cause of unplanned treatment interruption in patients with HNC undergoing RT or CCRT, because mucositis is often accompanied by pain, aspiration, inanition, and infection, leading to discontinuation of the treatment course to allow healing, but negatively impacting prognosis [64,65,66]. The incidence rate of mucositis toxicity was 39% in patients with RT and 43% in patients with CCRT [9], accounting for 11–47% of interruption events during the treatment course [65,66]. Patients with LAHNSCC showed a high prevalence of malnutrition at the time of diagnosis, and developed malnutrition during the treatment course [67]. Malnutrition is a risk factor for the development of mucositis [68]; therefore, appropriate nutritional intervention during the CCRT course (to reduce the incidence of mucositis and other toxicities, as well as the resultant treatment interruption) is a reasonable approach. Nonetheless, our observations regarding the interplay between nutritional status, mucositis toxicity, and interruption events among patients with HNC undergoing CCRT remain inconsistent with those of previous studies. Some investigations showed that patients with optimal nutritional status suffer less from mucositis toxicity [69,70], and those with less incidence of treatment-related toxicity experience fewer treatment interruption events [9,71,72,73,74]. Russo et al. reviewed the relationship between RT breaks and oral mucositis, and reported that nutritional counseling and diet therapy—such as nutritional supplements and feeding tube placement—could reduce mucositis toxicity and the resulting treatment interruption in patients with HNC undergoing RT or CCRT [9]. Paccagnella et al. reported that HNC patients undergoing CCRT and early nutritional intervention had a lower incidence of RT breaks >5 days than those receiving standard care [73]. Lewis et al. further showed that patients with LAHNSCC undergoing CCRT who received prophylactic tube placement had a higher rate of chemotherapy completion than those who did not undergo tube placement [72]. In contrast, some studies showed that nutrition counseling or diet therapy—such as nutritional supplements and feeding tube placement—in patients treated with CCRT may have no benefit in reducing the frequency of treatment interruption events [69,74]. Moreover, the application of oral nutritional supplements is not advantageous in treatment interruption in patients with HNC receiving RT or CCRT [75], and there is no difference in the treatment interruption rate of patients with HNC receiving CCRT under nutritional supply via feeding tube placement [76,77,78,79]. These discrepancies among studies could be attributed to the inevitable bias of the retrospective study design, heterogeneous enrollment, and varied nutritional approaches. Thus, the present study, with a prospective design, shows essential observations. First, patients with treatment interruptions received fewer chemotherapy doses and developed a longer RT duration than those without interruptions. Furthermore, treatment interruption had no association with clinicopathological variables, pretreatment data pertaining to blood cell count, biochemical function, anthropometric measurements, NIBs and HLOP metabolites, body composition, calorie intake (≥30 kcal/kg/day), or the duration and percentage of feeding tube placement. In contrast to the pretreatment variables, significant decreases in treatment-interval levels of several variables—including hemoglobin, uric acid, albumin, ASM, and histidine metabolites—were detected in patients with treatment interruptions. Additionally, there was a strong correlation between treatment-interval changes in these blood variables and body composition parameters. Lastly, grade 3/4 mucositis and the number of grade 3/4 toxicities remained the risk factors with significant differences between the treatment-interruption and no-treatment-interruption groups. Thus, the treatment-interval level changes in certain variables—such as hemoglobin, uric acid, albumin, ASM, and histidine—could significantly affect the relationship between these acute morbidities and treatment interruption.
Acute toxicity from CCRT needs further research, and remains a major limitation of continuous and uninterrupted CCRT courses [16,17,18]. In particular, grade 3/4 mucositis was reported as the root cause of unplanned treatment interruption in patients with HNC undergoing RT or CCRT, because mucositis is often accompanied by pain, aspiration, inanition, and infection, leading to discontinuation of the treatment course to allow healing, but negatively impacting prognosis [64,65,66]. The incidence rate of mucositis toxicity was 39% in patients with RT and 43% in patients with CCRT [9], accounting for 11–47% of interruption events during the treatment course [65,66]. Patients with LAHNSCC showed a high prevalence of malnutrition at the time of diagnosis, and developed malnutrition during the treatment course [67]. Malnutrition is a risk factor for the development of mucositis [68]; therefore, appropriate nutritional intervention during the CCRT course (to reduce the incidence of mucositis and other toxicities, as well as the resultant treatment interruption) is a reasonable approach. Nonetheless, our observations regarding the interplay between nutritional status, mucositis toxicity, and interruption events among patients with HNC undergoing CCRT remain inconsistent with those of previous studies. Some investigations showed that patients with optimal nutritional status suffer less from mucositis toxicity [69,70], and those with less incidence of treatment-related toxicity experience fewer treatment interruption events [9,71,72,73,74]. Russo et al. reviewed the relationship between RT breaks and oral mucositis, and reported that nutritional counseling and diet therapy—such as nutritional supplements and feeding tube placement—could reduce mucositis toxicity and the resulting treatment interruption in patients with HNC undergoing RT or CCRT [9]. Paccagnella et al. reported that HNC patients undergoing CCRT and early nutritional intervention had a lower incidence of RT breaks >5 days than those receiving standard care [73]. Lewis et al. further showed that patients with LAHNSCC undergoing CCRT who received prophylactic tube placement had a higher rate of chemotherapy completion than those who did not undergo tube placement [72]. In contrast, some studies showed that nutrition counseling or diet therapy—such as nutritional supplements and feeding tube placement—in patients treated with CCRT may have no benefit in reducing the frequency of treatment interruption events [69,74]. Moreover, the application of oral nutritional supplements is not advantageous in treatment interruption in patients with HNC receiving RT or CCRT [75], and there is no difference in the treatment interruption rate of patients with HNC receiving CCRT under nutritional supply via feeding tube placement [76,77,78,79]. These discrepancies among studies could be attributed to the inevitable bias of the retrospective study design, heterogeneous enrollment, and varied nutritional approaches. Thus, the present study, with a prospective design, shows essential observations. First, patients with treatment interruptions received fewer chemotherapy doses and developed a longer RT duration than those without interruptions. Furthermore, treatment interruption had no association with clinicopathological variables, pretreatment data pertaining to blood cell count, biochemical function, anthropometric measurements, NIBs and HLOP metabolites, body composition, calorie intake (≥30 kcal/kg/day), or the duration and percentage of feeding tube placement. In contrast to the pretreatment variables, significant decreases in treatment-interval levels of several variables—including hemoglobin, uric acid, albumin, ASM, and histidine metabolites—were detected in patients with treatment interruptions. Additionally, there was a strong correlation between treatment-interval changes in these blood variables and body composition parameters. Lastly, grade 3/4 mucositis and the number of grade 3/4 toxicities remained the risk factors with significant differences between the treatment-interruption and no-treatment-interruption groups. Thus, the treatment-interval level changes in certain variables—such as hemoglobin, uric acid, albumin, ASM, and histidine—could significantly affect the relationship between these acute morbidities and treatment interruption.
