Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art
Abstract
Simple Summary
Abstract
1. Introduction
2. Classification of Gliomas
3. Technical Overview
3.1. Pediatric Population
3.2. Older Children and Adults
3.3. Sequence Planning—1H-MRS
3.4. Sequence Planning—31P-MRS
4. Diagnosis and 1H-MRS
4.1. Pediatric Population
4.2. Adult Population
4.2.1. Low-Grade vs. High-Grade
4.2.2. Follow-Up and MRS
5. Future Aspects and 31P-MRS
6. Limitations
6.1. 1H-MRS
6.2. 31P-MRS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KIAA1549 | protein KIAA1549 |
| BRAF | proto-oncogene B-Raf |
| NF1 | NF1-Gene |
| ATRX | ATP-dependent helicase ATRX |
| CDKN2A/B | cyclin-dependent kinase inhibitor 2A |
| TSC2 | tuberous sclerosis 2 gene |
| PRKCA | Protein Kinase C Alpha |
| MN1 | meningioma (disrupted in balanced translocation) 1 |
| MYB | MYB proto-oncogene |
| MYBL1 | MYB proto-oncogene like 1 gene |
| FGFR | Fibroblast growth factor receptor |
| H3 K27 | 27th amino acid in Histone H3 |
| TP53 | transformation-related protein 53 |
| ACVR1 | Activin A receptor, type I |
| PDGFRA | platelet-derived growth factor receptor A |
| EGFR | epidermal growth factor receptor |
| EZHIP | EZH inhibitory protein |
| IDH | Isocitrate dehydrogenase |
| MYCN | N-myc proto-oncogene protein |
| NTRK | neurotrophe tyrosin-receptor kinase |
| ALK | anaplastic lymphoma kinase |
| ROS | Reactive oxygen species |
| MET | mesenchymal-epithelial transition factor |
| TERT | telomerase reverse transcriptase |
| CIC | capicua |
| FUBP1 | far upstream element binding protein 1 |
| NOTCH1 | Neurogenic locus notch homolog protein 1 |
References
- Brennan, P.; Butler, H.; Christie, L.; Hegarty, M.; Jenkinson, M.; Keerie, C.; Norrie, J.; o’Brien, R.; Palmer, D.; Smith, B.; et al. Early diagnosis of brain tumours using a novel spectroscopic liquid biopsy. Brain Commun. 2021, 3, fcab056. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Mawrin, C.; Scherlach, C.; Skalej, M.; Firsching, R. Gliomas in Adults. Dtsch. Ärzteblatt Int. 2010, 107, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Duc, N. The role of diffusion tensor imaging metrics in the discrimination between cerebellar medulloblastoma and brainstem glioma. Pediatr. Blood Cancer 2020, 67, e28468. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.M.; Huy, H.Q.; Nadarajan, C.; Keserci, B. The Role of Predictive Model Based on Quantitative Basic Magnetic Resonance Imaging in Differentiating Medulloblastoma from Ependymoma. Anticancer Res. 2020, 40, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Durmo, F.; Rydelius, A.; Baena, S.; Askaner, K.; Lätt, J.; Bengzon, J.; Englund, E.; Chenevert, T.; Björkman-Burtscher, I.; Maly Sundgren, P. Multivoxel H-MR Spectroscopy Biometrics for Preoprerative Differentiation Between Brain Tumors. Tomography 2018, 4, 172–181. [Google Scholar] [CrossRef]
