Imaging of Uveal Melanoma—Current Standard and Methods in Development
Abstract
:Simple Summary
Abstract
1. Introduction
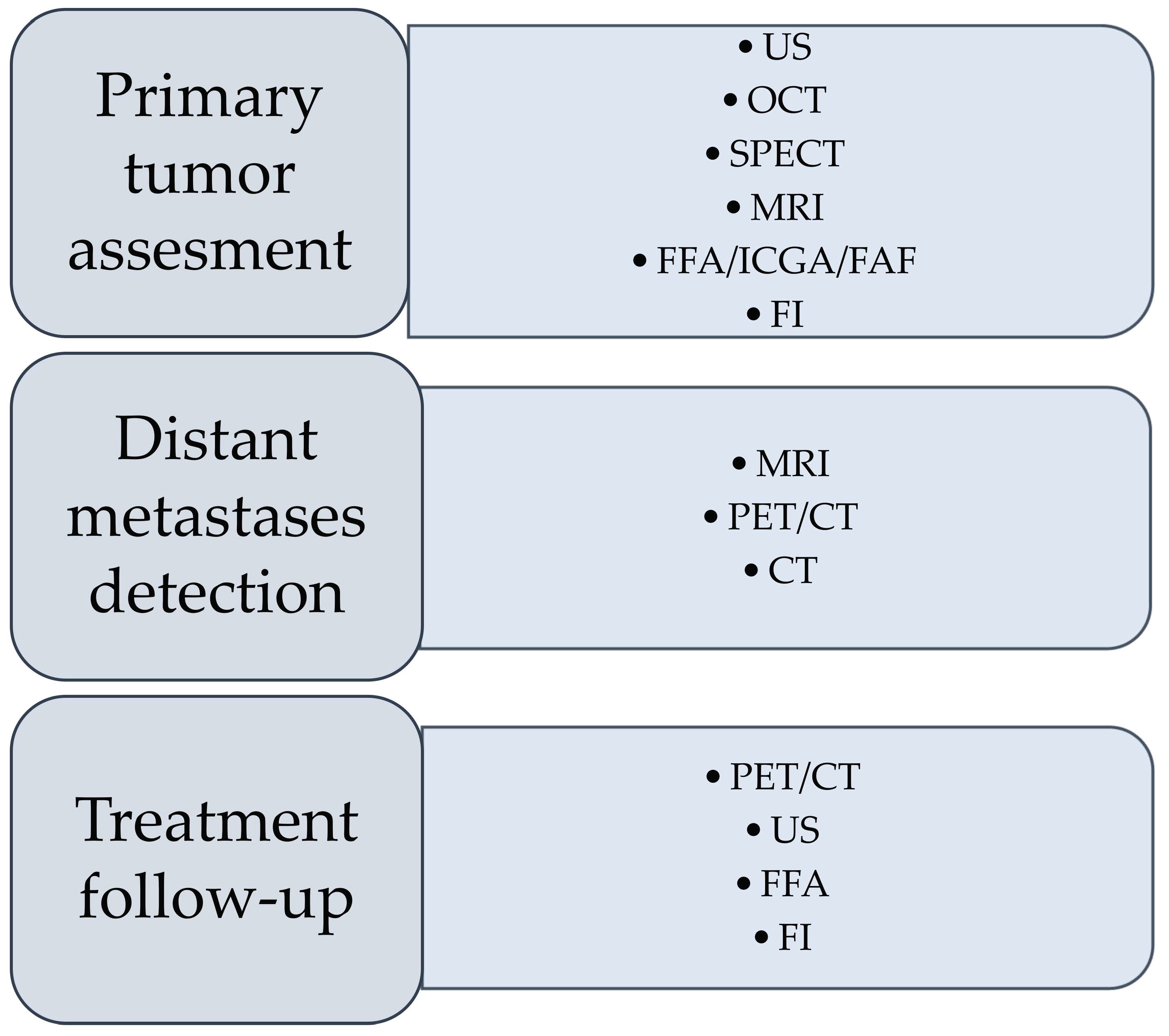
2. Imaging Techniques Currently Used in the Diagnosis of Uveal Melanoma
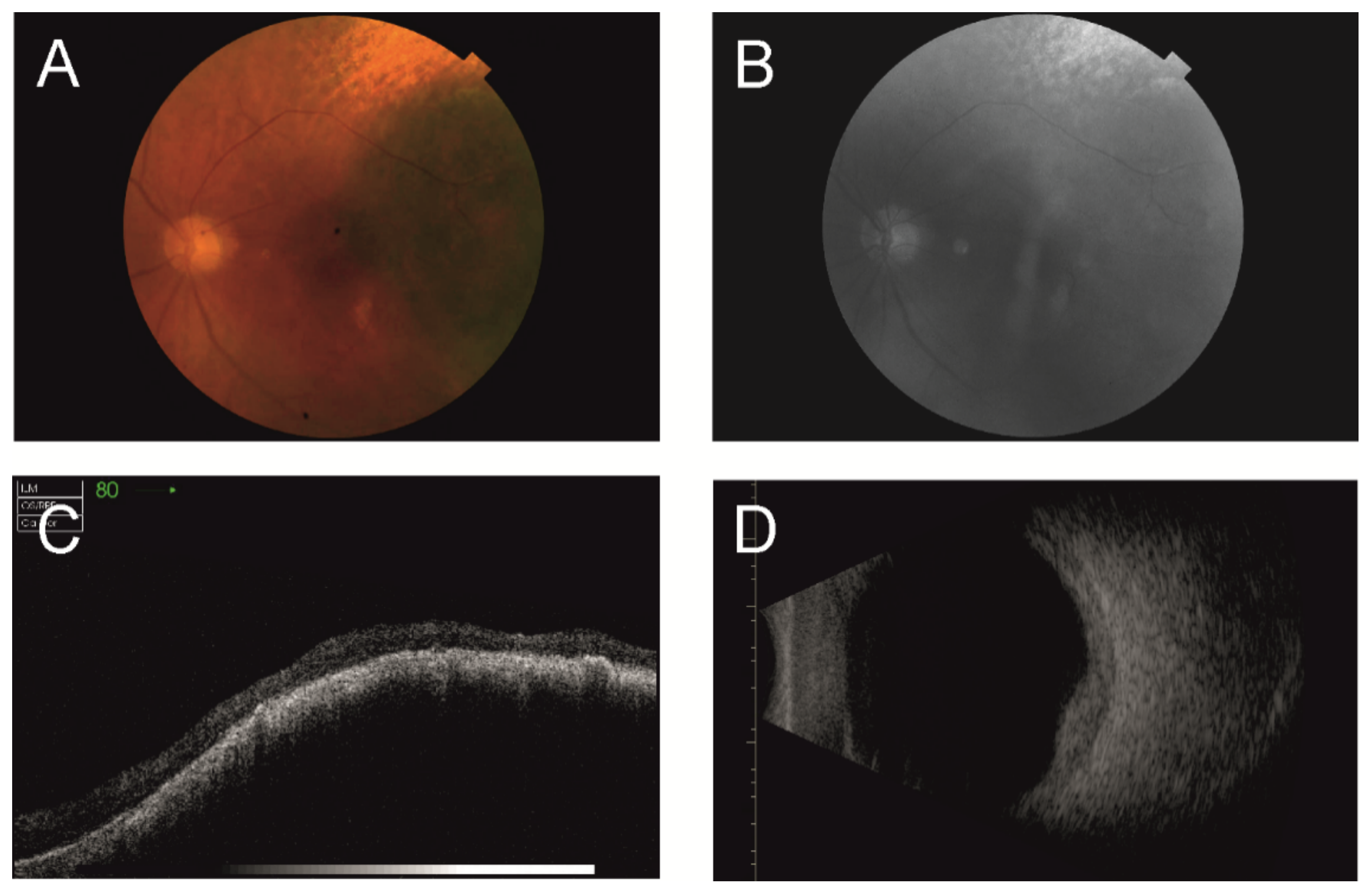
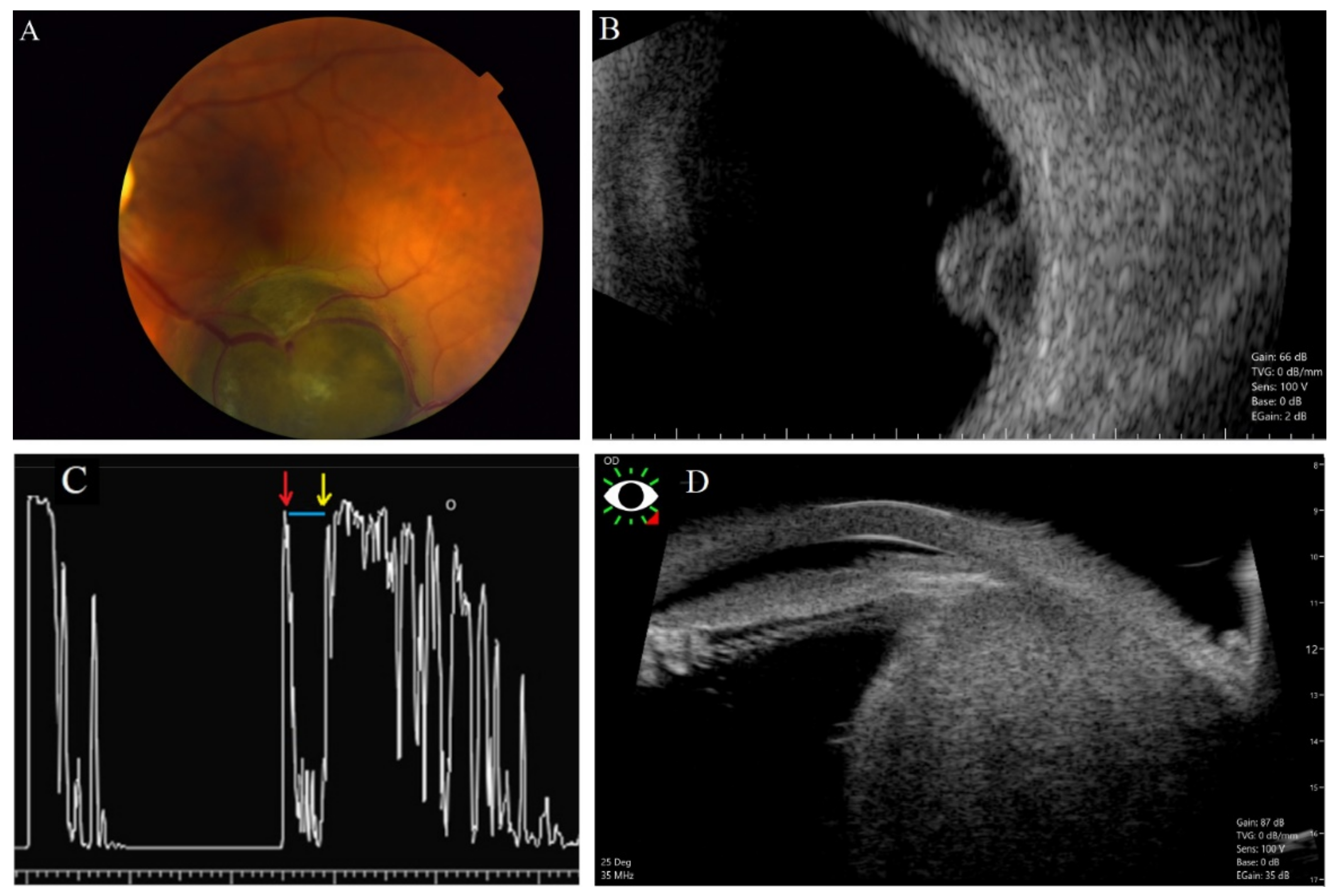
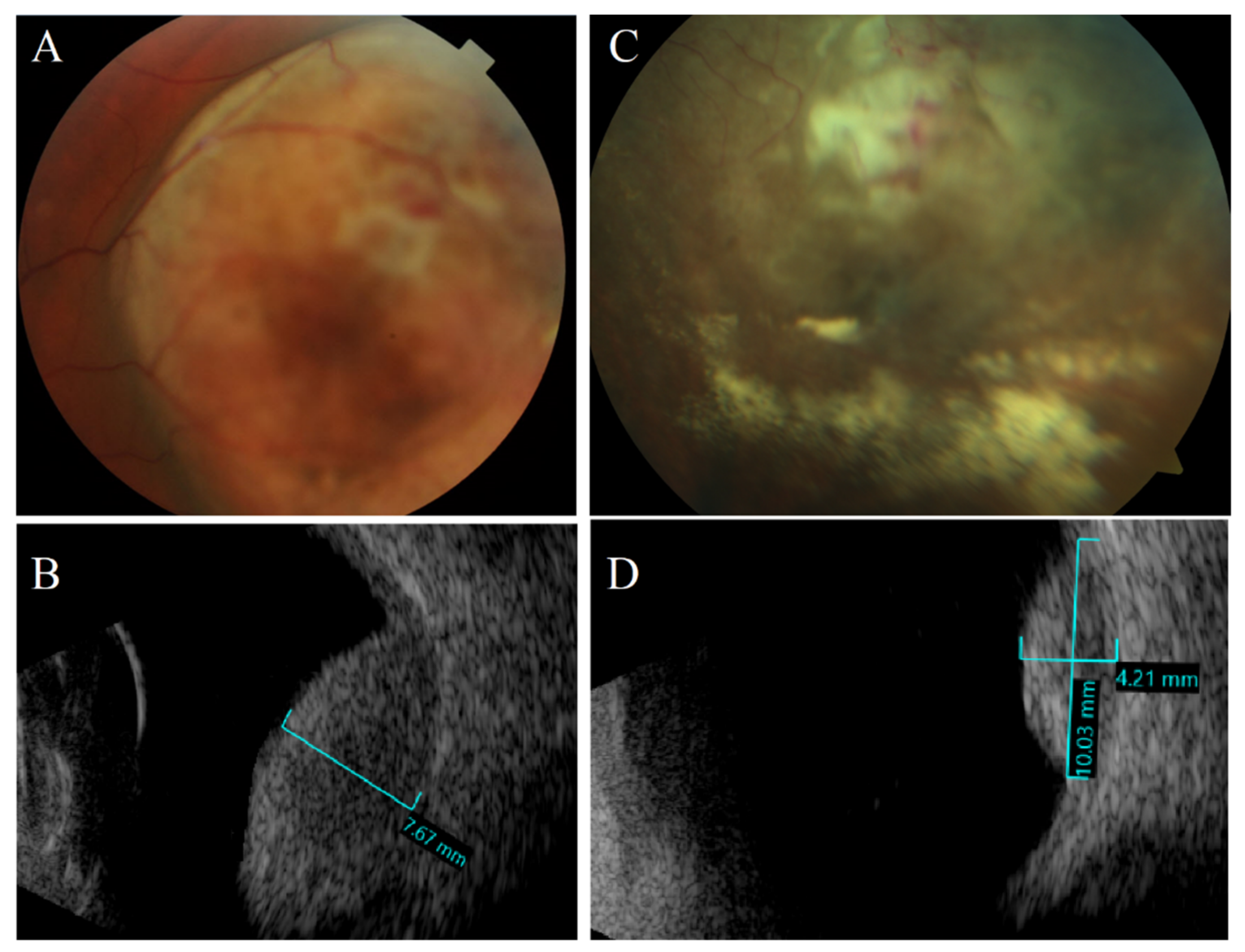
2.1. Ultrasonography
2.2. Optical Coherence Tomography
2.3. Fundus Imaging (FI)
2.4. Fundus Fluorescein Angiography, Indocyanine Green Angiography, and Fundus Autofluorescence
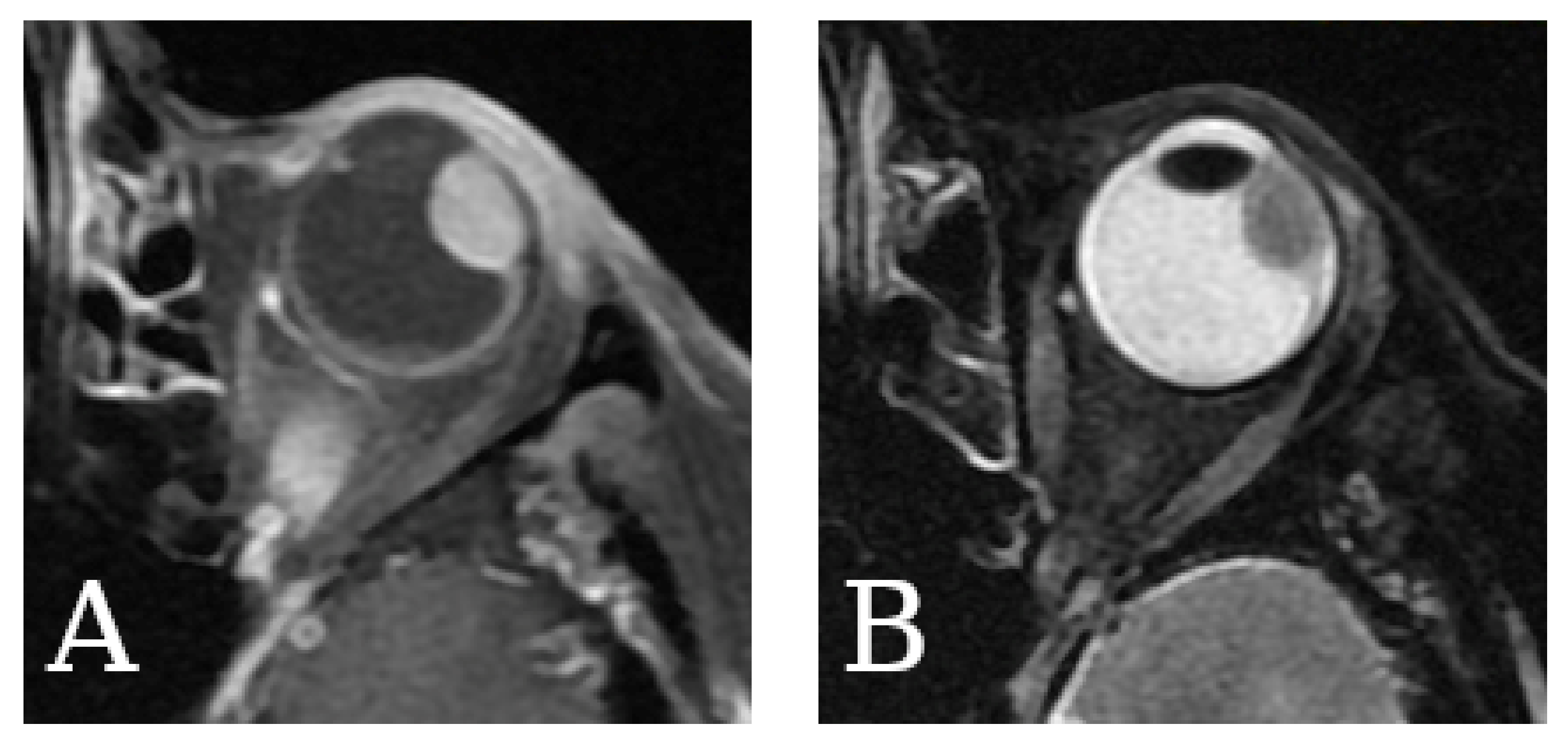
2.5. Magnetic Resonance Imaging
2.6. Computed Tomography
2.7. Single-Photon Emission Computed Tomography
2.8. Positron Emission Tomography/Computed Tomography
3. New and Experimental Imaging Techniques with Potential in UM Diagnostics
3.1. New Radiolabeled Radiotracers in PET
3.2. PET/MRI
3.3. Contrast Agents for MRI
3.4. Hyperpolarized MRI
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2016, 31, 241–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Zielinska, A.; Luis, M.; Carbone, C.; Martins-Gomes, C.; Souto, S.B.; Silva, A.M. Uveal melanoma: Physiopathology and new in situ-specific therapies. Cancer Chemother. Pharmacol. 2019, 84, 15–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogrusöz, M.; Jager, M.J.; Damato, B. Uveal Melanoma Treatment and Prognostication. Asia-Pac. J. Ophthalmol. 2017, 6, 186–196. [Google Scholar] [CrossRef]
- Blum, E.S.; Yang, J.; Komatsubara, K.M.; Carvajal, R.D. Clinical Management of Uveal and Conjunctival Melanoma. Oncology 2016, 30, 29. [Google Scholar]
- Bechrakis, N.E.; Sehu, K.W.; Lee, W.R.; Damato, B.E.; Foerster, M.H. Transformation of cell type in uveal melanomas: A quantitative histologic analysis. Arch. Ophthalmol. 2000, 118, 1406–1412. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.L.; Kaliki, S.; Cohen, M.N.; Shields, P.W.; Furuta, M.; Shields, J.A. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 Doyne Lecture. Eye 2015, 29, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Cormier, J.N.; Xing, Y.; Ding, M.; Lee, J.E.; Mansfield, P.F.; Gershenwald, J.E.; Ross, M.I.; Du, X.L. Ethnic differences among patients with cutaneous melanoma. Arch. Intern. Med. 2006, 166, 1907–1914. [Google Scholar] [CrossRef] [Green Version]
- Lamas, N.J.; Martel, A.; Nahon-Estève, S.; Goffinet, S.; Macocco, A.; Bertolotto, C.; Lassalle, S.; Hofman, P. Prognostic Biomarkers in Uveal Melanoma: The Status Quo, Recent Advances and Future Directions. Cancers 2021, 14, 96. [Google Scholar] [CrossRef]
- Griewank, K.G.; Murali, R. Pathology and genetics of uveal melanoma. Pathol.—J. RCPA 2013, 45, 18–27. [Google Scholar] [CrossRef]
- McLean, I.W.; Foster, W.D.; Zimmerman, L.E.; Gamel, J.W. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am. J. Ophthalmol. 1983, 96, 502–509. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.H.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Shields, J.A. Uveal melanoma: Estimating prognosis. Indian J. Ophthalmol. 2015, 63, 93–102. [Google Scholar] [CrossRef]
- Jovanovic, P.; Mihajlovic, M.; Djordjevic-Jocic, J.; Vlajkovic, S.; Cekic, S.; Stefanovic, V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013, 6, 1230–1244. [Google Scholar]
- Hu, D.N.; Yu, G.P.; McCormick, S.A.; Schneider, S.; Finger, P.T. Population-based incidence of uveal melanoma in various races and ethnic groups. Am. J. Ophthalmol. 2005, 140, 612–617. [Google Scholar] [CrossRef]
- Frenkel, S.; Hendler, K.; Pe’er, J. Uveal melanoma in Israel in the last two decades: Characterization, treatment and prognosis. Isr. Med. Assoc. J. 2009, 11, 280–285. [Google Scholar]
- Weis, E.; Shah, C.P.; Lajous, M.; Shields, J.A.; Shields, C.L. The association between host susceptibility factors and uveal melanoma: A meta-analysis. Arch. Ophthalmol. 2006, 124, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Nayman, T.; Bostan, C.; Logan, P.; Burnier, M.N., Jr. Uveal Melanoma Risk Factors: A Systematic Review of Meta-Analyses. Curr. Eye Res. 2017, 42, 1085–1093. [Google Scholar] [CrossRef]
- Shah, C.P.; Weis, E.; Lajous, M.; Shields, J.A.; Shields, C.L. Intermittent and chronic ultraviolet light exposure and uveal melanoma: A meta-analysis. Ophthalmology 2005, 112, 1599–1607. [Google Scholar] [CrossRef]