In multivariate analysis, we further elucidated that toxicity was not correlated with treatment interruption, i.e., interval changes in albumin and histidine levels during CCRT were independently and positively correlated with treatment interruption (Table 4). Furthermore, the change in albumin levels was positively correlated with the interval changes of transferrin and uric acid; the histidine change was positively correlated with the change in phenylalanine levels, but negatively correlated with the change in CRP levels (Table 5). The current analysis suggests that toxicity could appear to cause treatment interruption, but the underlying nutritional and/or inflammatory changes during CCRT may be responsible for the treatment interruption. Thus, the main factors behind alterations in nutrition and inflammation, mucositis toxicity, and treatment interruption could be substances with antioxidative, anti-inflammatory, and metal-ion-chelating effects in the blood. Serum albumin has multiple ligand-binding capacities and free-radical-scavenging properties; thus, it exerts antioxidant and anti-inflammatory functions, accounting for over 80% of the antioxidant activity in the blood [80,81]. Compared to other blood proteins, it is primarily exposed to reactive oxygen species (ROS) because of its free thiol group of the Cys34 residue [80,81]; it is the main extracellular molecule responsible for modulating the plasma redox state, augmenting intracellular glutathione levels, and regulating cell signaling through the ubiquitous transcription factor nuclear factor kappa B (NF-κB), which mediates pro-inflammatory stress [82,83,84]. The antioxidative and anti-inflammatory functions of serum albumin explain the pathological process, and the albumin supply improves the clinical condition in certain critical and chronically ill patients [84,85,86,87,88,89,90]. Ishizuka et al. also reported a strong correlation between serum levels and neutropenia toxicity in patients with HNC receiving cisplatin-based CCRT, and emphasized that decreased binding activity of albumin to metal ions at low serum albumin concentrations could result in increased cisplatin-related toxicity [6,81]. Furthermore, serum uric acid could serve as a free radical scavenger in the blood and perform normal-range antioxidative functions to protect erythrocytes and immune cells [91]. Chang et al. found that low pretreatment uric acid (<5.05 mg/dL) was associated with lower survival outcomes in Taiwanese patients with oral cavity squamous-cell carcinoma undergoing surgery [92]. Lin et al. reported that high post-treatment uric acid (>5.06 mg/dL) was correlated with better survival outcomes in Chinese patients with nasopharyngeal carcinoma undergoing RT or CCRT [93]. Additionally, serum transferrin performs its antioxidative function by binding with iron in an inactive redox form, because free iron ions can stimulate the production of free radicals [83]. Transferrin expression is also affected by inflammation and malnutrition in the blood [94,95]. Histidine—a dietary essential amino acid, and one of the least abundant amino acids in the human body—is commonly recognized as one of the most active and antioxidative amino acids [96,97]. It acts as a scavenger of the hydroxyl radicals produced by the metal-ion-mediated redox reaction and singlet oxygen via direct interactions with the histidine imidazole ring [98]. Histidine also acts as an anti-inflammatory agent by abolishing NF-κB-mediated production of pro-inflammatory cytokines [99] or downregulating prostaglandin E2 function by interacting with its imidazole ring [100]. The histidine residue in proteins of the body efficiently binds to cisplatin and carboplatin [101]. Finally, the relationship between histidine and phenylalanine is intimate in both molecular and clinical respects. A decrease in plasma histidine levels results in reduced phenylalanine oxidation in healthy individuals following a long-term low-histidine diet [102]. Changes in histidine and phenylalanine levels in patients with heart failure can be used to assess their responses to medications and nutritional interventions [49]. The transport of histidine and phenylalanine into cells requires L-type amino acid transporter 1 (LAT1), i.e., a membrane transporter responsible for transporting bulky amino acids, which is regulated by glutathione status and oxidative stress [103,104]. LAT1 is overexpressed in patients with oral cavity squamous-cell carcinoma [105]. Although the antioxidative ability of phenylalanine is not obvious [97], phenylalanine hydroxylase (PAH)—which is mainly present in the liver, and is active in other tissues such as the kidneys, brain, and pancreas—can quickly metabolize phenylalanine to tyrosine in healthy individuals [106]. Tyrosine is an amino acid with powerful antioxidative ability [97]. Thus, changes in the levels of certain proteins and amino acids in the blood during CCRT may provide a possible explanation for the relationship between mucositis toxicity and treatment interruption in LAHSCC patients undergoing CCRT.
Oxidative and inflammatory stresses—such as ROS and pro-inflammatory cytokines—induced by CCRT can potentially inflict harm on epithelial cells as well as other organs, including the liver, skin, muscle, and peripheral vascular tissue [69]. Subsequently, the body’s protective response prevents damage progression [83]. Antioxidants in the blood are important defensive and reparative systems of the human body. Thus, the induction of oxidative and inflammatory stress from CCRT could cause the consumption of serum antioxidants during the treatment course. Changes in serum levels of antioxidants may correspond to the magnitude of redox potential and inflammation caused by CCRT. Based on the increase or decrease in the CRP status over the CCRT course, we further analyzed the patients, because CRP’s sensitivity, specificity, and reproducibility are widely used to assess the severity of the systemic inflammatory response [107]; we found that all cases with treatment interruption were those of the increased-CRP group during CCRT. Meanwhile, the increased-CRP group revealed more treatment-interval losses in albumin, prealbumin, transferrin, uric acid, and histidine levels, along with LBM and ASM, and a higher proportion of mucositis toxicity (Table S1). Although further research is required, these analyses raise a hypothetical pathogenesis to decipher the relationship between nutritional–inflammatory changes and treatment toxicity and interruptions in patients with LAHNSCC undergoing CCRT: Serum albumin neutralizes the oxidative and inflammatory stresses from CCRT. When serum albumin levels fluctuate during the treatment course, serum proteins and metabolites such as transferrin and uric acid are utilized to maintain the optimal plasma antioxidative and anti-inflammatory function against CCRT (Table 5). Simultaneously, serum histidine counteracts CCRT-induced oxidative and inflammatory damage. The decrease in serum histidine levels might arouse the influx of serum phenylalanine into cells via LAT1, resulting in reduced phenylalanine levels. Within the cell, phenylalanine hydroxylase catalyzes phenylalanine to tyrosine, which performs antioxidative functions intracellularly and extracellularly [108,109]. When both serum albumin and histidine are spent and faded away, the muscle mass can carry out proteolysis and release albumin and histidine into the blood [110,111], while hemoglobin (another storehouse of histidine in the body) can also be degraded to maintain serum histidine levels [102,112,113].
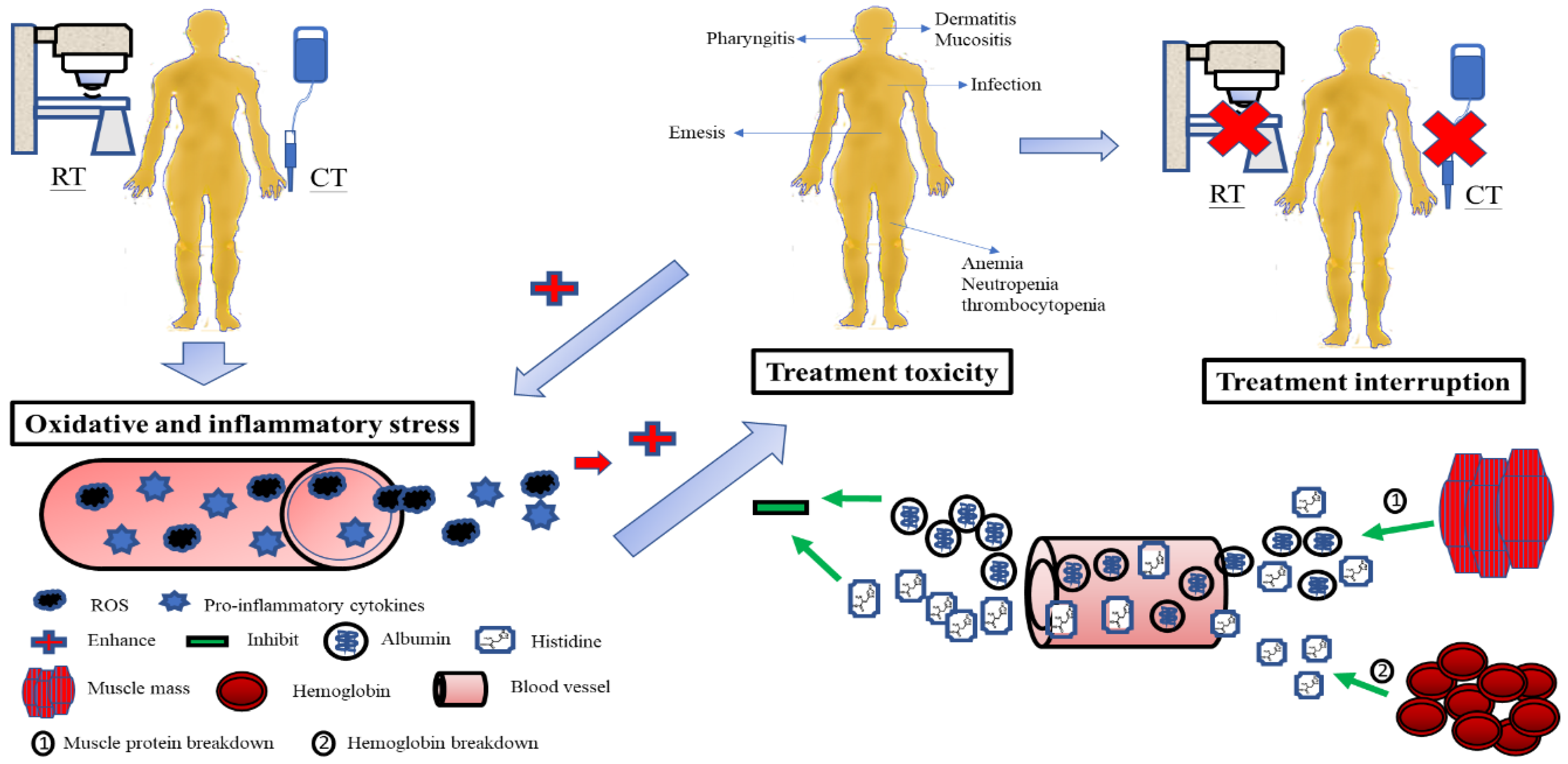
In the present study, patients with treatment interruptions may have developed more oxidative and inflammatory stress than those with no interruptions, leading to increased consumption of albumin and histidine, and subsequent muscle proteolysis and Hb breakdown. Although the release of albumin and histidine from the muscle and Hb was to compensate for the rapid loss of antioxidants in the blood, and to avoid the development of toxicity, the outcome was unsuccessful—possibly due to excessive oxidative and inflammatory damage. Accordingly, a reduced antioxidant pool could not protect the integrity and function of the mucosa and organs from toxicity. Once toxicities develop, they can deteriorate the status of oxidative and inflammatory insults from CCRT and make themselves worse. Finally, treatment interruption takes place in this vicious cycle (Figure 4).