- Weybright, P.; Maly Sundgren, P.; Maly, P.; Hassan, D.; Nan, B.; Rohrer, S.; Junck, L. Differentiation Between Brain Tumor Recurrence and Radiation Injury Using MR Spectroscopy. AJR Am. J. Roentgenol. 2005, 185, 1471–1476. [Google Scholar] [CrossRef]
- Ellingson, B.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.; Smits, M.; Nelson, S.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-Oncology 2015, 17, 1188–1198. [Google Scholar] [CrossRef]
- Malik, D.G.; Rath, T.J.; Urcuyo Acevedo, J.C.; Canoll, P.D.; Swanson, K.R.; Boxerman, J.L.; Quarles, C.C.; Schmainda, K.M.; Burns, T.C.; Hu, L.S. Advanced MRI Protocols to Discriminate Glioma From Treatment Effects: State of the Art and Future Directions. Front. Radiol. 2022, 2, 809373. [Google Scholar] [CrossRef]
- Louis, D.; Perry, A.; Reifenberger, G.; Deimling, A.; Figarella-Branger, D.; Cavenee, W.; Ohgaki, H.; Wiestler, O.; Kleihues, P.; Ellison, D. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.; Perry, A.; Wesseling, P.; Brat, D.; Cree, I.; Figarella-Branger, D.; Hawkins, C.; Ng, H.; Pfister, S.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Collins, V.; Jones, D.; Giannini, C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.; Perez, E.; Chirica, M.; Onken, J.; Kahn, J.; Brenner, W.; Ehret, F.; Euskirchen, P.; Koch, A.; Capper, D.; et al. High-grade astrocytoma with piloid features (HGAP): The Charité experience with a new central nervous system tumor entity. J. Neuro-Oncol. 2021, 153, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Brahmbhatt, N.; Kruser, T.J.; Kam, K.; Appin, C.; Wadhwani, N.; Chandler, J.; Md, P.; Lukas, R. Pleomorphic xanthoastrocytoma: A brief review. CNS Oncol. 2019, 8, CNS39. [Google Scholar] [CrossRef]
- Giannikou, K.; Zhu, Z.; Kim, J.; Winden, K.; Tyburczy, M.; Marron, D.; Parker, J.; Hebert, Z.; Bongaarts, A.; Taing, L.; et al. Subependymal giant cell astrocytomas are characterized by mTORC1 hyperactivation, a very low somatic mutation rate and a unique gene expression profile. Mod. Pathol. 2020, 34, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.; Mondal, G.; Hyun, M.; Ruiz, D.; Lin, Y.H.; Ziffle, J.; Joseph, N.; Onodera, C.; Talevich, E.; Grenert, J.; et al. A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat. Commun. 2018, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Orr, B.; Toprak, U.; Hovestadt, V.; Jones, D.; Capper, D.; Sill, M.; Buchhalter, I.; Northcott, P.; Leis, I.; et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 2016, 164, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Ellison, D.; Hawkins, C.; Jones, D.; Onar-Thomas, A.; Pfister, S.; Reifenberger, G.; Louis, D. cIMPACT-NOW update 4: Diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019, 137, 683–687. [Google Scholar] [CrossRef]