- Holly, E.A.; Aston, D.A.; Ahn, D.K.; Smith, A.H. Intraocular melanoma linked to occupations and chemical exposures. Epidemiology 1996, 7, 55–61. [Google Scholar] [CrossRef]
- Derrien, A.C.; Rodrigues, M.; Eeckhoutte, A.; Dayot, S.; Houy, A.; Mobuchon, L.; Gardrat, S.; Lequin, D.; Ballet, S.; Pierron, G.; et al. Germline MBD4 Mutations and Predisposition to Uveal Melanoma. J. Natl. Cancer Inst. 2021, 113, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rahman, M.H.; Sample, K.M.; Pilarski, R.; Walsh, T.; Grosel, T.; Kinnamon, D.; Boru, G.; Massengill, J.B.; Schoenfield, L.; Kelly, B.; et al. Whole Exome Sequencing Identifies Candidate Genes Associated with Hereditary Predisposition to Uveal Melanoma. Ophthalmology 2020, 127, 668–678. [Google Scholar] [CrossRef]
- Shields, C.L.; Dalvin, L.A.; Ancona-Lezama, D.A.; Yu, M.D.; Di Nicola, M.; Williams, B.K.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M.; Welch, R.J.; et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019, 39, 1840–1851. [Google Scholar] [CrossRef]
- Shields, C.L.; Lim, L.S.; Dalvin, L.A.; Shields, J.A. Small choroidal melanoma: Detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr. Opin. Ophthalmol. 2019, 30, 206–214. [Google Scholar] [CrossRef]
- Shields, C.L.; Dalvin, L.A.; Yu, M.D.; Ancona-Lezama, D.; Di Nicola, M.; Williams, B.K.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M.; Welch, R.J.; et al. Choroidal nevus transformation into melanoma per millimeter increment in thickness using multimodal imaging in 2355 cases: The 2019 Wendell L. Hughes Lecture. Retina 2019, 39, 1852–1860. [Google Scholar] [CrossRef]
- Freton, A.; Chin, K.J.; Raut, R.; Tena, L.B.; Kivelä, T.; Finger, P.T. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur. J. Ophthalmol. 2012, 22, 236–243. [Google Scholar] [CrossRef]
- Finger, P.T.; Kurli, M.; Reddy, S.; Tena, L.B.; Pavlick, A.C. Whole body PET/CT for initial staging of choroidal melanoma. Br. J. Ophthalmol. 2005, 89, 1270–1274. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Green, D.L.; Hawkins, B.S.; Hayman, J.; Jaiyesimi, I.; et al. Screening for metastasis from choroidal melanoma: The Collaborative Ocular Melanoma Study Group Report 23. J. Clin. Oncol. 2004, 22, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vidal, C.; Fernandez-Diaz, D.; Fernandez-Marta, B.; Lago-Baameiro, N.; Pardo, M.; Silva, P.; Paniagua, L.; Blanco-Teijeiro, M.J.; Piñeiro, A.; Bande, M. Treatment of Metastatic Uveal Melanoma: Systematic Review. Cancers 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallone, F.; Sacchetti, M.; Lambiase, A.; Moramarco, A. Molecular Insights and Emerging Strategies for Treatment of Metastatic Uveal Melanoma. Cancers 2020, 12, 2761. [Google Scholar] [CrossRef]
- Torres, V.; Triozzi, P.; Eng, C.; Tubbs, R.; Schoenfiled, L.; Crabb, J.W.; Saunthararajah, Y.; Singh, A.D. Circulating tumor cells in uveal melanoma. Future Oncol. 2011, 7, 101–109. [Google Scholar] [CrossRef]
- Amaro, A.; Gangemi, R.; Piaggio, F.; Angelini, G.; Barisione, G.; Ferrini, S.; Pfeffer, U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017, 36, 109–140. [Google Scholar] [CrossRef]
- Neupane, R.; Gaudana, R.; Boddu, S.H.S. Imaging Techniques in the Diagnosis and Management of Ocular Tumors: Prospects and Challenges. AAPS J. 2018, 20, 97. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Zhang, L.; Tang, F.; Wei, X. Application of Multimodal and Molecular Imaging Techniques in the Detection of Choroidal Melanomas. Front. Oncol. 2020, 10, 617868. [Google Scholar] [CrossRef]
- Berus, T.; Halon, A.; Markiewicz, A.; Orlowska-Heitzman, J.; Romanowska-Dixon, B.; Donizy, P. Clinical, Histopathological and Cytogenetic Prognosticators in Uveal Melanoma—A Comprehensive Review. Anticancer Res. 2017, 37, 6541–6549. [Google Scholar] [CrossRef]
- Bustamante, P.; Piquet, L.; Landreville, S.; Burnier, J.V. Uveal melanoma pathobiology: Metastasis to the liver. Semin. Cancer Biol. 2020, 71, 65–85. [Google Scholar] [CrossRef]
- Diener-West, M.; Hawkins, B.S.; Markowitz, J.A.; Schachat, A.P. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch. Ophthalmol 1992, 110, 245–250. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L.; Mashayekhi, A.; Ganesh, A.; Furuta, M.; Shields, J.A. Influence of age on prognosis of young patients with uveal melanoma: A matched retrospective cohort study. Eur. J. Ophthalmol. 2013, 23, 208–216. [Google Scholar] [CrossRef]