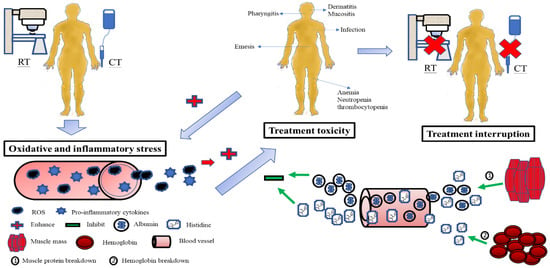
Figure 4.
Possible pathogenesis explains the relationship between changes in serum albumin and histidine levels during CCRT, treatment toxicity, and treatment interruption in patients with LAHNSCC undergoing CCRT. The oxidative and inflammatory stress from CCRT consumes serum albumin and histidine. Subsequently, muscle proteolysis and hemoglobin breakdown release albumin and histidine into the blood to compensate for the serum antioxidant loss and stop the development of toxicity. If serum antioxidants such as albumin and histidine fail to neutralize oxidative and inflammatory stress, the integrity and function of the mucosa and organs are no more protected from toxicity damage. Treatment interruption inevitably takes place. RT, radiotherapy; CT, chemotherapy; CCRT, concurrent chemoradiotherapy; LAHNSCC, locally advanced head and neck squamous-cell carcinoma.
The strengths of the present study are its homogeneous patient population treated with the standard CCRT protocol under the provision of recommended calories and protein over the treatment course, along with the simultaneous analysis of all confounding covariates. However, further research is needed for more detailed understanding. First, because patients with HNC undergoing CCRT require over 55,000 calories to complete the treatment course [114], the albumin and histidine released from the muscle mass and hemoglobin may be metabolized for energy fuel. For instance, histidine can be converted to glutamate, which can enter the glutamate–ornithine–proline pathway or the tricarboxylic acid cycle to produce energy [49]. In the present study, the mean BW was approximately 60 kg, and the average treatment duration was 42 days. Under the mandatory provision of at least 25 kcal/kg/day of energy, the patient would receive more than 60,000 calories throughout the entire CCRT course. The conversion of proteins and histidine to energy is trivial. Second, the changes in serum levels of albumin reflect the dynamic state between albumin synthesis and catabolism, including degradation and clearance in response to stress, inflammation, and diseases [115]. Fearon et al. demonstrated that the albumin synthesis rate is not changed in cancer patients even with low serum albumin concentrations [116]. Our results reported no grade 3/4 diarrhea toxicity, and there were no significant differences in interval changes regarding liver function, renal clearance (ΔALT, Δtotal bilirubin, and ΔeGFR), or the intake of calories, carbohydrates, proteins, and fat between the interruption and no-interruption groups. It is thus less likely that insufficient synthesis, increased gastrointestinal loss, and renal clearance could affect the interval changes in albumin levels during CCRT and lead to the resultant treatment interruption. Third, the decrease in treatment-interval serum histidine levels could be a consequence of enhanced histidine catabolism [117] or its increased accumulation in the muscle, where histidine is converted into strong antioxidative dipeptides such as carnosine [118] to cope with CCRT-induced oxidative stress causing muscle breakdown. Histidine metabolism is closely related to food and energy intake [117], and plasma histidine levels are positively correlated with protein synthesis [102]. Our data showed that under adequate energy and protein supply, there were no differences in histidine levels before and after CCRT (Table 2), no differences in the consumption of energy and protein between the interruption and no-interruption groups (Table 1), and a positive correlation between changes in histidine levels and muscle mass (Figure 2). This supports the hypothesis that serum histidine is consumed under oxidative and inflammatory stress, followed by muscle breakdown to supplement histidine loss, rather than being used to enhance catabolism or carnosine synthesis during CCRT. Additionally, the postulate pertaining to the PAH conversion of phenylalanine into tyrosine—which is assumed to be an antioxidant backup for histidine during CCRT—is debatable, because the activity of PAH could be impaired, and phenylalanine may produce abnormal tyrosine isomers that disrupt cellular homeostasis via oxidative and inflammatory stress from cancer and radiation [119,120]. However, Davis et al. analyzed the relationship between the structure and function of PAH under radiation exposure, and found that the activity of PAH might not be altered under regulation by the tetrahydrobiopterin cofactor [121,122]. Hence, the postulate could be true, and radiation exposure might modulate PAH function without completely impairing its ability to convert to tyrosine, because the provision of adequate calories and proteins throughout the entire CCRT course could replenish the consumption of the tetrahydrobiopterin cofactor during CCRT and, subsequently, maintain PAH function. Finally, leucine and ornithine concentrations decreased during CCRT. Leucine, an essential branched-chain amino acid, is among the most abundant amino acids in proteins. It stimulates protein synthesis via the mTOR signaling pathway, and inhibits proteolysis by downregulating the ubiquitin–proteasome proteolytic pathway [123,124]. Leucine can be obtained via protein catabolism from diet or from the breakdown of tissue protein stores. It acts as the major nitrogen donor to build common body proteins in tissues such as the skeletal muscle, liver, and pancreas [125,126]. Leucine, like other branched-chain amino acids, can reduce oxidative stress in chronic illnesses [127]. When muscles are exposed to chemotherapy or radiotherapy, muscle proteins undergo proteolysis, mainly via ubiquitin-dependent NF-κB-mediated or ROS-induced cytokine (e.g., tumor necrosis factor-α, interleukin-1, and interferon-γ) pathways [128]. Leucine supplementation activates mTOR signaling, which is a master regulator of muscle mass and function [129]. Our findings showed that two variables (TLC and leucine) at pretreatment and two (hemoglobin and ornithine) during the treatment course were associated with treatment-interval changes in leucine levels (Table S2). Thus, decreases in serum leucine levels during CCRT could be due to the shift of leucine from the blood to the intracellular microenvironment, where it repairs tissue protein damage from oxidative and inflammatory stress caused by CCRT. Pretreatment TLC was correlated with pretreatment levels of albumin (r = 0.32, p < 0.05), prealbumin (r = 0.40, p < 0.01), and ASM (r = 0.32, p < 0.05); these could represent the nutritional status. Pretreatment leucine levels are correlated with the total concentrations of all essential amino acids [130]. Both TLC and leucine pretreatment status may reflect the magnitude of nutritional status and leucine storage to cope with CCRT-induced tissue protein loss. Leucine is a major amino acid in hemoglobin, and it can improve anemia via mTOR signaling and iron metabolism [131,132]; thus, there could be a close correlation between leucine and hemoglobin during CCRT. The connection between leucine and ornithine during CCRT could be ascribed to the redistribution of leucine and other essential amino acids to maintain protein loss; it thus the influences the ornithine load that is converted from other amino acid pools. Ornithine, a nonessential amino acid, is an essential intermediate in the urea cycle, and plays an important role in the liver. The ornithine level is determined by the ornithine load generated from the metabolism of amino acids, clearance by the urea cycle, and ornithine catabolism—represented as the ornithine–spermine/spermidine (polyamine metabolite) pathway [133,134]. We found that only treatment-interval level changes in cholesterol were associated with those of ornithine (Table S2); thus, ornithine catabolism (ornithine–polyamine pathway) could cause changes in serum ornithine levels during CCRT. Although the ornithine load could be affected because other amino acids were utilized to repair damaged proteins, patients received adequate energy and protein supply, and their hepatic functions (ALT and