- Huse, J.; Snuderl, M.; Jones, D.; Brathwaite, C.; Altman, N.; Lavi, E.; Saffery, R.; Sexton-Oates, A.; Blümcke, I.; Capper, D.; et al. Polymorphous low-grade neuroepithelial tumor of the young (PLNTY): An epileptogenic neoplasm with oligodendroglioma-like components, aberrant CD34 expression, and genetic alterations involving the MAP kinase pathway. Acta Neuropathol. 2017, 133, 417–429. [Google Scholar] [CrossRef]
- Qaddoumi, I.; Orisme, W.; Wen, J.; Santiago, T.; Gupta, K.; Dalton, J.; Tang, B.; Haupfear, K.; Punchihewa, C.; Easton, J.; et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016, 131, 833–845. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Bartels, U.; Bouffet, E.; Becher, O.; Hawkins, C. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: Diagnostic and therapeutic implications. Acta Neuropathol. 2014, 128, 573–581. [Google Scholar] [CrossRef]
- Gianno, F.; Antonelli, M.; Dio, T.; Minasi, S.; Donofrio, V.; Buccoliero, A.; Gardiman, M.; Pollo, B.; Camassei, F.; Rossi, S.; et al. Correlation Between Immunohistochemistry and Sequencing in H3G34-Mutant Gliomas. Am. J. Surg. Pathol. 2021, 45, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Gessi, M.; Gielen, G.; Hammes, J.; Dörner, E.; Mühlen, A.; Waha, A.; Pietsch, T. H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: Possible diagnostic and therapeutic implications? J. Neuro-Oncol. 2013, 112, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.; Jones, D.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.; Khuong Quang, D.A.; Tönjes, M.; et al. Driver mutations in histone H3.3 and chromatin remodeling genes in pediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Schrimpf, D.; Ryzhova, M.; Sturm, D.; Chavez, L.; Hovestadt, V.; Sharma, T.; Habel, A.; Burford, A.; Jones, C.; et al. H3-/IDH-wild type pediatric glioblastoma is comprised of molecularly and prognostically distinct subtypes with associated oncogenic drivers. Acta Neuropathol. 2017, 134, 507–516. [Google Scholar] [CrossRef]
- Guerreiro Stucklin, A.; Ryall, S.; Fukuoka, K.; Zápotocký, M.; Lassaletta, A.; Li, C.; Bridge, T.; Kim, B.; Arnoldo, A.; Kowalski, P.; et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat. Commun. 2019, 10, 4343. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.; Jin, G.; Mclendon, R.; Rasheed, B.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, P.; Mansouri, A.; Das, S. The role of ATRX in glioma biology. Front. Oncol. 2017, 7, 236. [Google Scholar] [CrossRef]
- Capper, D.; Zentgraf, H.; Balss, J.; Hartmann, C.; Deimling, A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009, 118, 599–601. [Google Scholar] [CrossRef]
- Liu, X.; Gerges, N.; Korshunov, A.; Sabha, N.; Khuong Quang, D.A.; Fontebasso, A.; Fleming, A.; Hadjadj, D.; Schwartzentruber, J.; Majewski, J.; et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012, 124, 615–625. [Google Scholar] [CrossRef]