- Rietschel, P.; Panageas, K.S.; Hanlon, C.; Patel, A.; Abramson, D.H.; Chapman, P.B. Variates of survival in metastatic uveal melanoma. J. Clin. Oncol. 2005, 23, 8076–8080. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.-F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Bilmin, K.; Synoradzki, K.J.; Czarnecka, A.M.; Spałek, M.J.; Kujawska, T.; Solnik, M.; Merks, P.; Toro, M.D.; Rejdak, R.; Fiedorowicz, M. New Perspectives for Eye-Sparing Treatment Strategies in Primary Uveal Melanoma. Cancers 2021, 14, 134. [Google Scholar] [CrossRef]
- Young, T.A.; Rao, N.P.; Glasgow, B.J.; Moral, J.N.; Straatsma, B.R. Fluorescent in situ hybridization for monosomy 3 via 30-gauge fine-needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology 2007, 114, 142–146. [Google Scholar] [CrossRef]
- McCannel, T.A.; Burgess, B.L.; Rao, N.P.; Nelson, S.F.; Straatsma, B.R. Identification of candidate tumor oncogenes by integrative molecular analysis of choroidal melanoma fine-needle aspiration biopsy specimens. Arch. Ophthalmol. 2010, 128, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Dalvin, L.A.; Shields, C.L.; Ancona-Lezama, D.A.; Yu, M.D.; Di Nicola, M.; Williams, B.K., Jr.; Lucio-Alvarez, J.A.; Ang, S.M.; Maloney, S.M.; Welch, R.J.; et al. Combination of multimodal imaging features predictive of choroidal nevus transformation into melanoma. Br. J. Ophthalmol. 2019, 103, 1441–1447. [Google Scholar] [CrossRef]
- Coupland, S.E. Analysis of intraocular biopsies. Dev. Ophthalmol. 2012, 49, 96–116. [Google Scholar] [CrossRef]
- Shields, J.A.; Mashayekhi, A.; Ra, S.; Shields, C.L. Pseudomelanomas of the posterior uveal tract: The 2006 Taylor R. Smith Lecture. Retina 2005, 25, 767–771. [Google Scholar] [CrossRef]
- Silverman, R.H. Focused ultrasound in ophthalmology. Clin. Ophthalmol. 2016, 10, 1865–1875. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, E.M.; Rodriguez, A.; Garcia, I. Review of ocular ultrasonography. Vet. Radiol. Ultrasound 2001, 42, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Oberg, J.; Spenger, C.; Wang, F.H.; Andersson, A.; Westman, E.; Skoglund, P.; Sunnemark, D.; Norinder, U.; Klason, T.; Wahlund, L.O.; et al. Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiol. Aging 2008, 29, 1423–1433. [Google Scholar] [CrossRef]
- Lin, D.T.; Munk, P.L.; Maberley, A.L.; Cooperberg, P.L.; Rootman, J. Ultrasonography of pathologically proved choroidal melanoma with a high-resolution small-parts scanner. Can. J. Ophthalmol. 1987, 22, 161–164. [Google Scholar]
- Coleman, D.J. Ocular tumor patterns. Bibl. Ophthalmol. 1975, 83, 136–140. [Google Scholar]
- Kivelä, T. Diagnosis of uveal melanoma. Dev. Ophthalmol. 2012, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tarlan, B.; Kıratlı, H. Uveal Melanoma: Current Trends in Diagnosis and Management. Turk. J. Ophthalmol. 2016, 46, 123–137. [Google Scholar] [CrossRef]
- Blanco, G. Diagnosis and treatment of orbital invasion in uveal melanoma. Can. J. Ophthalmol. 2004, 39, 388–396. [Google Scholar] [CrossRef]
- Hosten, N.; Bornfeld, N.; Wassmuth, R.; Lemke, A.J.; Sander, B.; Bechrakis, N.E.; Felix, R. Uveal melanoma: Detection of extraocular growth with MR imaging and US. Radiology 1997, 202, 61–67. [Google Scholar] [CrossRef]
- Xu, G.; Xue, Y.; Özkurt, Z.G.; Slimani, N.; Hu, Z.; Wang, X.; Xia, K.; Ma, T.; Zhou, Q.; Demirci, H. Photoacoustic imaging features of intraocular tumors: Retinoblastoma and uveal melanoma. PLoS ONE 2017, 12, e0170752. [Google Scholar] [CrossRef]
- Margo, C.E. The Collaborative Ocular Melanoma Study: An overview. Cancer Control 2004, 11, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study: COMS report no. 21. Arch. Ophthalmol. 2003, 121, 1163–1171. [CrossRef] [Green Version]
- Ossoinig, K.C. Clinical echo-ophthalmology. In Current Concepts of Ophthalmology; Grzybowski, A., Ed.; CV Mosby Co.: St Louis, MO, USA, 1972; Volume III, pp. 101–130. [Google Scholar]
- Inc, M. Ultrasound. Available online: https://www.mdsave.com/procedures/ultrasound/d781f5ca (accessed on 22 April 2022).