total bilirubin levels) were not altered during the CCRT course. The conversion of ornithine to citrulline in the urea cycle should be not significantly affected. Thus, the ornithine–polyamine pathway may explain the unusual association between ornithine and cholesterol. Oxidative stress can increase the activity of spermidine/spermine N1-acetyltransferase (SSAT)—the rate-limiting enzyme in polyamine catabolism [135]. Jell et al. showed an accumulation of body fat and an increase in acetyl- and malonyl-CoA pools in the white adipose tissue of SSAT-knockout mice [136]. Kramer et al. reported that increased SSAT activity augments metabolic flux via the polyamine pathway owing to enhanced ornithine decarboxylase function, which reduces polyamines via SSAT [133,137]. This vicious cycle results in the fall of acetyl-CoA and malonyl-CoA—i.e., precursors of cholesterol and fatty acids—and eventually results in a substantial increase in glucose oxidation and fat tissue loss [133]. Overexpression of SSAT could also reduce plasma cholesterol levels via the enhanced hepatic cholesterol 7α-hydroxylase activity, which converts cholesterol to bile acid in transgenic animal models [138]. Thus, CCRT-induced oxidative and inflammatory stress might be responsible for the reduction in leucine and ornithine levels during the treatment course.
Our results for LAHNSCC were partially consistent with those of previous reports on other malignancies [139,140,141,142,143]. Patients with esophageal cancer receiving CCRT who exhibited increased albumin levels during the treatment course had better survival outcomes [139,143]. Kayauchi et al. analyzed 226 patients with lung cancer undergoing cytotoxic chemotherapy, and reported that patients with decreased albumin levels during treatment significantly suffered from febrile neutropenia toxicity [141]. The histidine degradation pathway is essential for determining the chemosensitivity of tumor cells to methotrexate; thus, the addition of histidine to methotrexate significantly reduces tumor growth in animal models [140]. Exogenous histidine treatment has antitumor effects on liver cancer cell lines, and can reverse sorafenib resistance by modulating LAT1 [142]. Additionally, some reports have shown that supplementation with certain amino acids might improve protein synthesis in cancer with cachexia [38,144]. Although these reports—including ours—suggest that albumin and histidine supplementation during the anticancer treatment could be a potential and reasonable strategy in daily practice, its clinical application remains ambiguous. ROS, ionizing radicals, and inflammation generated from radiation and chemotherapy mainly eradicate cancer cells and cause toxicity during the treatment course; thus, it is unclear whether exogenous antioxidants such as albumin and histidine improve or limit the overall treatment outcomes. Additionally, growing evidence indicates that, clinically speaking, reduced serum albumin levels are a result rather than an etiology for diseases [115]. Furthermore, there is uncertainty regarding the outcomes of histidine supplementation at the molecular level, such as variations in gene expression and different metabolic fates of excess histidine in various tissues. These concerns may leave a considerable gap in the application of albumin and histidine.
This study had several limitations. Although treatment interruption had a negative impact on the survival outcomes of cancer patients receiving RT or CCRT [19,20,21,22], patients with no treatment interruptions showed a marginal benefit in survival outcomes compared to those with interruptions (6-month recurrence rate: 8.6% vs. 33.3%, p = 0.054; one-year mortality rate: 11.4% vs. 33.3%, p = 0.109; two-year mortality rate: 17.14% vs. 44.4%, p = 0.081) in this study. This could be ascribed to treatment interruption as the primary endpoint. Therefore, the sample size may not be sufficient for survival analysis. Second, the status of oxidation and inflammation before and after CCRT is not fully understood. Although we used treatment-interval CRP changes to reflect the dynamic state of oxidative and inflammatory stress, and found some significant correlations between CRP changes, treatment toxicity, and interruptions, further research is required. For instance, patients with increased CRP levels developed a similar number of grade 3/4 toxicities as those with decreased CRP levels during CCRT (Table S1), but patients with interruptions developed more high-grade toxicities than those with no interruptions (Table 1). This discrepancy suggests that certain potential oxidative and inflammatory stress biomarkers—such as malondialdehyde, glutathione, and pro-inflammatory cytokines, which could correspond to the degree of oxidative and inflammatory stress—should be examined and used to reclassify the redox and inflammation status of patients. Furthermore, the enrolled patients were Taiwanese, and most of them were men (95.5%). The present results should be cautiously interpreted for different ethnic groups, females, varied treatment schedules, nutrition support plans, and regional susceptibility to this disease. Although the advantage of this study was a prospective design with homogeneous enrollment, standard protocol, and accurate sample size calculation that was based on the head and neck cancer registry of our institute, the issue that all patients were recruited from a single institution should be addressed. Because there might be the presence of selection bias, future investigation with a prospective large-scale and multi-institutional study is warranted. Additionally, a major weakness of this study was the lack of a comprehensive amino acid metabolite profile before and after CCRT. This drawback may leave the postulate regarding the conversion of phenylalanine into tyrosine up for debate. We believe that even if the entire amino acid panel is completed and histidine and phenylalanine are replaced, certain amino acids with antioxidative and anti-inflammatory functions can counteract CCRT-induced insults. The hypothesis regarding the contribution of serum albumin and amino acids against oxidative and inflammatory stress still holds. Lastly, although six patients failed to meet the criteria of the final analysis (two drop-outs and four with incomplete data collection during CCRT), there were no statistical differences in baseline characteristics between the complete-CCRT and incomplete-CCRT groups (Table S3). Thus, the impact of drop-outs and incomplete data collection was not considered in the present analysis.
5. Conclusions
This prospective observational study demonstrated that among patients with LAHNSCC receiving CCRT under the recommended energy and protein supply (≥25 kcal/kg/day and 1.0 g/kg/day of protein) over the treatment course, treatment interruption was associated with treatment-interval changes in serum albumin and histidine levels, both of which likely reduce the oxidative and inflammatory stress generated from CCRT, as well as the resultant treatment-associated toxicity. Follow-up of serum albumin and histidine levels during CCRT may be considered as a routine nutritional assessment for these patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14133112/s1: Table S1: Clinicopathological variables, biochemical and nutrition–inflammation data, anthropometric and body composition characteristics, serum HLOP metabolites, and treatment-related factors of 44 patients with LAHNSCC completing the CCRT course, stratified by the status of CRP change during the treatment course. Table S2: Associations of clinicopathological variables, NIBs, body composition parameters, treatment-related factors, and serum metabolites with treatment-interval changes in leucine and ornithine levels over the CCRT course in 44 patients with LAHNSCC completing CCRT. Table S3: Clinicopathological variables, biochemical and nutrition–inflammation data, anthropometric and body composition characteristics, and serum HLOP metabolite levels of 50 patients with LAHNSCC stratified by the status of complete CCRT and incomplete CCRT.
