Impact of Lung Metastasis versus Metastasis of Bone, Brain, or Liver on Overall Survival and Thyroid Cancer-Specific Survival of Thyroid Cancer Patients: A Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Primary Outcomes
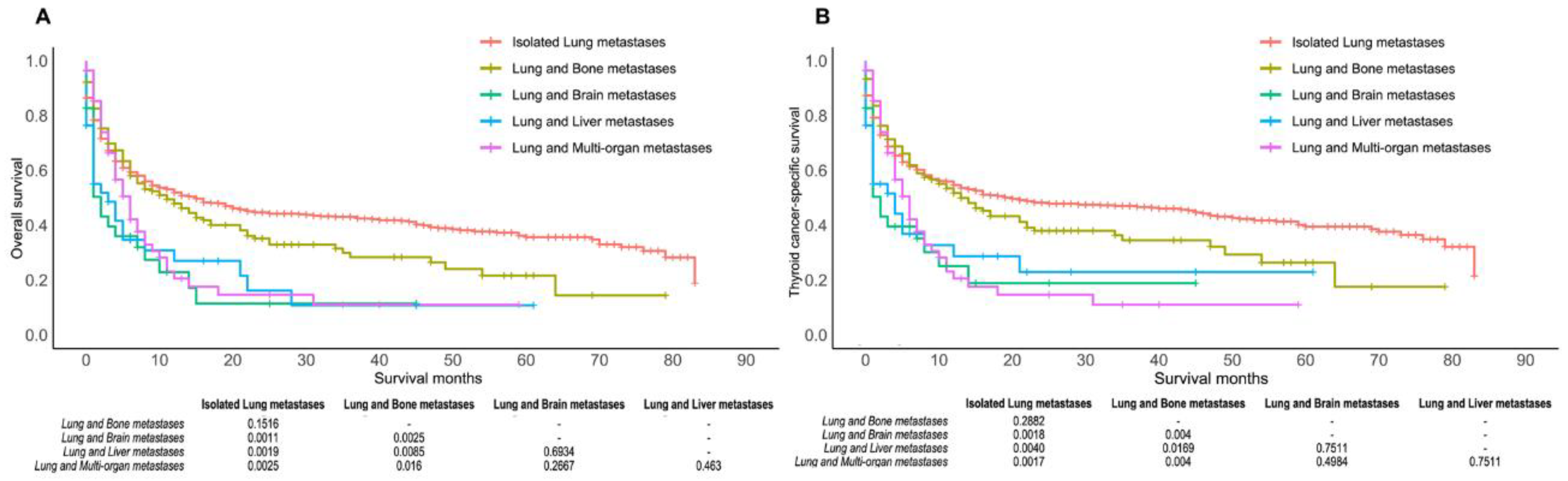
3.3. Secondary Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Observatory. Available online: who.int/gho/database/en/ (accessed on 29 May 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, J.M.; Mallone, S.; van Dijk, B.; Bielska-Lasota, M.; Otter, R.; Foschi, R.; Baudin, E.; Links, T.P. Carcinoma of endocrine organs: Results of the RARECARE project. Eur. J. Cancer 2012, 48, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hong, H.; Han, J.; Leng, S.X.; Zhang, H.; Yan, X. Comparison of Survival and Risk Factors of Differentiated Thyroid Cancer in the Geriatric Population. Front. Oncol. 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Thyroid Carcinoma; Version 3.2021; National Comprehensive Cancer Network: Fort Washington, PA, USA, 2021. [Google Scholar]
- Yu, X.M.; Wan, Y.; Sippel, R.S.; Chen, H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann. Surg. 2011, 254, 653–660. [Google Scholar] [CrossRef]
- Lang, B.H.; Lo, C.Y.; Chan, W.F.; Lam, K.Y.; Wan, K.Y. Prognostic factors in papillary and follicular thyroid carcinoma: Their implications for cancer staging. Ann. Surg. Oncol. 2007, 14, 730–738. [Google Scholar] [CrossRef]
- Sampson, E.; Brierley, J.D.; Le, L.W.; Rotstein, L.; Tsang, R.W. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 2007, 110, 1451–1456. [Google Scholar] [CrossRef]
- Ito, Y.; Kudo, T.; Kobayashi, K.; Miya, A.; Ichihara, K.; Miyauchi, A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: Analysis of 5768 patients with average 10-year follow-up. World J. Surg. 2012, 36, 1274–1278. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Mechlis-Frish, S.; Hirsch, D. Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J. Surg. 2006, 30, 1088–1095. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jeon, M.J.; Kim, M.; Hong, A.R.; Kim, H.K.; Shin, D.Y.; Kim, B.H.; Kim, W.B.; Shong, Y.K.; Kang, H.C. Unusual metastases from differentiated thyroid cancers: A multicenter study in Korea. PLoS ONE 2020, 15, e0238207. [Google Scholar] [CrossRef] [PubMed]
- Ohkuwa, K.; Sugino, K.; Nagahama, M.; Kitagawa, W.; Matsuzu, K.; Suzuki, A.; Tomoda, C.; Hames, K.; Akaishi, J.; Masaki, C.; et al. Risk stratification in differentiated thyroid cancer with RAI-avid lung metastases. Endocr. Connect. 2021, 10, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Kim, H.I.; Kim, Y.N.; Kim, T.H.; Kim, S.W.; Chung, J.H. Prognostic indicators of outcomes in patients with lung metastases from differentiated thyroid carcinoma during long-term follow-up. Clin. Endocrinol. 2018, 88, 318–326. [Google Scholar] [CrossRef]