- Silverman, R.H.; Coleman, D.J.; Rondeau, M.J.; Woods, S.M.; Lizzi, F.L. Measurement of ocular tumor volumes from serial, cross-sectional ultrasound scans. Retina 1993, 13, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.J. Reliability of ocular tumor diagnosis with ultrasound. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1973, 77, Op677–Op686. [Google Scholar]
- Foss, A.J.; Alexander, R.A.; Jefferies, L.W.; Hungerford, J.L.; Harris, A.L.; Lightman, S. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996, 56, 2900–2903. [Google Scholar]
- Beenakker, J.-W.M.; Ferreira, T.A.; Soemarwoto, K.P.; Genders, S.W.; Teeuwisse, W.M.; Webb, A.G.; Luyten, G.P.M. Clinical evaluation of ultra-high-field MRI for three-dimensional visualisation of tumour size in uveal melanoma patients, with direct relevance to treatment planning. Magn. Reson. Mater. Phys. Biol. Med. 2016, 29, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Bakri, S.J.; Sculley, L.; Singh, A.D. Imaging techniques for uveal melanoma. Int. Ophthalmol. Clin. 2006, 46, 1–13. [Google Scholar] [CrossRef]
- Silverman, R.H. High-Resolution Ultrasound Imaging of the Eye—A Review. Clin. Exp. Ophthalmol. 2009, 37, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Nanji, A.A.; Mercado, C.; Galor, A.; Dubovy, S.; Karp, C.L. Updates in Ocular Surface Tumor Diagnostics. Int. Ophthalmol. Clin. 2017, 57, 47–62. [Google Scholar] [CrossRef]
- Bianciotto, C.; Shields, C.L.; Guzman, J.M.; Romanelli-Gobbi, M.; Mazzuca, D., Jr.; Green, W.R.; Shields, J.A. Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases. Ophthalmology 2011, 118, 1297–1302. [Google Scholar] [CrossRef]
- Pavlin, C.J.; Vásquez, L.M.; Lee, R.; Simpson, E.R.; Ahmed, I.I.K. Anterior segment optical coherence tomography and ultrasound biomicroscopy in the imaging of anterior segment tumors. Am. J. Ophthalmol. 2009, 147, 214–219. [Google Scholar] [CrossRef]
- Scott, I.U.; Murray, T.G.; Hughes, J.R. Evaluation of imaging techniques for detection of extraocular extension of choroidal melanoma. Arch. Ophthalmol. 1998, 116, 897–899. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.A.; Robertson, D.M. Extrascleral extension of choroidal melanoma diagnosed by ultrasound. Ophthalmology 1983, 90, 1554–1559. [Google Scholar] [CrossRef]
- Bedi, D.G.; Gombos, D.S.; Ng, C.S.; Singh, S. Sonography of the eye. AJR Am. J. Roentgenol. 2006, 187, 1061–1072. [Google Scholar] [CrossRef]
- Damianidis, C.H.; Konstantinou, D.; Kyriakou, V.; Arvaniti, M.; Kotziamani, N.; Rodokalakis, G.; Agriou, A.; Emmanouilidou, M.; Tsitouridis, I. Magnetic resonance imaging and ultrasonographic evaluation of retinal detachment in orbital uveal melanomas. Neuroradiol. J. 2010, 23, 329–338. [Google Scholar] [CrossRef]
- Shields, C.L.; Pellegrini, M.; Kligman, B.E.; Bianciotto, C.; Shields, J.A. Ciliary body and choroidal pseudomelanoma from ultrasonographic imaging of hypermature cataract in 20 cases. Ophthalmology 2013, 120, 2546–2551. [Google Scholar] [CrossRef]
- Byrne, S.F.; Green, R.L. Ultrasound of the Eye and Orbit, 2e, 2nd ed.; Mosby Inc.: St. Louis, MO, USA, 2002; p. 576. [Google Scholar]
- Grech Fonk, L.; Ferreira, T.A.; Webb, A.G.; Luyten, G.P.M.; Beenakker, J.M. The Economic Value of MR-Imaging for Uveal Melanoma. Clin. Ophthalmol. 2020, 14, 1135–1143. [Google Scholar] [CrossRef]
- Sobottka, B.; Kreissig, I. Ultrasonography of metastases and melanomas of the choroid. Curr. Opin. Ophthalmol. 1999, 10, 164–167. [Google Scholar] [CrossRef]
- Walter, U.; Niendorf, T.; Graessl, A.; Rieger, J.; Krüger, P.C.; Langner, S.; Guthoff, R.F.; Stachs, O. Ultrahigh field magnetic resonance and colour Doppler real-time fusion imaging of the orbit—a hybrid tool for assessment of choroidal melanoma. Eur. Radiol. 2014, 24, 1112–1117. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Hossain, P.; Anderson, D.F. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br. J. Ophthalmol. 2007, 91, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Aumann, S.; Donner, S.; Fischer, J.; Müller, F. Optical Coherence Tomography (OCT): Principle and Technical Realization. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 59–85. [Google Scholar] [CrossRef] [Green Version]
- Photonics Media. Available online: https://www.photonics.com/a36339/Is_OCT_Worth_It (accessed on 22 April 2022).