- Pekmezci, M.; Rice, T.; Molinaro, A.; Hansen, H.; McCoy, L.; Tihan, T.; Giannini, C.; Eckel-Passow, J.; Lachance, D.; Wiencke, J.; et al. OS07.8 Adult infiltrating giomas with WHO 2016 integrated diagnosis: Additional prognostic roles of ATRX and TERT. Neuro-Oncology 2017, 19, iii15. [Google Scholar] [CrossRef][Green Version]
- Siemens. Syngo MR B19, Basic Manual-Spectroscopy; Booklet; Siemens Healthineers AG: Munich, Germany, 2012. [Google Scholar]
- Pinggera, D.; Steiger, R.; Bauer, M.; Kerschbaumer, J.; Luger, M.; Beer, R.; Rietzler, A.; Grams, A.; Gizewski, E.; Thomé, C.; et al. Cerebral Energy Status and Altered Metabolism in Early Severe TBI: First Results of a Prospective 31P-MRS Feasibility Study. Neurocrit. Care 2020, 34, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Rietzler, A.; Steiger, R.; Mangesius, S.; Walchhofer, L.M.; Gothe, R.M.; Schocke, M.; Gizewski, E.R.; Grams, A.E. Energy metabolism measured by 31P magnetic resonance spectroscopy in the healthy human brain. J. Neuroradiol. 2021, in press. [CrossRef] [PubMed]
- Hattingen, E.; Lanfermann, H.; Menon, S.; Neumann-Haefelin, T.; DuMesnil de Rochement, R.; Stamelou, M.; Höglinger, G.; Magerkurth, J.; Pilatus, U. Combined 1H and 31P MR spectroscopic imaging: Impaired energy metabolism in severe carotid stenosis and changes upon treatment. Magn. Reson. Mater. Phys. Biol. Med. 2009, 22, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Alrayahi, J.; Zápotocký, M.; Ramaswamy, V.; Hanagandi, P.; Branson, H.; Mubarak, W.; Raybaud, C.; Laughlin, S. Pediatric Brain Tumor Genetics: What Radiologists Need to Know. RadioGraphics 2018, 38, 7. [Google Scholar] [CrossRef]
- Donia, M.; Abougabal, A.; Zakaria, Y.; Farhoud, A. Role of proton magnetic resonance spectroscopy in diagnosis of pilocytic astrocytoma in children. Alex. J. Med. 2012, 48, 131–137. [Google Scholar] [CrossRef]
- Porto, L.; Kieslich, M.; Franz, K.; Lehrnbecher, T.; Zanella, F.; Pilatus, U.; Hattingen, E. MR spectroscopy differentiation between high and low grade astrocytomas: A comparison between paediatric and adult tumours. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2010, 15, 214–221. [Google Scholar] [CrossRef]
- Hasan, A.M.; Hasan, A.; Megally, H.; Khallaf, M.; Haseib, A. The combined role of MR spectroscopy and perfusion imaging in preoperative differentiation between high- and low-grade gliomas. Egypt. J. Radiol. Nucl. Med. 2019, 50, 72. [Google Scholar] [CrossRef]
- Shakir, T.; Fengli, L.; Chenguang, G.; Chen, N.; Zhang, M.; Shaohui, M. 1H-MR spectroscopy in grading of cerebral glioma: A new view point, MRS image quality assessment. Acta Radiol. Open 2022, 11, 205846012210770. [Google Scholar] [CrossRef]
- Grossman, R.; Yousem, D. Neuroradiology: The Requisites; Requisites in Radiology; Mosby: St. Louis, MO, USA, 2003. [Google Scholar]