- Yousuf, K.; Archibald, S.D. Brain metastases from papillary adenocarcinoma of the thyroid. J. Otolaryngol. 2006, 35, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, K.; Raffel, A.; Ramp, U.; Wolf, A.; Donner, A.; Krausch, M.; Eisenberger, C.F.; Knoefel, W.T. Synchronous occurrence of a follicular, papillary and medullary thyroid carcinoma in a recurrent goiter. Endocr. J. 2005, 52, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Erickson, L.A.; Jin, L.; Nakamura, N.; Bridges, A.G.; Markovic, S.N.; Lloyd, R.V. Clinicopathologic features and BRAF(V600E) mutation analysis in cutaneous metastases from well-differentiated thyroid carcinomas. Cancer 2007, 109, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Brogioni, S.; Viacava, P.; Tomisti, L.; Martino, E.; Macchia, E. A special case of bilateral ovarian metastases in a woman with papillary carcinoma of the thyroid. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2007, 115, 397–400. [Google Scholar] [CrossRef]
- Aïssaoui, R.; Turki, Z.; Achiche, A.; Balti, M.H.; Ben Slama, C.; Zbiba, M. Adrenal metastasis of a papillary thyroid cancer. Ann. D’endocrinol. 2006, 67, 364–367. [Google Scholar] [CrossRef]
- Khang, A.R.; Cho, S.W.; Choi, H.S.; Ahn, H.Y.; Yoo, W.S.; Kim, K.W.; Kang, K.W.; Yi, K.H.; Park, D.J.; Lee, D.S.; et al. The risk of second primary malignancy is increased in differentiated thyroid cancer patients with a cumulative (131)I dose over 37 GBq. Clin. Endocrinol. 2015, 83, 117–123. [Google Scholar] [CrossRef]
- Cho, S.W.; Choi, H.S.; Yeom, G.J.; Lim, J.A.; Moon, J.H.; Park, D.J.; Chung, J.K.; Cho, B.Y.; Yi, K.H.; Park, Y.J. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid Off. J. Am. Thyroid Assoc. 2014, 24, 277–286. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Ren, Y.; Dai, C.; Zheng, H.; Zhou, F.; She, Y.; Jiang, G.; Fei, K.; Yang, P.; Xie, D.; Chen, C. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 2016, 7, 53245–53253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, R.K.; Brewer, T.M.; Theriault, R.L.; Ueno, N.T. Bone Metastasis of Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 105–129. [Google Scholar] [CrossRef]
- Thureau, S.; Marchesi, V.; Vieillard, M.H.; Perrier, L.; Lisbona, A.; Leheurteur, M.; Tredaniel, J.; Culine, S.; Dubray, B.; Bonnet, N.; et al. Efficacy of extracranial stereotactic body radiation therapy (SBRT) added to standard treatment in patients with solid tumors (breast, prostate and non-small cell lung cancer) with up to 3 bone-only metastases: Study protocol for a randomised phase III trial (STEREO-OS). BMC Cancer 2021, 21, 117. [Google Scholar] [CrossRef]
- Qutbi, M.; Shafeie, B.; Amoui, M.; Tabeie, F.; Azizmohammadi, Z.; Mahmoud-Pashazadeh, A.; Javadi, H.; Assadi, M.; Asli, I.N. Evaluation of Prognostic Factors Associated With Differentiated Thyroid Carcinoma With Pulmonary Metastasis. Clin. Nucl. Med. 2016, 41, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Hussein, M.H.; Zerfaoui, M.; Attia, A.S.; Marzouk Ellythy, A.; Mostafa, A.; Ruiz, E.M.L.; Shama, M.A.; Russell, J.O.; Randolph, G.W.; et al. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Daniels, G.H. Follicular Variant of Papillary Thyroid Carcinoma: Hybrid or Mixture? Thyroid Off. J. Am. Thyroid Assoc. 2016, 26, 872–874. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.M.; Schneider, D.F.; Leverson, G.; Chen, H.; Sippel, R.S. Follicular variant of papillary thyroid carcinoma is a unique clinical entity: A population-based study of 10,740 cases. Thyroid Off. J. Am. Thyroid Assoc. 