- Popescu, D.P.; Choo-Smith, L.P.; Flueraru, C.; Mao, Y.; Chang, S.; Disano, J.; Sherif, S.; Sowa, M.G. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys. Rev. 2011, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Sayyad, F.E.; Galor, A.; Dubovy, S.; Karp, C.L. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul. Surf. 2015, 13, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, B.J.; Galor, A.; Nanji, A.A.; El Sayyad, F.; Wang, J.; Dubovy, S.R.; Joag, M.G.; Karp, C.L. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul. Surf. 2014, 12, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscat, S.; Srinivasan, S.; Sampat, V.; Kemp, E.; Parks, S.; Keating, D. Optical coherence tomography in the diagnosis of subclinical serous detachment of the macula secondary to a choroidal nevus. Ophthalmic Surg. Lasers 2001, 32, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.L.; Brugnoni, N.; Kaiser, P.K.; Singh, A.D. Optical coherence tomography enhanced depth imaging of choroidal tumors. Am. J. Ophthalmol. 2011, 151, 586–593.e582. [Google Scholar] [CrossRef]
- Say, E.A.T.; Ferenczy, S.; Magrath, G.N.; Samara, W.A.; Khoo, C.T.L.; Shields, C.L. Image quality and artifacts on optical coherence tomography angiography: Comparison of Pathologic and Paired Fellow Eyes in 65 Patients with Unilateral Choroidal Melanoma Treated with Plaque Radiotherapy. Retina 2017, 37, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.P.; Kibele, N.X.; Prasuhn, M.; Kakkassery, V.; Toro, M.D.; Ranjbar, M.; Grisanti, S.; Becker, M.; Rommel, F. Evaluating Retinal and Choroidal Perfusion Changes after Isometric and Dynamic Activity Using Optical Coherence Tomography Angiography. Diagnostics 2021, 11, 808. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Ortisi, E.; Scollo, D.; Reibaldi, M.; Russo, A.; Pizzo, A.; Faro, G.; Macchi, I.; Fallico, M.; Toro, M.D.; et al. Vascular changes after vitrectomy for rhegmatogenous retinal detachment: Optical coherence tomography angiography study. Acta. Ophthalmol. 2019, 98, e563–e569. [Google Scholar] [CrossRef]
- Wrzesińska, D.; Nowomiejska, K.; Nowakowska, D.; Toro, M.D.; Bonfiglio, V.; Reibaldi, M.; Avitabile, T.; Rejdak, R. Secondary Vitrectomy with Internal Limiting Membrane Plug due to Persistent Full-Thickness Macular Hole OCT-Angiography and Microperimetry Features: Case Series. J. Ophthalmol. 2020, 2020, 2650873. [Google Scholar] [CrossRef]
- Neroev, V.V.; Saakyan, S.V.; Myakoshina, E.B.; Okhotsimskaya, T.D.; Fadeeva, V.A. Role of optical coherence tomography angiography in diagnostics of early choroidal melanoma and circumscribed choroidal hemangioma. Vestn. Oftalmol. 2018, 134, 4–18. [Google Scholar] [CrossRef]
- Ghassemi, F.; Mirshahi, R.; Fadakar, K.; Sabour, S. Optical coherence tomography angiography in choroidal melanoma and nevus. Clin. Ophthalmol. 2018, 12, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valverde-Megías, A.; Say, E.A.; Ferenczy, S.R.; Shields, C.L. Differential Macular Features on Optical Coherence Tomography Angiography in Eyes with Choroidal Nevus and Melanoma. Retina 2017, 37, 731–740. [Google Scholar] [CrossRef] [PubMed]
- de Carlo, T.E.; Mieler, W.F. Automated quantitative OCTA measurements of uveal melanoma-treated eyes with and without radiation retinopathy after I-125 plaque therapy and of nonirradiated fellow eyes. Eye 2021, 35, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Skalet, A.H.; Liu, L.; Binder, C.; Miller, A.K.; Crilly, R.; Hung, A.Y.; Wilson, D.J.; Huang, D.; Jia, Y. Longitudinal Detection of Radiation-Induced Peripapillary and Macular Retinal Capillary Ischemia Using OCT Angiography. Ophthalmol. Retin. 2020, 4, 320–326. [Google Scholar] [CrossRef]
- Skalet, A.H.; Liu, L.; Binder, C.; Miller, A.K.; Wang, J.; Wilson, D.J.; Crilly, R.; Thomas, C.R., Jr.; Hung, A.Y.; Huang, D.; et al. Quantitative OCT Angiography Evaluation of Peripapillary Retinal Circulation after Plaque Brachytherapy. Ophthalmol. Retin. 2018, 2, 244–250. [Google Scholar] [CrossRef]
- Matet, A.; Daruich, A.; Zografos, L. Radiation Maculopathy After Proton Beam Therapy for Uveal Melanoma: Optical Coherence Tomography Angiography Alterations Influencing Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3851–3861. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, Q.; Chen, M.X.; Yan, Y.N.; Zhou, W.J.; Liu, Y.M.; Wei, W.B. Retinal Microvascular Changes in Uveal Melanoma Following Conbercept Injection after Plaque Radiotherapy as Detected by Optical Coherence Tomographic Angiography. Retina 2021, 41, 2605–2611. [Google Scholar] [CrossRef]
- Materin, M.A.; Raducu, R.; Bianciotto, C.; Shields, C.L. Fundus autofluorescence and optical coherence tomography findings in choroidal melanocytic lesions. Middle East Afr. J. Ophthalmol. 2010, 17, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Vishnevskia-Dai, V.; Zur, D.; Yaacobi, S.; Moroz, I.; Newman, H.; Neudorfer, M. Optical Coherence Tomography: An Adjunctive Tool for Differentiating between Choroidal Melanoma and Metastasis. J. Ophthalmol. 2016, 2016, 9803547. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Koizumi, H.; Pozzoni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Shields, C.L.; Kaliki, S.; Furuta, M.; Mashayekhi, A.; Shields, J.A. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012, 32, 1363–1372. [Google Scholar] [CrossRef]
- Shah, S.U.; Kaliki, S.; Shields, C.L.; Ferenczy, S.R.; Harmon, S.A.; Shields, J.A. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology 2012, 119, 1066–1072. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A. Choroidal melanoma. Oman J. Ophthalmol. 2012, 5, 3–9. [Google Scholar] [CrossRef]
- Grisanti, S.; Tura, A. Uveal melanoma. In Noncutaneous Melanoma; Scott, J.F., Gerstenblith, M.R., Eds.; Codon Publications: Brisbane, Australia, 2018. [Google Scholar] [CrossRef]
- Timberlake, G.T.; Kennedy, M. Thedirect Ophthalmoscope How It Worksand How to Use It. Available online: https://web.media.mit.edu/~raskar/Eye/TheDirectOphthalmoscope.pdf (accessed on 22 April 2022).
- Abràmoff, M.D.; Garvin, M.K.; Sonka, M. Retinal imaging and image analysis. IEEE Rev. Biomed. Eng. 2010, 3, 169–208. [Google Scholar] [CrossRef] [Green Version]
- Mirchia, K.; Turell, M.; Singh, A. Imaging Modalities for Uveal Melanoma. Eur. Ophthalmic. Rev. 2012, 6, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Fallico, M.; Raciti, G.; Longo, A.; Reibaldi, M.; Bonfiglio, V.; Russo, A.; Caltabiano, R.; Gattuso, G.; Falzone, L.; Avitabile, T. Current molecular and clinical insights into uveal melanoma (Review). Int. J. Oncol. 2021, 58, 10. [Google Scholar] [CrossRef]
- Callaway, N.F.; Mruthyunjaya, P. Widefield imaging of retinal and choroidal tumors. Int. J. Retin. Vitr. 2019, 5, 49. [Google Scholar] [CrossRef]
- Jain, A.; Shah, S.P.; Tsui, I.; McCannel, T.A. The value of Optos Panoramic 200MA imaging for the monitoring of large suspicious choroidal lesions. Semin. Ophthalmol. 2009, 24, 43–44. [Google Scholar] [CrossRef]
- Mackenzie, P.J.; Russell, M.; Ma, P.E.; Isbister, C.M.; Maberley, D.A. Sensitivity and specificity of the optos optomap for detecting peripheral retinal lesions. Retina 2007, 27, 1119–1124. [Google Scholar] [CrossRef]
- Lamirel, C.; Bruce, B.B.; Wright, D.W.; Newman, N.J.; Biousse, V. Nonmydriatic digital ocular fundus photography on the iPhone 3G: The FOTO-ED study. Arch. Ophthalmol. 2012, 130, 939–940. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Wang, E.H.; Pokabla, M.J.; Noecker, R.J. Teleophthalmology assessment of diabetic retinopathy fundus images: Smartphone versus standard office computer workstation. Telemed. J. E-Health. 2012, 18, 158–162. [Google Scholar] [CrossRef]
- Bastawrous, A.; Giardini, M.E.; Bolster, N.M.; Peto, T.; Shah, N.; Livingstone, I.A.; Weiss, H.A.; Hu, S.; Rono, H.; Kuper, H.; et al. Clinical Validation of a Smartphone-Based Adapter for Optic Disc Imaging in Kenya. JAMA Ophthalmol. 2016, 134, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Hurley, B.R.; Regillo, C.D. Fluorescein Angiography: General Principles and Interpretation. In Retinal Angiography and Optical Coherence Tomography; Arevalo, J.F., Ed.; Springer: New York, NY, USA, 2009; pp. 27–42. [Google Scholar] [CrossRef]
- Dithmar, S.; Holz, F.G. Fluorescence Angiography in Ophthalmology; Springer Medizin Verlag: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Meyer, K.; Augsburger, J.J. Independent diagnostic value of fluorescein angiography in the evaluation of intraocular tumors. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 489–494. [Google Scholar] [CrossRef]
- Kapoor, A.; Beniwal, V.; Beniwal, S.; Mathur, H.; Kumar, H.S. Management of uveal tract melanoma: A comprehensive review. J. Egypt Natl. Canc. Inst. 2016, 28, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Kawali, A.; Pichi, F.; Avadhani, K.; Invernizzi, A.; Hashimoto, Y.; Mahendradas, P. Multimodal Imaging of the Normal Eye. Ocul. Immunol. Inflamm. 2017, 25, 721–731. [Google Scholar] [CrossRef]
- Invernizzi, A.; Pellegrini, M.; Cornish, E.; Yi Chong Teo, K.; Cereda, M.; Chabblani, J. Imaging the Choroid: From Indocyanine Green Angiography to Optical Coherence Tomography Angiography. Asia-Pac. J. Ophthalmol. 2020, 9, 335–348. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; De Potter, P. Patterns of indocyanine green videoangiography of choroidal tumours. Br. J. Ophthalmol. 1995, 79, 237–245. [Google Scholar] [CrossRef] [Green Version]