Author Contributions
Conceptualization, K.-Y.Y.; data curation, K.-Y.Y., H.H.L. and C.-H.W.; formal analysis, K.-Y.Y. and Y.-P.P.; funding acquisition, K.-Y.Y. and C.-H.W.; investigation, K.-Y.Y., H.H.L., P.-H.C. and Y.-C.L.; methodology, K.-Y.Y. and C.-H.W.; project administration, C.-H.W.; resources, K.-Y.Y., H.H.L. and P.-H.C.; software, K.-Y.Y. and C.-L.P.; supervision, K.-Y.Y., M.-H.L. and C.-H.W.; validation, K.-Y.Y., W.-C.C. and C.-H.W.; visualization, K.-Y.Y., M.-H.L. and Y.-C.L.; writing—original draft, C.-H.W. and K.-Y.Y.; writing—review and editing, K.-Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Chang Gung Memorial Hospital, Keelung (CMRPG2G0611).
Institutional Review Board Statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB approval numbers: 101-4047B, date of approval 2012; and 201700158B0, date of approval 2017).
Informed Consent Statement
Written informed consent to publish this paper was obtained from all patients.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank all of the patients who participated in this study. We are grateful to Ting-Wei Ye, Ting-An Ye, Chien-Ting Chiang, and Ya-Huei Lin for artwork and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Giraldi, L.; Pastorino, R.; Arzani, D.; Petrelli, L.; Wunsch-Filho, V.; Toporcov, T.N.; Moyses, R.A.; et al. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: A multicentre study within the INHANCE consortium. Eur. J. Epidemiol. 2018, 33, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Saxton, J.P.; Lavertu, P.; Tuason, L.; Wood, B.G.; Wanamaker, J.R.; Eliachar, I.; Strome, M.; Van Kirk, M.A. A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer: Preliminary results. Head Neck 1997, 19, 567–575. [Google Scholar] [CrossRef]
- El-Sayed, S.; Nelson, N. Adjuvant and adjunctive chemotherapy in the management of squamous cell carcinoma of the head and neck region. A meta-analysis of prospective and randomized trials. J. Clin. Oncol. 1996, 14, 838–847. [Google Scholar] [CrossRef]
- Bese, N.S.; Hendry, J.; Jeremic, B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Groome, P.A.; O’Sullivan, B.; Mackillop, W.J.; Jackson, L.D.; Schulze, K.; Irish, J.C.; Warde, P.R.; Schneider, K.M.; Mackenzie, R.G.; Hodson, D.I.; et al. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer: Population-based outcomes study supporting need for intensified treatment schedules. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1002–1012. [Google Scholar] [CrossRef]
- Ishizuka, M.; Fujimoto, Y.; Itoh, Y.; Kitagawa, K.; Sano, M.; Miyagawa, Y.; Ando, A.; Hiramatsu, M.; Hirasawa, N.; Ishihara, S.; et al. Relationship between hematotoxicity and serum albumin level in the treatment of head and neck cancers with concurrent chemoradiotherapy using cisplatin. Jpn. J. Clin. Oncol. 2011, 41, 973–979. [Google Scholar] [CrossRef]
- Mazul, A.L.; Stepan, K.O.; Barrett, T.F.; Thorstad, W.L.; Massa, S.; Adkins, D.R.; Daly, M.D.; Rich, J.T.; Paniello, R.C.; Pipkorn, P.; et al. Duration of radiation therapy is associated with worse survival in head and neck cancer. Oral Oncol. 2020, 108, 104819. [Google Scholar] [CrossRef]
- McCloskey, S.A.; Jaggernauth, W.; Rigual, N.R.; Hicks, W.L., Jr.; Popat, S.R.; Sullivan, M.; Mashtare, T.L., Jr.; Khan, M.K.; Loree, T.R.; Singh, A.K. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am. J. Clin. Oncol. 2009, 32, 587–591. [Google Scholar] [CrossRef]
- Russo, G.; Haddad, R.; Posner, M.; Machtay, M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 2008, 13, 886–898. [Google Scholar] [CrossRef]
- Shaikh, T.; Handorf, E.A.; Murphy, C.T.; Mehra, R.; Ridge, J.A.; Galloway, T.J. The impact of radiation treatment time on survival in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 967–975. [Google Scholar] [CrossRef]
- Wendt, T.G.; Grabenbauer, G.G.; Rodel, C.M.; Thiel, H.J.; Aydin, H.; Rohloff, R.; Wustrow, T.P.; Iro, H.; Popella, C.; Schalhorn, A. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: A randomized multicenter study. J. Clin. Oncol. 1998, 16, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef]
- Lee, A.W.; Lau, W.H.; Tung, S.Y.; Chua, D.T.; Chappell, R.; Xu, L.; Siu, L.; Sze, W.M.; Leung, T.W.; Sham, J.S.; et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong nasopharyngeal cancer study group. J. Clin. Oncol. 2005, 23, 6966–6975. [Google Scholar] [CrossRef]
- Giddings, A. Treatment interruptions in radiation therapy for head-and-neck cancer: Rates and causes. J. Med. Imaging Radiat. Sci. 2010, 41, 222–229. [Google Scholar] [CrossRef]
- James, N.D.; Robertson, G.; Squire, C.J.; Forbes, H.; Jones, K.; Cottier, B.; Sub-committee, R.C.R.C.O.A. A national audit of radiotherapy in head and neck cancer. Clin. Oncol. (R. Coll. Radiol.) 2003, 15, 41–46. [Google Scholar] [CrossRef][Green Version]
- James, N.D.; Williams, M.V.; Summers, E.T.; Jones, K.; Cottier, B.; Royal College of Radiologists Clinical Audit Subcommittee. The management of interruptions to radiotherapy in head and neck cancer: An audit of the effectiveness of national guidelines. Clin. Oncol. (R. Coll. Radiol.) 2008, 20, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.B.; Keane, T.J.; Gadalla, T.; Maki, E. The effect of treatment time and treatment interruption on tumour control following radical radiotherapy of laryngeal cancer. Radiother. Oncol. 1992, 23, 137–143. [Google Scholar] [CrossRef]
- Fowler, J.F.; Lindstrom, M.J. Loss of local control with prolongation in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 457–467. [Google Scholar] [CrossRef]
- Herrmann, T.; Jakubek, A.; Trott, K.R. The importance of the timing of a gap in radiotherapy of squamous cell carcinomas of the head and neck. Strahlenther. Onkol. 1994, 170, 545–549. [Google Scholar] [PubMed]
- Suwinski, R.; Sowa, A.; Rutkowski, T.; Wydmanski, J.; Tarnawski, R.; Maciejewski, B. Time factor in postoperative radiotherapy: A multivariate locoregional control analysis in 868 patients. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 399–412. [Google Scholar] [CrossRef]
- Withers, H.R.; Taylor, J.M.; Maciejewski, B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988, 27, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Tsang, N.M.; Tseng, C.K.; Lin, S.Y. Causes of interruption of radiotherapy in nasopharyngeal carcinoma patients in Taiwan. Jpn. J. Clin. Oncol. 2000, 30, 230–234. [Google Scholar] [CrossRef]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Chasen, M.R.; Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support. Care Cancer 2009, 17, 1345–1351. [Google Scholar] [CrossRef]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef]
- Nakano, J.; Ishii, S.; Fukushima, T.; Natsuzako, A.; Sakamoto, F.; Natsuzako, A.; Sakamoto, J.; Okitaet, M. Factors affecting muscle strength in cancer patients receiving chemotherapy. J. Nov. Physiother. Rehabil. 2017, 1, 56–66. [Google Scholar] [CrossRef]
- Orell-Kotikangas, H.; Osterlund, P.; Makitie, O.; Saarilahti, K.; Ravasco, P.; Schwab, U.; Makitie, A.A. Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Otolaryngol. 2017, 137, 778–785. [Google Scholar] [CrossRef]