2013, 23, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Passler, C.; Prager, G.; Scheuba, C.; Niederle, B.E.; Kaserer, K.; Zettinig, G.; Niederle, B. Follicular variant of papillary thyroid carcinoma: A long-term follow-up. Arch. Surg. 2003, 138, 1362–1366. [Google Scholar] [CrossRef]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid Off. J. Am. Thyroid Assoc. 2019, 29, 311–321. [Google Scholar] [CrossRef]
- Limaiem, F.; Kashyap, S.; Naing, P.T.; Giwa, A.O. Anaplastic Thyroid Cancer; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Treasure, T.; Milošević, M.; Fiorentino, F.; Macbeth, F. Pulmonary metastasectomy: What is the practice and where is the evidence for effectiveness? Thorax 2014, 69, 946–949. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, U.; Buyse, M.; Friedel, G.; Ginsberg, R.J.; Girard, P.; Goldstraw, P.; Johnston, M.; McCormack, P.; Pass, H.; Putnam, J.B., Jr. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 1997, 113, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Jee, Y.S. Solitary metastasis of myxoid liposarcoma from the thigh to intraperitoneum: A case report. World J. Surg. Oncol. 2019, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Le, U.T.; Bronsert, P.; Picardo, F.; Riethdorf, S.; Haager, B.; Rylski, B.; Czerny, M.; Beyersdorf, F.; Wiesemann, S.; Pantel, K.; et al. Intraoperative detection of circulating tumor cells in pulmonary venous blood during metastasectomy for colorectal lung metastases. Sci. Rep. 2018, 8, 8751. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [Green Version]
- Harari, A.; Li, N.; Yeh, M.W. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Weeks, K.S.; Kahl, A.R.; Lynch, C.F.; Charlton, M.E. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007–2014. Cancer 2018, 124, 1483–1491. [Google Scholar] [CrossRef]
- Asban, A.; Chung, S.K.; Xie, R.; Lindeman, B.M.; Balentine, C.J.; Kirklin, J.K.; Chen, H. Gender and Racial Disparities in Survival After Surgery Among Papillary and Patients With Follicular Thyroid Cancer: A 45-Year Experience. Clin. Med. Insights. Endocrinol. Diabetes 2019, 12, 117955141986619. [Google Scholar] [CrossRef]
- Rubinstein, J.C.; Herrick-Reynolds, K.; Dinauer, C.; Morotti, R.; Solomon, D.; Callender, G.G.; Christison-Lagay, E.R. Recurrence and Complications in Pediatric and Adolescent Papillary Thyroid Cancer in a High-Volume Practice. J. Surg. Res. 2020, 249, 58–66. [Google Scholar] [CrossRef]
- Tang, J.; Kong, D.; Cui, Q.; Wang, K.; Zhang, D.; Liao, X.; Gong, Y.; Wu, G. Racial disparities of differentiated thyroid carcinoma: Clinical behavior, treatments, and long-term outcomes. World J. Surg. Oncol. 2018, 16, 45. [Google Scholar] [CrossRef]
- Suresh, R.; Sethi, S.; Ali, S.; Giorgadze, T.; Sarkar, F.H. Differential Expression of MicroRNAs in Papillary Thyroid Carcinoma and Their Role in Racial Disparity. J. Cancer Sci. Ther. 2015, 7, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef] [PubMed]



| Variable | Lung Metastasis from Thyroid Cancer | p | ||