- Toyooka, M.; Kimura, H.; Uematsu, H.; Kawamura, Y.; Takeuchi, H.; Itoh, H. Tissue characterization of glioma by proton magnetic resonance spectroscopy and perfusion-weighted magnetic resonance imaging: Glioma grading and histological correlation. Clin. Imaging 2008, 32, 251–258. [Google Scholar] [CrossRef]
- Öz, G.; Alger, J.; Barker, P.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.; Brindle, K.; Cudalbu, C.; Dincer, A.; et al. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.; Wind, J.; Iorgulescu, J.; Chan, T.; Yamada, Y.; Sherman, J. Radiation necrosis following treatment of high grade glioma—A review of the literature and current understanding. Acta Neurochir. 2011, 154, 191–201; discussion 201. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Glantz, M.J.; Chalmers, L.; Van Horn, A.; Sloan, A.E. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J. Neuro-Oncol. 2007, 82, 81–83. [Google Scholar] [CrossRef]
- Easaw, J.; Mason, W.; Perry, J.; Laperriere, N.; Eisenstat, D.; Del Maestro, R.; Belanger, K.; Fulton, D.; Macdonald, D. Canadian Recommendations for the Treatment of Recurrent or Progressive Glioblastoma Multiforme. Curr. Oncol. (Toronto Ont.) 2011, 18, e126–e136. [Google Scholar] [CrossRef]
- Hygino da Cruz, L.C.; Rodriguez, I.; Domingues, R.; Gasparetto, E.; Sorensen, A. Pseudoprogression and Pseudoresponse: Imaging Challenges in the Assessment of Posttreatment Glioma. AJNR. Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef]
- Kazda, T.; Bulik, M.; Pospisil, P.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. NeuroImage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef]
- Smith, E.; Carlos, R.; Junck, L.; Tsien, C.; Elias, A.; Maly Sundgren, P. Developing a Clinical Decision Model: MR Spectroscopy to Differentiate Between Recurrent Tumor and Radiation Change in Patients with New Contrast-Enhancing Lesions. AJR Am. J. Roentgenol. 2009, 192, W45–W52. [Google Scholar] [CrossRef]
- Elias, A.E.; Carlos, R.C.; Smith, E.A.; Frechtling, D.; George, B.; Maly, P.; Sundgren, P.C. MR Spectroscopy Using Normalized and Non-normalized Metabolite Ratios for Differentiating Recurrent Brain Tumor from Radiation Injury. Acad. Radiol. 2011, 18, 1101–1108. [Google Scholar] [CrossRef]
- Bulik, M.; Kazda, T.; Slampa, P.; Jancalek, R. The Diagnostic Ability of Follow-Up Imaging Biomarkers after Treatment of Glioblastoma in the Temozolomide Era: Implications from Proton MR Spectroscopy and Apparent Diffusion Coefficient Mapping. BioMed Res. Int. 2015, 2015, 641023. [Google Scholar] [CrossRef]