- Silver, H.J.; Dietrich, M.S.; Murphy, B.A. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 2007, 29, 893–900. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2005, 9 (Suppl. 2), S51–S63. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.C.H.; Hoeben, A.; Lalisang, R.I.; Van Helvoort, A.; Wesseling, F.W.R.; Hoebers, F.; Baijens, L.W.J.; Schols, A. Disease-induced and treatment-induced alterations in body composition in locally advanced head and neck squamous cell carcinoma. J. Cachexia Sarcopenia Muscle 2020, 11, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.; Gentile, P.C.; Bianciardi, F.; Tosti, M.; Palladino, A.; Di Palma, M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support. Care Cancer 2010, 18, 433–437. [Google Scholar] [CrossRef]
- Miyawaki, E.; Naito, T.; Nakashima, K.; Miyawaki, T.; Mamesaya, N.; Kawamura, T.; Shota, K.; Omori, H.; Wakuda, K.; Ono, A.; et al. Management of anorexia prevents skeletal muscle wasting during cisplatin-based chemotherapy for thoracic malignancies. JCSM Clin. Rep. 2020, 5, 8–15. [Google Scholar] [CrossRef]
- Lazzari, G.; De Cillis, M.A.; Buccoliero, G.; Silvano, G. Competing morbidities in advanced head and neck squamous cell carcinoma concurrent chemoradiotherapy: A strong implication of a multidisciplinary team approach. Cancer Manag. Res. 2019, 11, 9771–9782. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Haraf, D.J.; List, M.A.; Kocherginsky, M.; Mittal, B.B.; Rosen, F.; Brockstein, B.; Williams, R.; Witt, M.E.; Stenson, K.M.; et al. High survival and organ function rates after primary chemoradiotherapy for intermediate-stage squamous cell carcinoma of the head and neck treated in a multicenter phase II trial. J. Clin. Oncol. 2006, 24, 3438–3444. [Google Scholar] [CrossRef][Green Version]
- Wong, W.W.; Mick, R.; Haraf, D.J.; Weichselbaum, R.R.; Vokes, E.E. Time-dose relationship for local tumor control following alternate week concomitant radiation and chemotherapy of advanced head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 153–162. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Lin, Y.C.; Ling, H.H.; Chang, P.H.; Pan, Y.P.; Wang, C.H.; Chou, W.C.; Chen, F.P.; Yeh, K.Y. Concurrent chemoradiotherapy induces body composition changes in locally advanced head and neck squamous cell carcinoma: Comparison between oral cavity and non-oral cavity cancer. Nutrients 2021, 13, 2969. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Ling, H.H.; Ng, S.H.; Wang, C.H.; Chang, P.H.; Chou, W.C.; Chen, F.P.; Lin, Y.C. Role of the appendicular skeletal muscle index for predicting the recurrence-free survival of head and neck cancer. Diagnostics 2021, 11, 309. [Google Scholar] [CrossRef]
- Wendrich, A.W.; Swartz, J.E.; Bril, S.I.; Wegner, I.; de Graeff, A.; Smid, E.J.; de Bree, R.; Pothen, A.J. Low skeletal muscle mass is a predictive factor for chemotherapy dose-limiting toxicity in patients with locally advanced head and neck cancer. Oral Oncol. 2017, 71, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wang, C.H.; Ling, H.H.; Pan, Y.P.; Chang, P.H.; Chou, W.C.; Chen, F.P.; Yeh, K.Y. Inflammation status and body composition predict two-year mortality of patients with locally advanced head and neck squamous cell carcinoma under provision of recommended energy intake during concurrent chemoradiotherapy. Biomedicines 2022, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Metabolomics of head and neck cancer: A mini-review. Front. Physiol. 2016, 7, 526. [Google Scholar] [CrossRef] [PubMed]
- Tiziani, S.; Lopes, V.; Gunther, U.L. Early stage diagnosis of oral cancer using 1H NMR-based metabolomics. Neoplasia 2009, 11, 269–276, IN7–IN10. [Google Scholar] [CrossRef]
- Xie, G.X.; Chen, T.L.; Qiu, Y.P.; Shi, P.; Zheng, X.J.; Su, M.M.; Zhao, A.H.; Zhou, Z.T.; Jia, W. Urine metabolite profiling offers potential early diagnosis of oral cancer. Metabolomics 2012, 8, 220–231. [Google Scholar] [CrossRef]
- Yonezawa, K.; Nishiumi, S.; Kitamoto-Matsuda, J.; Fujita, T.; Morimoto, K.; Yamashita, D.; Saito, M.; Otsuki, N.; Irino, Y.; Shinohara, M.; et al. Serum and tissue metabolomics of head and neck cancer. Cancer Genom. Proteom. 2013, 10, 233–238. [Google Scholar]
- Zhou, J.; Xu, B.; Huang, J.; Jia, X.; Xue, J.; Shi, X.; Xiao, L.; Li, W. 1H NMR-based metabonomic and pattern recognition analysis for detection of oral squamous cell carcinoma. Clin. Chim. Acta 2009, 401, 8–13. [Google Scholar] [CrossRef]
- Wang, C.H.; Cheng, M.L.; Liu, M.H. Simplified plasma essential amino acid-based profiling provides metabolic information and prognostic value additive to traditional risk factors in heart failure. Amino Acids 2018, 50, 1739–1748. [Google Scholar] [CrossRef]
- Wang, C.H.; Cheng, M.L.; Liu, M.H. Amino acid-based metabolic panel provides robust prognostic value additive to b-natriuretic peptide and traditional risk factors in heart failure. Dis. Markers 2018, 2018, 3784589. [Google Scholar] [CrossRef]
- Huang, S.S.; Lin, J.Y.; Chen, W.S.; Liu, M.H.; Cheng, C.W.; Cheng, M.L.; Wang, C.H. Phenylalanine- and leucine-defined metabolic types identify high mortality risk in patients with severe infection. Int. J. Infect. Dis. 2019, 85, 143–149. [Google Scholar] [CrossRef]
- Kuo, W.K.; Liu, Y.C.; Chu, C.M.; Hua, C.C.; Huang, C.Y.; Liu, M.H.; Wang, C.H. Amino acid-based metabolic indexes identify patients with chronic obstructive pulmonary disease and further discriminates patients in advanced BODE stages. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Yen, C.H.; Wu, I.W.; Liu, M.H.; Cheng, H.Y.; Lin, Y.T.; Lee, C.C.; Hsu, K.H.; Sun, C.Y.; Chen, C.Y.; et al. The association between low protein diet and body composition, muscle function, inflammation, and amino acid-based metabolic profile in chronic kidney disease stage 3-5 patients. Clin. Nutr. ESPEN 2021, 46, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Tsai, M.H.; Chiang, C.J.; Tsai, S.T.; Liu, T.W.; Lou, P.J.; Liao, C.T.; Lin, J.C.; Chang, J.T.; Tsai, M.H.; et al. Adjuvant radiotherapy after curative surgery for oral cavity squamous cell carcinoma and treatment effect of timing and duration on outcome-A taiwan cancer registry national database analysis. Cancer Med. 2018, 7, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.J.; Jin, Y.N.; Wang, S.Y.; Zhang, F.; Zhou, G.Q.; Zhang, W.J.; Zhi, B.; Cheng; Ma, J.; Qi, Z.Y.; et al. The detrimental effects of radiotherapy interruption on local control after concurrent chemoradiotherapy for advanced T-stage nasopharyngeal carcinoma: An observational, prospective analysis. BMC Cancer 2018, 18, 740. [Google Scholar] [CrossRef] [PubMed]
- Boje, C.R.; Dalton, S.O.; Primdahl, H.; Kristensen, C.A.; Andersen, E.; Johansen, J.; Andersen, L.J.; Overgaard, J. Evaluation of comorbidity in 9388 head and neck cancer patients: A national cohort study from the DAHANCA database. Radiother. Oncol. 2014, 110, 91–97. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef]
- Araki, K.; Ito, Y.; Fukada, I.; Kobayashi, K.; Miyagawa, Y.; Imamura, M.; Kira, A.; Takatsuka, Y.; Egawa, C.; Suwa, H.; et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer 2018, 18, 982. [Google Scholar] [CrossRef]
- Pan, Y.P.; Chang, P.H.; Fan, C.W.; Tseng, W.K.; Huang, J.S.; Chen, C.H.; Chou, W.C.; Wang, C.H.; Yeh, K.Y. Relationship between pre-treatment nutritional status, serum glutamine, arginine levels and clinicopathological features in Taiwan colorectal cancer patients. Asia Pac. J. Clin. Nutr. 2015, 24, 598–604. [Google Scholar] [CrossRef]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The official positions of the international society for clinical densitometry: Acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, H.M.; Pang, Y.P.; Yeh, K.Y. Early nutritional support in non-metastatic stage IV oral cavity cancer patients undergoing adjuvant concurrent chemoradiotherapy: Analysis of treatment tolerance and outcome in an area endemic for betel quid chewing. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2012, 20, 1169–1174. [Google Scholar] [CrossRef]