|---|---|---|---|---|
| Yes | No | All | ||
| (n = 998) | (n = 76,324) | (n = 77,322) | ||
| Age | <0.0001 | |||
| ≥55 | 249 (24.9%) | 50,765 (66.5%) | 51,014 (66.0%) | |
| <55 | 749 (75.1%) | 25,559 (33.5%) | 26,308 (34.0%) | |
| Race | <0.0001 | |||
| White | 745 (74.7%) | 60,807 (79.7%) | 61,552 (79.6%) | |
| Black | 100 (10.0%) | 5413 (7.1%) | 5513 (7.1%) | |
| Unknown | 4 (0.4%) | 1308 (1.7%) | 1312 (1.7%) | |
| Others * | 149 (14.9%) | 8796 (11.5%) | 8945 (11.6%) | |
| Sex | <0.0001 | |||
| Male | 458 (45.9%) | 17,547 (23.0%) | 18,005 (23.3%) | |
| Female | 540 (54.1%) | 58,777 (77.0%) | 59,317 (76.7%) | |
| Married | 0.5408 | |||
| Married | 4 (0.4%) | 177 (0.2%) | 181 (0.2%) | |
| Unmarried | 994 (99.6%) | 76,145 (99.8%) | 77,139 (99.8%) | |
| Unknown | 0 | 2 (0.0%) | 2 (0.0%) | |
| Grade | <0.0001 | |||
| I | 74 (7.4%) | 14,684 (19.2%) | 14,758 (19.1%) | |
| II | 39 (3.9%) | 2748 (3.6%) | 2787 (3.6%) | |
| III | 104 (10.4%) | 754 (1.0%) | 858 (1.1%) | |
| VI | 295 (29.6%) | 492 (0.6%) | 787 (1.0%) | |
| Unknown | 486 (48.7%) | 57,646 (75.5%) | 58,132 (75.2%) | |
| Histologic ICD-O-3 for thyroid cancer | <0.0001 | |||
| Carcinoma, undiff., NOS | 215 (21.5%) | 327 (0.4%) | 542 (0.7%) | |
| Follicular Adenocarcinoma, NOS | 99 (9.9%) | 3632 (4.8%) | 3731 (4.8%) | |
| Medullary carcinoma, NOS | 39 (3.9%) | 1186 (1.6%) | 1225 (1.6%) | |
| Oxyphilic adenocarcinoma | 28 (2.8%) | 1338 (1.8%) | 1366 (1.8%) | |
| Papillary and follicular adenoca. | 137 (13.7%) | 26,399 (34.6%) | 26,536 (34.3%) | |
| Papillary adenocarcinoma, NOS | 312 (31.3%) | 40,869 (53.6%) | 41,181 (53.3%) | |
| Papillary carcinoma, NOS | 10 (1.0%) | 1686 (2.2%) | 1696 (2.2%) | |
| Others | 158 (15.8%) | 887 (1.2%) | 1045 (1.4%) | |
| Stage group | <0.0001 | |||
| I | 0 | 53,510 (70.1%) | 53,510 (69.2%) | |
| II | 92 (9.2%) | 5540 (7.3%) | 5632 (7.3%) | |
| III | 0 | 9761 (12.8%) | 9761 (12.6%) | |
| VI | 896 (89.8%) | 4989 (6.5%) | 5885 (7.6%) | |
| Unknown | 10 (1.0%) | 2524 (3.3%) | 2534 (3.3%) | |
| T stage | <0.0001 | |||
| T0 | 6 (0.6%) | 107 (0.1%) | 113 (0.2%) | |
| T1 | 47 (4.7%) | 43,527 (57.0%) | 43,574 (56.4%) | |
| T2 | 48 (4.8%) | 12,815 (16.8%) | 12,863 (16.6%) | |
| T3 | 209 (20.9%) | 15,575 (20.4%) | 15,784 (20.4%) | |
| T4 | 560 (56.1%) | 2420 (3.2%) | 2980 (3.9%) | |
| Tx | 128 (12.8%) | 1880 (2.5%) | 2008 (2.6%) | |
| N stage | <0.0001 | |||
| N0 | 279 (28.0%) | 56,099 (73.5%) | 56,378 (72.9%) | |
| N1 | 613 (61.4%) | 18,044 (23.6%) | 18,657 (24.1%) | |
| Nx | 106 (10.6%) | 2181 (2.9%) | 2287 (3.0%) | |
| M stage | <0.0001 | |||
| M0 | 0 | 75,590 (99.0%) | 75,590 (97.8%) | |
| M1 | 988 (99.0%) | 603 (0.8%) | 1591 (2.1%) | |
| Mx | 10 (1.0%) | 131 (0.2%) | 141 (0.2%) | |
| Regional | <0.0001 | |||
| Positive | 407 (40.8%) | 17,729 (23.2%) | 18,136 (23.5%) | |
| Negative | 75 (7.5%) | 23,536 (30.8%) | 23,611 (30.5%) | |
| Unknown | 516 (51.7%) | 35,059 (45.9%) | 35,575 (46.0%) | |
| SP | <0.0001 | |||
| Yes | 543 (54.4%) | 74,221 (97.2%) | 74,764 (96.7%) | |
| No | 454 (45.5%) | 2067 (2.7%) | 2521 (3.3%) | |
| Unknown | 1 (0.1%) | 36 (0.1%) | 37 (0.1%) | |
| SD | <0.0001 | |||