- HyvÄRinen, L.E.A.; Flower, R.W. INDOCYANINE GREEN FLUORESCENCE ANGIOGRAPHY. Acta. Ophthalmol. 1980, 58, 528–538. [Google Scholar] [CrossRef]
- Sato, T.; Karasawa, Y.; Ishikawa, S.; Taguchi, M.; Muraoka, T.; Ito, M.; Takeuchi, M. Potential Phototoxicity of Indocyanine Green in Retinal Pigment Epithelial Cells after Angiography under Ambient Illumination. Oxid. Med. Cell. Longev. 2018, 2018, 6065285. [Google Scholar] [CrossRef]
- Lavinsky, D.; Belfort, R.N.; Navajas, E.; Torres, V.; Martins, M.C.; Belfort, R., Jr. Fundus autofluorescence of choroidal nevus and melanoma. Br. J. Ophthalmol. 2007, 91, 1299–1302. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.; Kaliki, S.; Shields, C.L. Autofluorescence of intraocular tumours. Curr. Opin. Ophthalmol. 2013, 24, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Nivison-Smith, L.; Assaad, N.; Kalloniatis, M. Fundus Autofluorescence in Age-related Macular Degeneration. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2017, 94, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, R.B.; Davidorf, F.H.; McAdoo, J.F.; Chakeres, D.W. Magnetic resonance imaging of uveal melanomas. Arch. Ophthalmol. 1987, 105, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Crooks, L.E.; Mills, C.M.; Davis, P.L.; Brant-Zawadzki, M.; Hoenninger, J.; Arakawa, M.; Watts, J.; Kaufman, L. Visualization of cerebral and vascular abnormalities by NMR imaging. The effects of imaging parameters on contrast. Radiology 1982, 144, 843–852. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Wełniak-Kamińska, M.; Świątkiewicz, M.; Orzeł, J.; Chorągiewicz, T.; Toro, M.D.; Rejdak, R.; Bogorodzki, P.; Grieb, P. Changes of Ocular Dimensions as a Marker of Disease Progression in a Murine Model of Pigmentary Glaucoma. Front. Pharmacol. 2020, 11, 573238. [Google Scholar] [CrossRef]
- Richdale, K.; Wassenaar, P.; Teal Bluestein, K.; Abduljalil, A.; Christoforidis, J.A.; Lanz, T.; Knopp, M.V.; Schmalbrock, P. 7 Tesla MR imaging of the human eye in vivo. J. Magn. Reson. Imaging 2009, 30, 924–932. [Google Scholar] [CrossRef] [Green Version]
- Hosten, N.; Lemke, A.J.; Bornfeld, N.; Wassmuth, R.; Schweiger, U.; Terstegge, K.; Felix, R. Fast spin-echo MR imaging of the eye. Eur. Radiol. 1996, 6, 900–903. [Google Scholar] [CrossRef]
- Houle, V.; Bélair, M.; Allaire, G.S. AIRP best cases in radiologic-pathologic correlation: Choroidal melanoma. Radiographics 2011, 31, 1231–1236. [Google Scholar] [CrossRef]
- Hirunpat, P.; Sanghan, N.; Hirunpat, S. White matter: A good reference for the signal intensity evaluation in magnetic resonance imaging for the diagnosis of uveal melanoma. Neuroradiol. J. 2020, 34, 113–119. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Harms, S.E. Surface-coil MR imaging of orbital neoplasms. AJNR Am. J. Neuroradiol. 1986, 7, 29–34. [Google Scholar]
- de Keizer, R.J.; Vielvoye, G.J.; de Wolff-Rouendaal, D. Nuclear magnetic resonance imaging of intraocular tumors. Am. J. Ophthalmol. 1986, 102, 438–441. [Google Scholar] [CrossRef]
- Commoner, B.; Townsend, J.; Pake, G.E. Free radicals in biological materials. Nature 1954, 174, 689–691. [Google Scholar] [CrossRef]
- Gomori, J.M.; Grossman, R.I.; Shields, J.A.; Augsburger, J.J.; Joseph, P.M.; DeSimeone, D. Choroidal melanomas: Correlation of NMR spectroscopy and MR imaging. Radiology 1986, 158, 443–445. [Google Scholar] [CrossRef]
- Tailor, T.D.; Gupta, D.; Dalley, R.W.; Keene, C.D.; Anzai, Y. Orbital neoplasms in adults: Clinical, radiologic, and pathologic review. Radiographics 2013, 33, 1739–1758. [Google Scholar] [CrossRef]
- Mafee, M.F.; Peyman, G.A.; Grisolano, J.E.; Fletcher, M.E.; Spigos, D.G.; Wehrli, F.W.; Rasouli, F.; Capek, V. Malignant uveal melanoma and simulating lesions: MR imaging evaluation. Radiology 1986, 160, 773–780. [Google Scholar] [CrossRef]
- Stroszczynski, C.; Hosten, N.; Bornfeld, N.; Wiegel, T.; Schueler, A.; Foerster, P.; Lemke, A.J.; Hoffmann, K.T.; Felix, R. Choroidal hemangioma: MR findings and differentiation from uveal melanoma. AJNR Am. J. Neuroradiol. 1998, 19, 1441–1447. [Google Scholar]
- Schueler, A.O.; Hosten, N.; Bechrakis, N.E.; Lemke, A.J.; Foerster, P.; Felix, R.; Foerster, M.H.; Bornfeld, N. High resolution magnetic resonance imaging of retinoblastoma. Br. J. Ophthalmol. 2003, 87, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, B.H.; Ricks, C.; Harrie, R.P. Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma. BMC Ophthalmol. 2018, 18, 320. [Google Scholar] [CrossRef]
- Récsán, Z.; Karlinger, K.; Fodor, M.; Zalatnai, A.; Papp, M.; Salacz, G. MRI for the evaluation of scleral invasion and extrascleral extension of uveal melanomas. Clin. Radiol. 2002, 57, 371–376. [Google Scholar] [CrossRef]
- Beenakker, J.W.; van Rijn, G.A.; Luyten, G.P.; Webb, A.G. High-resolution MRI of uveal melanoma using a microcoil phased array at 7 T. NMR Biomed. 2013, 26, 1864–1869. [Google Scholar] [CrossRef]
- Lindner, T.; Langner, S.; Falke, K.; Walter, U.; Krüger, P.C.; Pohlmann, A.; Zimpfer, A.; Stahnke, T.; Hadlich, S.; Guthoff, R.; et al. Anatomic and pathological characterization of choroidal melanoma using multimodal imaging: What is practical, what is needed? Melanoma Res. 2015, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.M.; McCaffery, S.; Rowley, H.A.; Fischbein, N.J.; Shimikawa, A.; O’Brien, J.M. High-resolution 3D T2-weighted fast spin echo: New applications in the orbit. Neuroradiology 2003, 45, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Fanea, L.; Fagan, A.J. Review: Magnetic resonance imaging techniques in ophthalmology. Mol. Vis. 2012, 18, 2538–2560. [Google Scholar] [PubMed]
- Bert, R.J.; Patz, S.; Ossiani, M.; Caruthers, S.D.; Jara, H.; Krejza, J.; Freddo, T. High-resolution MR imaging of the human eye 2005. Acad. Radiol. 2006, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.; Romaniuk, C.; Ghaneh, P.; Wong, H.; McKay, M.; Chopra, M.; Coupland, S.E.; Damato, B.E. MRI in the detection of hepatic metastases from high-risk uveal melanoma: A prospective study in 188 patients. Br. J. Ophthalmol. 2013, 97, 159–163. [Google Scholar] [CrossRef]
- Servois, V.; Mariani, P.; Malhaire, C.; Petras, S.; Piperno-Neumann, S.; Plancher, C.; Levy-Gabriel, C.; Lumbroso-le Rouic, L.; Desjardins, L.; Salmon, R.J. Preoperative staging of liver metastases from uveal melanoma by magnetic resonance imaging (MRI) and fluorodeoxyglucose-positron emission tomography (FDG-PET). Eur. J. Surg. Oncol. 2010, 36, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, J.H.; Catalanotti, F.; Landa, J.; Barker, C.A.; Shoushtari, A.N.; Abramson, D.H. Hepatic abnormalities identified by staging MRI and accuracy of MRI of patients with uveal melanoma. Br. J. Ophthalmol. 2019, 103, 1266–1271. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Grech Fonk, L.; Jaarsma-Coes, M.G.; van Haren, G.G.R.; Marinkovic, M.; Beenakker, J.-W.M. MRI of Uveal Melanoma. Cancers 2019, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Jaarsma-Coes, M.G.; Goncalves Ferreira, T.A.; van Haren, G.R.; Marinkovic, M.; Beenakker, J.M. MRI enables accurate diagnosis and follow-up in uveal melanoma patients after vitrectomy. Melanoma Res. 2019, 29, 655–659. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef] [Green Version]
- Erb-Eigner, K.; Willerding, G.; Taupitz, M.; Hamm, B.; Asbach, P. Diffusion-weighted imaging of ocular melanoma. Investig. Radiol. 2013, 48, 702–707. [Google Scholar] [CrossRef]
- Kamrava, M.; Sepahdari, A.R.; Leu, K.; Wang, P.C.; Roberts, K.; Demanes, D.J.; McCannel, T.; Ellingson, B.M. Quantitative multiparametric MRI in uveal melanoma: Increased tumor permeability may predict monosomy 3. Neuroradiology 2015, 57, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Ciller, C.; De Zanet, S.I.; Rüegsegger, M.B.; Pica, A.; Sznitman, R.; Thiran, J.P.; Maeder, P.; Munier, F.L.; Kowal, J.H.; Cuadra, M.B. Automatic Segmentation of the Eye in 3D Magnetic Resonance Imaging: A Novel Statistical Shape Model for Treatment Planning of Retinoblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Foti, P.V.; Longo, A.; Reibaldi, M.; Russo, A.; Privitera, G.; Spatola, C.; Raffaele, L.; Salamone, V.; Farina, R.; Palmucci, S.; et al. Uveal melanoma: Quantitative evaluation of diffusion-weighted MR imaging in the response assessment after proton-beam therapy, long-term follow-up. Radiol. Med. 2017, 122, 131–139. [Google Scholar] [CrossRef]
- Mazonakis, M.; Damilakis, J. Computed tomography: What and how does it measure? Eur. J. Radiol. 2016, 85, 1499–1504. [Google Scholar] [CrossRef]
- Eguren, M.; Holguin, A.; Diaz, K.; Vidalon, J.; Linan, C.; Pereira, C.; Lagravere Vich, M.O. Can gray values be converted to Hounsfield units? A systematic review. Dentomaxillofac. Radiol. 2021, 51, 20210140. [Google Scholar] [CrossRef]
- Blackham, K.A.; Vidal, L.L.M. Computed Tomography. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 848–853. [Google Scholar] [CrossRef]