- Frank, M.P.; Powers, R.W. Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 852, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Pappa-Louisi, A.; Nikitas, P.; Agrafiotou, P.; Papageorgiou, A. Optimization of separation and detection of 6-aminoquinolyl derivatives of amino acids by using reversed-phase liquid chromatography with on line UV, fluorescence and electrochemical detection. Anal. Chim. Acta 2007, 593, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, A.; Torres-Lagares, D.; Robles-Garcia, M.; Pachon-Ibanez, J.; Gonzalez-Padilla, D.; Gutierrez-Perez, J.L. Cancer treatment-induced oral mucositis: A critical review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Keefe, D.M.; Rassias, G.; O’Neil, L.; Gibson, R.J. Severe mucositis: How can nutrition help? Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 627–631. [Google Scholar] [CrossRef]
- Hunter, M.; Kellett, J.; Toohey, K.; D’Cunha, N.M.; Isbel, S.; Naumovski, N. Toxicities caused by head and neck cancer treatments and their influence on the development of malnutrition: Review of the literature. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 935–949. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Zhao, Q.; Zhang, Y.; Liu, S.; Liu, Z.; Meng, L.; Xin, Y.; Jiang, X. The effects of early nutritional intervention on oral mucositis and nutritional status of patients with head and neck cancer treated with radiotherapy. Front. Oncol. 2020, 10, 595632. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Brody, R.; Touger-Decker, R.; Parrott, J.S.; Epstein, J. Feeding tube use in patients with head and neck cancer. Head Neck 2014, 36, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support. Care Cancer 2010, 18, 837–845. [Google Scholar] [CrossRef]
- Valentini, V.; Marazzi, F.; Bossola, M.; Micciche, F.; Nardone, L.; Balducci, M.; Dinapoli, N.; Bonomo, P.; Autorino, R.; Silipigni, S.; et al. Nutritional counselling and oral nutritional supplements in head and neck cancer patients undergoing chemoradiotherapy. J. Hum. Nutr. Diet. 2012, 25, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef]
- Chen, A.M.; Li, B.Q.; Lau, D.H.; Farwell, D.G.; Luu, Q.; Stuart, K.; Newman, K.; Purdy, J.A.; Vijayakumar, S. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1026–1032. [Google Scholar] [CrossRef]
- Nugent, B.; Parker, M.J.; McIntyre, I.A. Nasogastric tube feeding and percutaneous endoscopic gastrostomy tube feeding in patients with head and neck cancer. J. Hum. Nutr. Diet. 2010, 23, 277–284. [Google Scholar] [CrossRef]
- Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Larsson, S.; Hammerlid, E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: A randomized study. Head Neck 2012, 34, 1–9. [Google Scholar] [CrossRef]
- Williams, G.F.; Teo, M.T.; Sen, M.; Dyker, K.E.; Coyle, C.; Prestwich, R.J. Enteral feeding outcomes after chemoradiotherapy for oropharynx cancer: A role for a prophylactic gastrostomy? Oral Oncol. 2012, 48, 434–440. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Batalova, A.A.; Goncharov, N.V. Serum Albumin. Encyclopedia 2021, 1, 65–75. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The universal soldier: Enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Dubois, M.J.; Orellana-Jimenez, C.; Melot, C.; De Backer, D.; Berre, J.; Leeman, M.; Brimioulle, S.; Appoloni, O.; Creteur, J.; Vincent, J.L. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit. Care Med. 2006, 34, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16 (Suppl. S5), 6–11. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Margarson, M.P.; Mumby, S.; Evans, T.W.; Gutteridge, J.M. Administration of albumin to patients with sepsis syndrome: A possible beneficial role in plasma thiol repletion. Clin. Sci. 1998, 95, 459–465. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Mumby, S.; Martin, G.S.; Bernard, G.R.; Gutteridge, J.M.; Evans, T.W. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit. Care Med. 2004, 32, 755–759. [Google Scholar] [CrossRef]
- Soejima, A.; Matsuzawa, N.; Miyake, N.; Karube, M.; Fukuoka, K.; Nakabayashi, K.; Kitamoto, K.; Nagasawa, T. Hypoalbuminemia accelerates erythrocyte membrane lipid peroxidation in chronic hemodialysis patients. Clin. Nephrol. 1999, 51, 92–97. [Google Scholar]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Yang, C.-Y.; Lin, Y.-C.; Lin, C.-H.; Kuo, C.-S.; Li, Y.-H.; Chen, Y.-W. Pretreatment body mass index and serum uric acid and albumin levels as prognostic predictors in patients with oral squamous cell carcinoma. J. Med. Sci. 2021, 41, 295–304. [Google Scholar] [CrossRef]
- Lin, H.; Lin, H.X.; Ge, N.; Wang, H.Z.; Sun, R.; Hu, W.H. Plasma uric acid and tumor volume are highly predictive of outcome in nasopharyngeal carcinoma patients receiving intensity modulated radiotherapy. Radiat. Oncol. 2013, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Sun, X.; Jones, H., Jr.; Martin, V.I.; Joles, J.A.; Tsukamoto, H.; Couser, W.G.; al-Bander, H. Non-iron mediated alteration in hepatic transferrin gene expression in the nephrotic rat. Kidney Int. 1995, 47, 1068–1077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morlese, J.F.; Forrester, T.; Del Rosario, M.; Frazer, M.; Jahoor, F. Transferrin kinetics are altered in children with severe protein-energy malnutrition. J. Nutr. 1997, 127, 1469–1474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tessari, P. Nonessential amino acid usage for protein replenishment in humans: A method of estimation. Am. J. Clin. Nutr. 2019, 110, 255–264. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Son, D.O.; Satsu, H.; Shimizu, M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005, 579, 4671–4677. [Google Scholar] [CrossRef]
- Peterson, J.W.; King, D.; Ezell, E.L.; Rogers, M.; Gessell, D.; Hoffpauer, J.; Reuss, L.; Chopra, A.K.; Gorenstein, D. Cholera toxin-induced PGE(2) activity is reduced by chemical reaction with L-histidine. Biochim. Biophys. Acta 2001, 1537, 27–41. [Google Scholar] [CrossRef]
- Tanley, S.W.; Helliwell, J.R. Structural dynamics of cisplatin binding to histidine in a protein. Struct. Dyn. 2014, 1, 034701. [Google Scholar] [CrossRef] [PubMed]
- Kriengsinyos, W.; Rafii, M.; Wykes, L.J.; Ball, R.O.; Pencharz, P.B. Long-term effects of histidine depletion on whole-body protein metabolism in healthy adults. J. Nutr. 2002, 132, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D. Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009, 121, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Li, D.; Fu, L.; Zhang, X.; Bao, Y.; Zheng, L. Review of the correlation of LAT1 with diseases: Mechanism and treatment. Front. Chem. 2020, 8, 564809. [Google Scholar] [CrossRef]
- Kim, D.K.; Ahn, S.G.; Park, J.C.; Kanai, Y.; Endou, H.; Yoon, J.H. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in oral squamous cell carcinoma and its precusor lesions. Anticancer Res. 2004, 24, 1671–1675. [Google Scholar]