- Zeng, Q.S.; Li, C.F.; Liu, H.; Zhen, J.H.; Feng, D.C. Distinction Between Recurrent Glioma and Radiation Injury Using Magnetic Resonance Spectroscopy in Combination with Diffusion-Weighted Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Moustafa, H.; Ahmed, E.; El-Toukhy, M. Glioma residual or recurrence versus radiation necrosis: Accuracy of pentavalent technetium-99m-dimercaptosuccinic acid [Tc-99m (V) DMSA] brain SPECT compared to proton magnetic resonance spectroscopy (1H-MRS): Initial results. J. Neuro-Oncol. 2011, 106, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sawlani, V.; Taylor, R.; Rowley, K.; Redfern, R.; Martin, J.; Poptani, H. Magnetic Resonance Spectroscopy for Differentiating Pseudo-Progression from True Progression in GBM on Concurrent Chemoradiotherapy. Neuroradiol. J. 2012, 25, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Walecki, J.; Sokól, M.; Pieniążek, P.; Maciejewski, B.; Tarnawski, R.; Krupska, T.; Wydmański, J.; Brzeziński, J.; Grieb, P. Role of short TE 1H-MR spectroscopy in monitoring of post-operation irradiated patients. Eur. J. Radiol. 1999, 30, 154–161. [Google Scholar] [CrossRef]
- Rock, J.; Hearshen, D.; Scarpace, L.; Croteau, D.; Gutierrez, J.; Fisher, J.; Rosenblum, M.; Mikkelsen, T. Correlations between Magnetic Resonance Spectroscopy and Image-guided Histopathology, with Special Attention to Radiation Necrosis. Neurosurgery 2002, 51, 912–919; discussion 919. [Google Scholar] [CrossRef]
- Barajas, R.; Chang, J.; Segal, M.; Parsa, A.; Mcdermott, M.; Berger, M.; Cha, S. Differentiation of Recurrent Glioblastoma Multiforme from Radiation Necrosis after External Beam Radiation Therapy with Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef]
- Van Dijken, B.; Laar, P.; Holtman, G.; Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef]
- Fèvre, C.; Constans, J.M.; Chambrelant, I.; Antoni, D.; Bund, C.; Leroy-Freschini, B.; Schott, R.; Cebula, H.; Noël, G. Pseudoprogression versus true progression in glioblastoma patients: A multiapproach literature review. Part 2—Radiological features and metric markers. Crit. Rev. Oncol. 2021, 159, 103230. [Google Scholar] [CrossRef]
- Anbarloui, M.; Ghodsi, S.; Khoshnevisan, A.; Khadivi, M.; Abdollahzade, S.; Aoude, A.; Naderi, S.; Najafi, Z.; Faghih-Jouibari, M. Accuracy of magnetic resonance spectroscopy in distinction between radiation necrosis and recurrence of brain tumors. Iran. J. Neurol. 2015, 14, 29–34. [Google Scholar]
- Galijašević, M.; Steiger, R.; Radović, I.; Birkl-Toeglhofer, A.M.; Birkl, C.; Deeg, L.; Mangesius, S.; Rietzler, A.; Regodić, M.; Stockhammer, G.; et al. Phosphorous Magnetic Resonance Spectroscopy and Molecular Markers in IDH1 Wild Type Glioblastoma. Cancers 2021, 13, 3569. [Google Scholar] [CrossRef]
- Wenger, K.; Steinbach, J.; Bähr, O.; Pilatus, U.; Hattingen, E. Lower Lactate Levels and Lower Intracellular pH in Patients with IDH-Mutant versus Wild-Type Gliomas. Am. J. Neuroradiol. 2020, 41, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Wenger, K.; Hattingen, E.; Franz, K.; Steinbach, J.; Bähr, O.; Pilatus, U. In vivo Metabolic Profiles as Determined by 31P and short TE 1H MR-Spectroscopy. Clin. Neuroradiol. 