- Naik, M.N.; Tourani, K.L.; Sekhar, G.C.; Honavar, S.G. Interpretation of computed tomography imaging of the eye and orbit. A systematic approach. Indian J. Ophthalmol. 2002, 50, 339–353. [Google Scholar]
- de Keizer, R.J.; Vielvoye, G.J.; de Wolff-Rouendaal, D. CT-scan and intraocular tumours: Detection and assessment of size and extrascleral growth of uveal melanomas. Doc. Ophthalmol. 1986, 61, 285–294. [Google Scholar] [CrossRef]
- Lin, E.; Alessio, A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr. 2009, 3, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Mafee, M.F.; Peyman, G.A.; McKusick, M.A. Malignant uveal melanoma and similar lesions studied by computed tomography. Radiology 1985, 156, 403–408. [Google Scholar] [CrossRef]
- Sallet, G.; Serop, S.; Verbraeken, H.; Hanssens, M. The value of medical imaging techniques in the diagnosis of extra-ocular extension of malign melanoma of the choroid: A case report. Bull. Soc. Belge Ophtalmol. 1993, 248, 53–58. [Google Scholar] [PubMed]
- Khan, S.N.; Sepahdari, A.R. Orbital masses: CT and MRI of common vascular lesions, benign tumors, and malignancies. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2012, 26, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurli, M.; Reddy, S.; Tena, L.B.; Pavlick, A.C.; Finger, P.T. Whole body positron emission tomography/computed tomography staging of metastatic choroidal melanoma. Am. J. Ophthalmol. 2005, 140, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Holly, T.A.; Abbott, B.G.; Al-Mallah, M.; Calnon, D.A.; Cohen, M.C.; DiFilippo, F.P.; Ficaro, E.P.; Freeman, M.R.; Hendel, R.C.; Jain, D.; et al. Single photon-emission computed tomography. J. Nucl. Cardiol. 2010, 17, 941–973. [Google Scholar] [CrossRef]
- Wernick, M.N.; Aarsvold, J.N. CHAPTER 2—Introduction to Emission Tomography. In Emission Tomography; Wernick, M.N., Aarsvold, J.N., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 11–23. [Google Scholar] [CrossRef]
- Nolte, T.; Gross-Weege, N.; Schulz, V. (Hybrid) SPECT and PET Technologies. Recent Results Cancer Res. 2020, 216, 111–133. [Google Scholar] [CrossRef]
- Alonso, O.; Núñez, M.; Cánepa, J.; Guisoli, P.; Mut, F.; Lago, G.; Touya, E. Evaluation of ocular tumors with technetium-99m-MIBI: Planar pinhole technique or SPECT? J. Nucl. Med. Technol. 2000, 28, 85–87. [Google Scholar]
- Abe, K.; Sasaki, M.; Koga, H.; Kaneko, K.; Sawamoto, H.; Yoshikawa, H.; Honda, H. Clinical role of 123I-IMP SPECT for the differential diagnosis of ocular malignant melanoma: A time-course analysis. Nucl. Med. Commun. 2007, 28, 567–573. [Google Scholar] [CrossRef]
- Goto, H. Clinical efficacy of 123I-IMP SPECT for the diagnosis of malignant uveal melanoma. Int. J. Clin. Oncol. 2004, 9, 74–78. [Google Scholar] [CrossRef]
- Kato, K.; Kubota, T.; Ikeda, M.; Tadokoro, M.; Abe, S.; Nakano, S.; Nishino, M.; Kobayashi, H.; Ishigaki, T. Low efficacy of 18F-FDG PET for detection of uveal malignant melanoma compared with 123I-IMP SPECT. J. Nucl. Med. 2006, 47, 404–409. [Google Scholar]
- Yamazaki, K.; Nishii, R.; Maeda, T.; Makishima, H.; Kasuya, G.; Chang, T.; Tamura, K.; Wakatsuki, M.; Tsuji, H.; Murakami, K.; et al. Assessment of SPECT-CT fusion images and semi-quantitative evaluation using SUV in (123)I-IMP SPECT in patients with choroidal melanoma. Ann. Nucl. Med. 2020, 34, 864–872. [Google Scholar] [CrossRef]
- Brooks, D.J. Positron emission tomography and single-photon emission computed tomography in central nervous system drug development. NeuroRx 2005, 2, 226–236. [Google Scholar] [CrossRef]
- Salvatori, M.; Rizzo, A.; Rovera, G.; Indovina, L.; Schillaci, O. Radiation dose in nuclear medicine: The hybrid imaging. Radiol. Med. 2019, 124, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Kalemaki, M.S.; Karantanas, A.H.; Exarchos, D.; Detorakis, E.T.; Zoras, O.; Marias, K.; Millo, C.; Bagci, U.; Pallikaris, I.; Stratis, A.; et al. PET/CT and PET/MRI in ophthalmic oncology (Review). Int. J. Oncol. 2020, 56, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Omami, G.; Tamimi, D.; Branstetter, B.F. Basic principles and applications of (18)F-FDG-PET/CT in oral and maxillofacial imaging: A pictorial essay. Imaging Sci. Dent. 2014, 44, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Moses, W.W. Fundamental Limits of Spatial Resolution in PET. Nucl. Instrum. Methods Phys. Res. A 2011, 648 (Suppl. S1), S236–S240. [Google Scholar] [CrossRef] [Green Version]
- Hui, K.H.; Pfeiffer, M.L.; Esmaeli, B. Value of positron emission tomography/computed tomography in diagnosis and staging of primary ocular and orbital tumors. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2012, 26, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Griffeth, L.K. Use of PET/CT scanning in cancer patients: Technical and practical considerations. Bayl. Univ. Med. Cent. Proc. 2005, 18, 321–330. [Google Scholar] [CrossRef]
- Kapoor, V.; McCook, B.M.; Torok, F.S. An introduction to PET-CT imaging. Radiographics 2004, 24, 523–543. [Google Scholar] [CrossRef]
- Reddy, S.; Kurli, M.; Tena, L.B.; Finger, P.T. PET/CT imaging: Detection of choroidal melanoma. Br. J. Ophthalmol. 2005, 89, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Finger, P.T.; Chin, K.; Iacob, C.E. 18-Fluorine-labelled 2-deoxy-2-fluoro-D-glucose positron emission tomography/computed tomography standardised uptake values: A non-invasive biomarker for the risk of metastasis from choroidal melanoma. Br. J. Ophthalmol. 2006, 90, 1263–1266. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Ogino, Y.; Ichimura, K.; Tanaka, T.; Kaji, M. Clinicopathological correlation for the role of fluorodeoxyglucose positron emission tomography computed tomography in detection of choroidal malignant melanoma. Int. J. Clin. Oncol. 2014, 19, 230–239. [Google Scholar] [CrossRef] [PubMed]
- McCannel, T.A.; Reddy, S.; Burgess, B.L.; Auerbach, M. Association of positive dual-modality positron emission tomography/computed tomography imaging of primary choroidal melanoma with chromosome 3 loss and tumor size. Retina 2010, 30, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Papastefanou, V.P.; Islam, S.; Szyszko, T.; Grantham, M.; Sagoo, M.S.; Cohen, V.M. Metabolic activity of primary uveal melanoma on PET/CT scan and its relationship with monosomy 3 and other prognostic factors. Br. J. Ophthalmol. 2014, 98, 1659–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingenstein, A.; Haug, A.R.; Nentwich, M.M.; Tiling, R.; Schaller, U.C. Whole-body F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography imaging in the follow-up of metastatic uveal melanoma. Melanoma Res. 2010, 20, 511–516. [Google Scholar] [CrossRef]
- Strobel, K.; Bode, B.; Dummer, R.; Veit-Haibach, P.; Fischer, D.R.; Imhof, L.; Goldinger, S.; Steinert, H.C.; von Schulthess, G.K. Limited value of 18F-FDG PET/CT and S-100B tumour marker in the detection of liver metastases from uveal melanoma compared to liver metastases from cutaneous melanoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1774–1782. [Google Scholar] [CrossRef] [Green Version]
- Orcurto, V.; Denys, A.; Voelter, V.; Schalenbourg, A.; Schnyder, P.; Zografos, L.; Leyvraz, S.; Delaloye, A.B.; Prior, J.O. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: Results from a pilot study. Melanoma Res. 2012, 22, 63–69. [Google Scholar] [CrossRef]
- Huang, B.; Law, M.W.; Khong, P.L. Whole-body PET/CT scanning: Estimation of radiation dose and cancer risk. Radiology 2009, 251, 166–174. [Google Scholar] [CrossRef]
- Núñez Miller, R.; Pozo, M.A. NonFDG PET in oncology. Clin. Transl. Oncol. 2011, 13, 780–786. [Google Scholar] [CrossRef]
- Eisenmenger, L.B. NonFDG Radiopharmaceuticals in Head and Neck PET Imaging: Current Techniques and Future Directions. Semin. Ultrasound CT MR 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Bastawrous, S.; Bhargava, P.; Behnia, F.; Djang, D.S.; Haseley, D.R. Newer PET application with an old tracer: Role of 18F-NaF skeletal PET/CT in oncologic practice. Radiographics 2014, 34, 1295–1316. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Dong, Z.; Xu, Y.; Huang, Q.; Pan, D.; Wang, L.; Yang, M. The diagnostic performance of (18)F-fluoride PET/CT in bone metastases detection: A meta-analysis. Clin. Radiol. 2019, 74, 196–206. [Google Scholar] [CrossRef]
- Shen, C.T.; Qiu, Z.L.; Han, T.T.; Luo, Q.Y. Performance of 18F-fluoride PET or PET/CT for the detection of bone metastases: A meta-analysis. Clin. Nucl. Med. 2015, 40, 103–110. [Google Scholar] [CrossRef]
- Feng, H.; Xia, X.; Li, C.; Song, Y.; Qin, C.; Liu, Q.; Zhang, Y.; Lan, X. Imaging malignant melanoma with (18)F-5-FPN. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 113–122. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Zhang, Y.; Zhang, F.; Liu, C.; Song, Y.; Zhang, Y.; Lan, X. Detection of melanoma metastases with PET—Comparison of 18F-5-FPN with 18F–FDG. Nucl. Med. Biol. 2017, 50, 33–38. [Google Scholar] [CrossRef]