- Lichter-Konecki, U.; Hipke, C.M.; Konecki, D.S. Human phenylalanine hydroxylase gene expression in kidney and other nonhepatic tissues. Mol. Genet. Metab. 1999, 67, 308–316. [Google Scholar] [CrossRef]
- Morrison, L.; Laukkanen, J.A.; Ronkainen, K.; Kurl, S.; Kauhanen, J.; Toriola, A.T. Inflammatory biomarker score and cancer: A population-based prospective cohort study. BMC Cancer 2016, 16, 80. [Google Scholar] [CrossRef]
- Gulcin, I. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef]
- van Overveld, F.W.; Haenen, G.R.; Rhemrev, J.; Vermeiden, J.P.; Bast, A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem. Biol. Interact. 2000, 127, 151–161. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Romero, L.; Garry, P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996, 64, 552–558. [Google Scholar] [CrossRef]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower serum albumin concentration and change in muscle mass: The health, aging and body composition study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.; Chothia, C. Haemoglobin: The structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979, 129, 175–220. [Google Scholar] [CrossRef]
- Cummings, A.J.; Flynn, F.V. Amino acid composition of serum proteins in health and disease. J. Clin. Pathol. 1955, 8, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubrak, C.; Olson, K.; Jha, N.; Scrimger, R.; Parliament, M.; McCargar, L.; Koski, S.; Baracos, V.E. Clinical determinants of weight loss in patients receiving radiation and chemoirradiation for head and neck cancer: A prospective longitudinal view. Head Neck 2013, 35, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef]
- Fearon, K.C.; Falconer, J.S.; Slater, C.; McMillan, D.C.; Ross, J.A.; Preston, T. Albumin synthesis rates are not decreased in hypoalbuminemic cachectic cancer patients with an ongoing acute-phase protein response. Ann. Surg. 1998, 227, 249–254. [Google Scholar] [CrossRef]
- Moro, J.; Tome, D.; Schmidely, P.; Demersay, T.C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Histidine metabolism and function. J. Nutr. 2020, 150, 2570S–2575S. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the tyrosine isomers meta-tyrosine and ortho-tyrosine in oxidative stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef]
- Neurauter, G.; Grahmann, A.V.; Klieber, M.; Zeimet, A.; Ledochowski, M.; Sperner-Unterweger, B.; Fuchs, D. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. 2008, 272, 141–147. [Google Scholar] [CrossRef]
- Davis, M.D.; Parniak, M.A.; Kaufman, S.; Kempner, E. The role of phenylalanine in structure-function relationships of phenylalanine hydroxylase revealed by radiation target analysis. Proc. Natl. Acad. Sci. USA 1997, 94, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Parniak, M.A.; Davis, M.; Kaufman, S.; Kempner, E.S. Radiation target analysis indicates that phenylalanine hydroxylase in rat liver extracts is a functional monomer. FEBS Lett. 1999, 449, 49–52. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Nofal, M.; Commisso, C.; Hackett, S.R.; Lu, W.; Grabocka, E.; Vander Heiden, M.G.; Miller, G.; Drebin, J.A.; Bar-Sagi, D.; et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015, 75, 544–553. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Y.; Luo, W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene 2020, 39, 6747–6756. [Google Scholar] [CrossRef]
- Iwasa, M.; Kobayashi, Y.; Mifuji-Moroka, R.; Hara, N.; Miyachi, H.; Sugimoto, R.; Tanaka, H.; Fujita, N.; Gabazza, E.C.; Takei, Y. Branched-chain amino acid supplementation reduces oxidative stress and prolongs survival in rats with advanced liver cirrhosis. PLoS ONE 2013, 8, e70309. [Google Scholar] [CrossRef]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-induced skeletal muscle dysfunction: Mechanisms and counteracting therapeutic strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef]
- Mahoney, S.J.; Narayan, S.; Molz, L.; Berstler, L.A.; Kang, S.A.; Vlasuk, G.P.; Saiah, E. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nat. Commun. 2018, 9, 548. [Google Scholar] [CrossRef]
- Cheng, M.L.; Wang, C.H.; Shiao, M.S.; Liu, M.H.; Huang, Y.Y.; Huang, C.Y.; Mao, C.T.; Lin, J.F.; Ho, H.Y.; Yang, N.I. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J. Am. Coll. Cardiol. 2015, 65, 1509–1520. [Google Scholar] [CrossRef]
- Enko, D.; Moro, T.; Holasek, S.; Baranyi, A.; Schnedl, W.J.; Zelzer, S.; Mangge, H.; Herrmann, M.; Meinitzer, A. Branched-chain amino acids are linked with iron metabolism. Ann. Transl. Med. 2020, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Knight, Z.A.; Schmidt, S.F.; Birsoy, K.; Tan, K.; Friedman, J.M. A critical role for mTORC1 in erythropoiesis and anemia. eLife 2014, 3, e01913. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2020, 8, 603837. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Wallace, H.M. Induction of spermidine/spermine N1-acetyltransferase in human cancer cells in response to increased production of reactive oxygen species. Biochem. Pharmacol. 1998, 55, 1119–1123. [Google Scholar] [CrossRef]
- Jell, J.; Merali, S.; Hensen, M.L.; Mazurchuk, R.; Spernyak, J.A.; Diegelman, P.; Kisiel, N.D.; Barrero, C.; Deeb, K.K.; Alhonen, L.; et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J. Biol. Chem. 2007, 282, 8404–8413. [Google Scholar] [CrossRef]
- Kramer, D.L.; Diegelman, P.; Jell, J.; Vujcic, S.; Merali, S.; Porter, C.W. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J. Biol. Chem. 2008, 283, 4241–4251. [Google Scholar] [CrossRef]
- Pirinen, E.; Gylling, H.; Itkonen, P.; Yaluri, N.; Heikkinen, S.; Pietila, M.; Kuulasmaa, T.; Tusa, M.; Cerrada-Gimenez, M.; Pihlajamaki, J.; et al. Activated polyamine catabolism leads to low cholesterol levels by enhancing bile acid synthesis. Amino Acids 2010, 38, 549–560. [Google Scholar] [CrossRef]
- Di Fiore, A.; Lecleire, S.; Gangloff, A.; Rigal, O.; Benyoucef, A.; Blondin, V.; Sefrioui, D.; Quiesse, M.; Iwanicki-Caron, I.; Michel, P.; et al. Impact of nutritional parameter variations during definitive chemoradiotherapy in locally advanced oesophageal cancer. Dig. Liver Dis. 2014, 46, 270–275. [Google Scholar] [CrossRef]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef]
- Kayauchi, N.; Nakagawa, Y.; Oteki, T.; Kagohashi, K.; Satoh, H. Change in Body Weight and Serum Albumin Levels in Febrile Neutropenic Lung Cancer Patients. Asian Pac. Isl. Nurs. J. 2020, 5, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Han, Y.; Kim, D.; Cho, S.; Kim, W.; Hwang, H.; Lee, H.W.; Han, D.H.; Kim, K.S.; Yun, M.; et al. Impact of Exogenous Treatment with Histidine on Hepatocellular Carcinoma Cells. Cancers 2022, 14, 1205. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Umezawa, R.; Takahashi, N.; Matsushita, H.; Kozumi, M.; Ishikawa, Y.; Yamamoto, T.; Takeda, K.; Jingu, K. Impact of change in serum albumin level during and after chemoradiotherapy in patients with locally advanced esophageal cancer. Esophagus 2018, 15, 190–197. [Google Scholar] [CrossRef]
- Tayek, J.A.; Bistrian, B.R.; Hehir, D.J.; Martin, R.; Moldawer, L.L.; Blackburn, G.L. Improved protein kinetics and albumin synthesis by branched chain amino acid-enriched total parenteral nutrition in cancer cachexia. A prospective randomized crossover trial. Cancer 1986, 58, 147–157. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).