| Yes | 118 (11.8%) | 1025 (1.3%) | 1143 (1.5%) | |
| No | 879 (88.1%) | 75,177 (98.5%) | 76,056 (98.4%) | |
| Unknown | 1 (0.1%) | 122 (0.2%) | 123 (0.2%) | |
| Variable | Overall Survival | Thyroid Cancer-Specific Survival | |||
|---|---|---|---|---|---|
| Hazard Ratio (95%CI) | p | Hazard Ratio (95%CI) | p | ||
| Age | ≥55 | 1.00 (reference) | 1.00 (reference) | ||
| <55 | 0.5205 (0.3845–0.7045) | p < 0.0001 | 0.5645 (0.4126–0.7723) | 0.0003 | |
| Race | White | 1.00 (reference) | 1.00 (reference) | ||
| Black | 0.8355 (0.5649–1.2355) | 0.3678 | 0.7233 (0.4711–1.1106) | 0.1387 | |
| Others | 0.7582 (0.5706–1.0073) | 0.0562 | 0.7166 (0.5305–0.9680) | 0.0298 | |
| Sex | Female | 1.00 (reference) | 1.00 (reference) | ||
| Male | 0.832 (0.0.6792–1.0191) | 0.0756 | 0.7944 (0.6424–0.9825) | 0.0338 | |
| Married | Married | 1.00 (reference) | 1.00 (reference) | ||
| Unmarried | 0.4467 (0.1400–1.4251) | 0.1734 | 0.3828 (0.1198–1.2234) | 0.1053 | |
| T stage | T1 | 1.00 (reference) | 1.00 (reference) | ||
| T0 | - # | - # | - # | - # | |
| T2 | 0.8199 (0.2624–2.5620) | 0.7327 | 0.7737 (0.1919–3.1201) | 0.7184 | |
| T3 | 1.2327 (0.5131–2.9615) | 0.6399 | 1.5424 (0.5375–4.4258) | 0.4204 | |
| T4 | 5.0814 (2.2417–11.5184) | 0.0001 | 7.1042 (2.6239–19.2344) | 0.0001 | |
| Tx | 2.5526 (1.0826–6.0185) | 0.0322 | 3.3817 (1.2042–9.4965) | 0.0207 | |
| N stage | N0 | 1.00 (reference) | 1.00 (reference) | ||
| N1 | 1.1283 (0.8793–1.4476) | 0.3427 | 1.0394 (0.8023–1.3465) | 0.7700 | |
| Nx | 1.3251 (0.9408–1.8663) | 01072 | 1.2687 (0.9034–1.8327) | 0.1624 | |
| SP | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 0.2273 (0.1779–0.2904) | <0.0001 | 0.2294 (0.1776–0.2963) | <0.0001 | |
| SD | No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 0.5713 (0.3763–0.8674) | 0.0086 | 0.5302 (0.3371–0.8347) | 0.0061 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, M.; Khan, F.Z.; He, X.; Cui, L.; Lei, K.; Ge, M. Impact of Lung Metastasis versus Metastasis of Bone, Brain, or Liver on Overall Survival and Thyroid Cancer-Specific Survival of Thyroid Cancer Patients: A Population-Based Study. Cancers 2022, 14, 3133. https://doi.org/10.3390/cancers14133133
Zhong M, Khan FZ, He X, Cui L, Lei K, Ge M. Impact of Lung Metastasis versus Metastasis of Bone, Brain, or Liver on Overall Survival and Thyroid Cancer-Specific Survival of Thyroid Cancer Patients: A Population-Based Study. Cancers. 2022; 14(13):3133. https://doi.org/10.3390/cancers14133133
Chicago/Turabian StyleZhong, Miaochun, Farhana Zerin Khan, Xianghong He, Lingfei Cui, Kefeng Lei, and Minghua Ge. 2022. "Impact of Lung Metastasis versus Metastasis of Bone, Brain, or Liver on Overall Survival and Thyroid Cancer-Specific Survival of Thyroid Cancer Patients: A Population-Based Study" Cancers 14, no. 13: 3133. https://doi.org/10.3390/cancers14133133
APA StyleZhong, M., Khan, F. Z., He, X., Cui, L., Lei, K., & Ge, M. (2022). Impact of Lung Metastasis versus Metastasis of Bone, Brain, or Liver on Overall Survival and Thyroid Cancer-Specific Survival of Thyroid Cancer Patients: A Population-Based Study. Cancers, 14(13), 3133. https://doi.org/10.3390/cancers14133133