- Denoyer, D.; Greguric, I.; Roselt, P.; Neels, O.C.; Aide, N.; Taylor, S.R.; Katsifis, A.; Dorow, D.S.; Hicks, R.J. High-contrast PET of melanoma using (18)F-MEL050, a selective probe for melanin with predominantly renal clearance. J. Nucl. Med. 2010, 51, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Rizzo-Padoin, N.; Chaussard, M.; Vignal, N.; Kotula, E.; Tsoupko-Sitnikov, V.; Vaz, S.; Hontonnou, F.; Liu, W.Q.; Poyet, J.L.; Vidal, M.; et al. [(18)F]MEL050 as a melanin-targeted PET tracer: Fully automated radiosynthesis and comparison to (18)F-FDG for the detection of pigmented melanoma in mice primary subcutaneous tumors and pulmonary metastases. Nucl. Med. Biol. 2016, 43, 773–780. [Google Scholar] [CrossRef]
- Denoyer, D.; Potdevin, T.; Roselt, P.; Neels, O.C.; Kirby, L.; Greguric, I.; Katsifis, A.; Dorow, D.S.; Hicks, R.J. Improved detection of regional melanoma metastasis using 18F-6-fluoro-N-[2-(diethylamino)ethyl] pyridine-3-carboxamide, a melanin-specific PET probe, by perilesional administration. J. Nucl. Med. 2011, 52, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yuan, L.; Yin, L.; Jiang, Y.; Gai, Y.; Liu, Q.; Wang, Y.; Zhang, Y.; Lan, X. Synthesis and Preclinical Evaluation of (18)F-PEG(3)-FPN for the Detection of Metastatic Pigmented Melanoma. Mol. Pharm. 2017, 14, 3896–3905. [Google Scholar] [CrossRef]
- Bashir, U.; Mallia, A.; Stirling, J.; Joemon, J.; MacKewn, J.; Charles-Edwards, G.; Goh, V.; Cook, G.J. PET/MRI in Oncological Imaging: State of the Art. Diagnostics 2015, 5, 333–357. [Google Scholar] [CrossRef] [Green Version]
- Nensa, F.; Beiderwellen, K.; Heusch, P.; Wetter, A. Clinical applications of PET/MRI: Current status and future perspectives. Diagn. Interv. Radiol. 2014, 20, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Donati, O.F.; Hany, T.F.; Reiner, C.S.; von Schulthess, G.K.; Marincek, B.; Seifert, B.; Weishaupt, D. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: Comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J. Nucl. Med. 2010, 51, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Beiderwellen, K.; Gomez, B.; Buchbender, C.; Hartung, V.; Poeppel, T.D.; Nensa, F.; Kuehl, H.; Bockisch, A.; Lauenstein, T.C. Depiction and characterization of liver lesions in whole body [¹⁸F]-FDG PET/MRI. Eur. J. Radiol. 2013, 82, e669–e675. [Google Scholar] [CrossRef]
- Reiner, C.S.; Stolzmann, P.; Husmann, L.; Burger, I.A.; Hüllner, M.W.; Schaefer, N.G.; Schneider, P.M.; von Schulthess, G.K.; Veit-Haibach, P. Protocol requirements and diagnostic value of PET/MR imaging for liver metastasis detection. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Lambiase, A.; Mantelli, F.; Sacchetti, M.; Rossi, S.; Aloe, L.; Bonini, S. Clinical applications of NGF in ocular diseases. Arch. Ital. Biol. 2011, 149, 283–292. [Google Scholar] [CrossRef]
- Pereira, P.R.; Odashiro, A.N.; Lim, L.A.; Miyamoto, C.; Blanco, P.L.; Odashiro, M.; Maloney, S.; De Souza, D.F.; Burnier, M.N., Jr. Current and emerging treatment options for uveal melanoma. Clin. Ophthalmol. 2013, 7, 1669–1682. [Google Scholar] [CrossRef] [Green Version]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, II: Characteristics of patients enrolled and not enrolled. COMS Report No. 17. Arch. Ophthalmol. 2001, 119, 951–965. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.F.; Hodes, B.L. Ultrasonographic diagnosis of choroidal malignant melanoma. Surv. Ophthalmol. 1977, 22, 29–40. [Google Scholar] [CrossRef]



| Imaging Method | Advantages | Limitations | Resolution |
|---|---|---|---|
| US | noninvasive wide availability elementary tool in the initial diagnosis visualization of lesion shape, structure, and vascularization good imaging of retinal detachment, vascular malformations, and hemorrhage good assessment of extrascleral extension and adjacent structures involvement ultrasound biomicroscopy as a developed method in diagnostics relatively low price, between USD 155 and USD 721 | operator-dependent method diagnostic difficulties in case of muscle atypical localization or vortex vein enlargement conditions mimicking uveal melanoma such as choroidal naevus, choroidal nevi, metastatic neoplasms, choroidal hemangiomas, disciform lesions, and a choroidal hemorrhages tumors less than 1 mm in thickness could be unnoticed overestimation of the tumor dimension compared to MR | 150 and 450 µm, 30 and 60 µm (UBM) |
| OCT | noninvasive radiation-free high spatial resolution can provide images of tissue microstructure can visualize small lesions in the anterior eye segment low price of around USD 200 on average | small tissue depth that can be visualized not suitable for imaging of pigmented lesions as image shadowing occurs limited use for detection and measurements of posterior eye segment tumors | 10 μm or up to 1 μm in UHR-OCT |
| FFA/ICGA/FAF | noninvasive radiation-free easily accessible possible differential diagnosis use in treatment follow-up (FAF) or assessing the proper placement of radioactive plaque (FAF) | motion artifacts subjective and nonquantitative interpretation of images low diagnostic accuracy when used alone | 7.4 to 5 μm |
| SPECT | noninvasive more sensitive than PET in primary tumor diagnosis suitable for small uveal melanoma diagnosis possible diagnosis in atypical manifestation or ocular complications use of melanic-specific radionuclides (123I-IMP) less expensive (USD 1900 on average) and more available than PET/CT | accurate diagnosis is highly time-dependent (up to 48 h) not suitable for amelanotic melanoma diagnosis exposure to radiopharmaceuticals and X-ray radiation with the use of SPECT and CT hybrid | 9.3 mm full-width at half maximum (FWHM) |
| FI | noninvasive, safe, easy to perform, and cost-effective, digital photos can be easily stored and transmitted for consultation | Inability to access deeper layers of the retina, optical aberrations, or cataract influence on image quality, 2D representation of the spatial structure (possible artifacts and peripheral aberrations) | Depends on CCD or phone camera resolution (14 µm per pixel) |
| CT | high sensitivity, whole-body imaging, a large area covered during acquisition, moderate/high-resolution, less movement artifacts, scanning of a broader range of patients (with metal depositions, pacemaker, etc.), short time of scan (anxiety patients or with claustrophobia) | false positives, radiation exposure, high cost of replacement X-ray tubes, and time-consuming analysis of data | 500–625 µm |
| PET/CT | noninvasive diagnosis of medium and large primary tumors sensitive in the diagnosis of distant metastases, especially in the liver suitable for metastatic disease prognostication can evaluate early treatment response with higher sensitivity than MRI | not widely available risk of falsely positive results in inflammation, infection, and trauma not suitable for diagnosis of small primary tumors exposure to radiation resulting in increased cancer risk very expensive (USD 2227 on average) | PET cameras can provide images with a spatial resolution of approximately 2.4 mm full-width at half maximum (FWHM) |
| MRI | noninvasive without exposition to ionizing radiation favorable soft tissue contrast a convenient method to visualize intra- and extraocular involvement good to measure the tumor used to detect distant metastases, especially in the liver used in choosing the therapeutic method | difficult to distinguish amelanotic melanoma from melanotic ones choroidal metastases could mimic a uveal melanoma difficult to distinguish tumor from vitreous hemorrhage movement artifacts relatively long duration time of approximately 20 min the relatively high price of EUR 200–EUR 1000 | 3T MRI 800 µm, 7T 500 to 650 µm, and 32 μm obtained by 9.4T MRI |
| Feature | 18F-NaF | 18F-5-FPN | 18F-MEL050 | 18F-PEG3-FPN |
|---|---|---|---|---|
| Molecular target | Bone remodeling | Melanin | Melanin | Melanin |
| Current use | Detection of bone metastases in prostate, breast, lung, and thyroid cancer, and diagnosis of fibrous dysplasia | Preclinical studies detecting small metastases from malignant melanoma in lymph nodes and lungs | Preclinical studies detecting small metastases from malignant melanoma in lymph nodes and lungs | Preclinical studies detecting small metastases from malignant melanoma in lungs and liver |
| Potential use in uveal melanoma | Detection of bone metastases, assessing extrascleral extension of the primary tumor | Detecting submillimeter metastases in lungs and lymph nodes | Detecting submillimeter metastases in lungs and lymph nodes | Detecting submillimeter metastases in liver and lungs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solnik, M.; Paduszyńska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Chorągiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of Uveal Melanoma—Current Standard and Methods in Development. Cancers 2022, 14, 3147. https://doi.org/10.3390/cancers14133147
Solnik M, Paduszyńska N, Czarnecka AM, Synoradzki KJ, Yousef YA, Chorągiewicz T, Rejdak R, Toro MD, Zweifel S, Dyndor K, et al. Imaging of Uveal Melanoma—Current Standard and Methods in Development. Cancers. 2022; 14(13):3147. https://doi.org/10.3390/cancers14133147
Chicago/Turabian StyleSolnik, Małgorzata, Natalia Paduszyńska, Anna M. Czarnecka, Kamil J. Synoradzki, Yacoub A. Yousef, Tomasz Chorągiewicz, Robert Rejdak, Mario Damiano Toro, Sandrine Zweifel, Katarzyna Dyndor, and et al. 2022. "Imaging of Uveal Melanoma—Current Standard and Methods in Development" Cancers 14, no. 13: 3147. https://doi.org/10.3390/cancers14133147