2019, 29, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wenger, K.; Hattingen, E.; Franz, K.; Steinbach, J.; Bähr, O.; Pilatus, U. Intracellular pH measured by 31 P-MR-spectroscopy might predict site of progression in recurrent glioblastoma under antiangiogenic therapy: Intracellular pH Measured by 31 P-MR-S. J. Magn. Reson. Imaging 2017, 46, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Grams, A.E.; Mangesius, S.; Steiger, R.; Radovic, I.; Rietzler, A.; Walchhofer, L.M.; Galijašević, M.; Mangesius, J.; Nowosielski, M.; Freyschlag, C.F.; et al. Changes in Brain Energy and Membrane Metabolism in Glioblastoma following Chemoradiation. Curr. Oncol. 2021, 28, 5041–5053. [Google Scholar] [CrossRef] [PubMed]
- Walchhofer, L.M.; Steiger, R.; Rietzler, A.; Kerschbaumer, J.; Freyschlag, C.; Stockhammer, G.; Gizewski, E.; Grams, A. Phosphorous Magnetic Resonance Spectroscopy to Detect Regional Differences of Energy and Membrane Metabolism in Naïve Glioblastoma Multiforme. Cancers 2021, 13, 2598. [Google Scholar] [CrossRef]
- Hnilicova, P.; Richterova, R.; Zelenak, K.; Kolarovszki, B.; Majercikova, Z.; Hatok, J. Noninvasive study of brain tumours metabolism using phosphorus-31 magnetic resonance spectroscopy. Bratisl. Lek. Listy 2020, 121, 488–492. [Google Scholar] [CrossRef]
- Maintz, D.; Heindel, W.; Kugel, H.; Jaeger, R.; Lackner, K.J. Phosphorus-31 MR spectroscopy of normal adult human brain and brain tumours. NMR Biomed. 2002, 15, 18–27. [Google Scholar] [CrossRef]
- Bulakbasi, N.; Kocaoglu, M.; Sanal, H.; Tayfun, C. Efficacy of in vivo 31Phosphorus Magnetic Resonance Spectroscopy in Differentiation and Staging of Adult Human Brain Tumors. Neuroradiol. J. 2007, 20, 646–655. [Google Scholar] [CrossRef]
- Hattingen, E.; Jurcoane, A.; Bähr, O.; Rieger, J.; Magerkurth, J.; Anti, S.; Steinbach, J.; Pilatus, U. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: A 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro-Oncology 2011, 13, 1349–1363. [Google Scholar] [CrossRef]
- Ha, D.H.; Choi, S.; Oh, J.; Yoon, S.K.; Kang, M.; Kim, K.U. Application of 31P MR Spectroscopy to the Brain Tumors. Korean J. Radiol. Off. J. Korean Radiol. Soc. 2013, 14, 477–486. [Google Scholar] [CrossRef]
- Kamble, R.B.; Jayakumar Peruvumba, N.; Shivashankar, R. Energy status and metabolism in intracranial space occupying lesions: A prospective 31p spectroscopic study. J. Clin. Diagn. Res. JCDR 2014, 8, RC05. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Chawla, S.; Mohan, S.; Wang, S.; Nasrallah, M.; Sheriff, S.; Desai, A.; Brem, S.; O’Rourke, D.; Wolf, R.; et al. Three-dimensional echo planar spectroscopic imaging for differentiation of true progression from pseudoprogression in patients with glioblastoma. NMR Biomed. 2018, 32, e4042. [Google Scholar] [CrossRef] [PubMed]
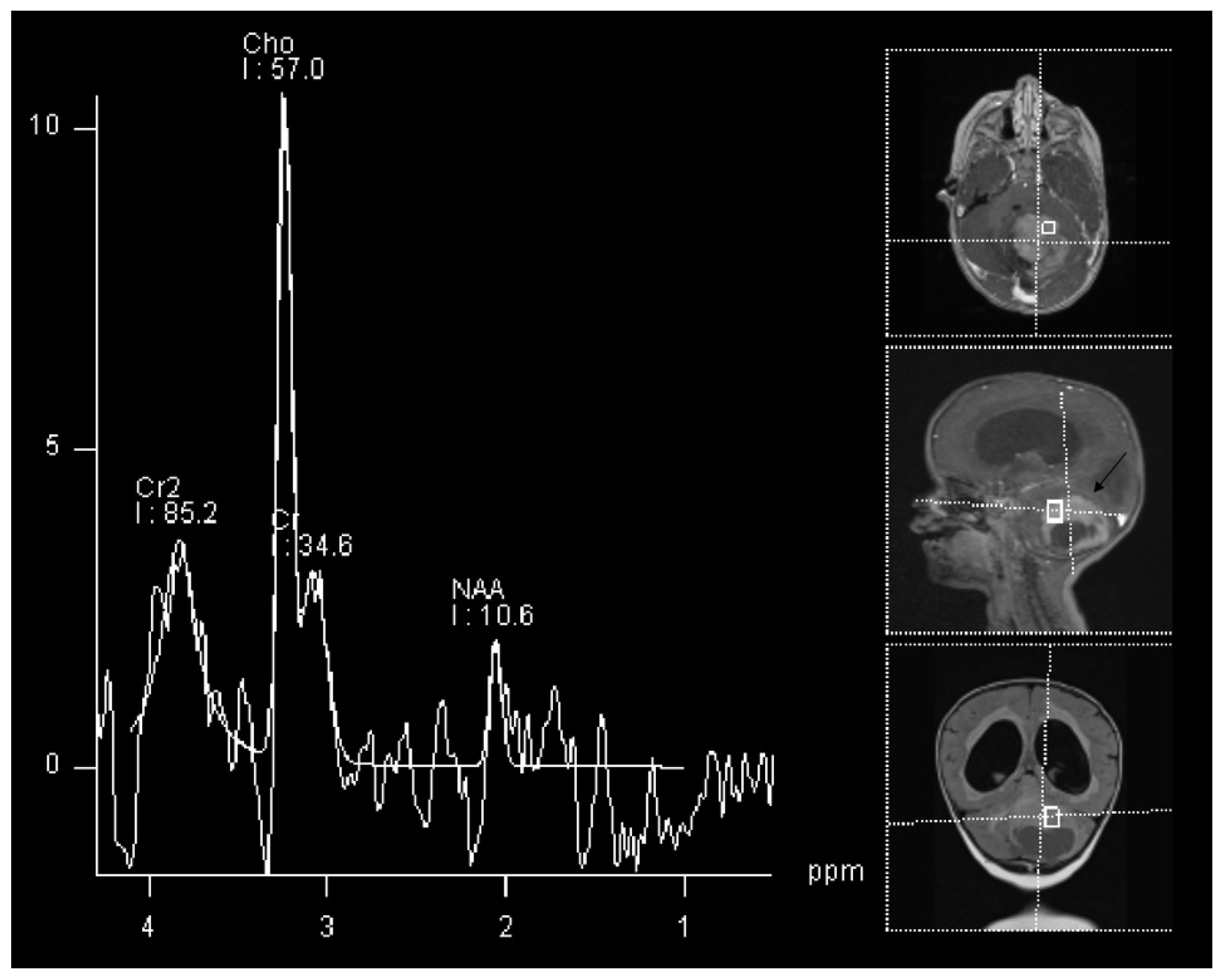

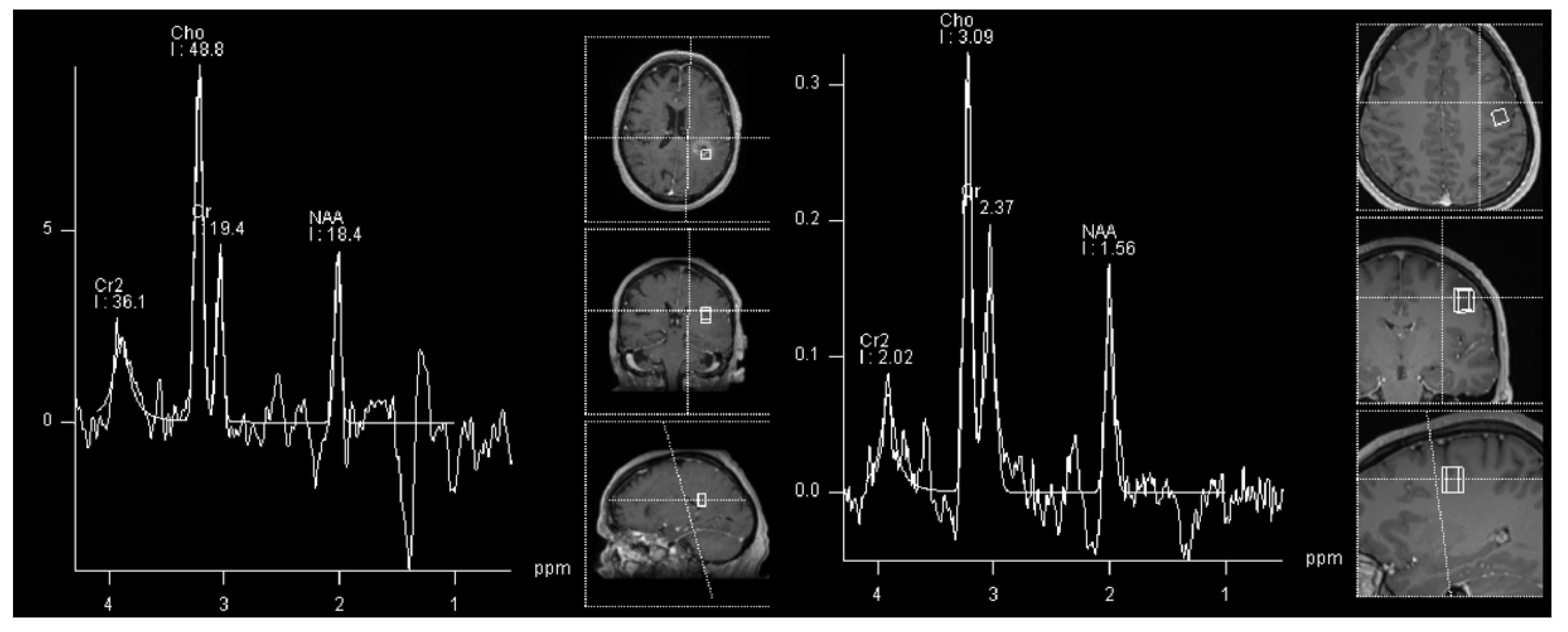
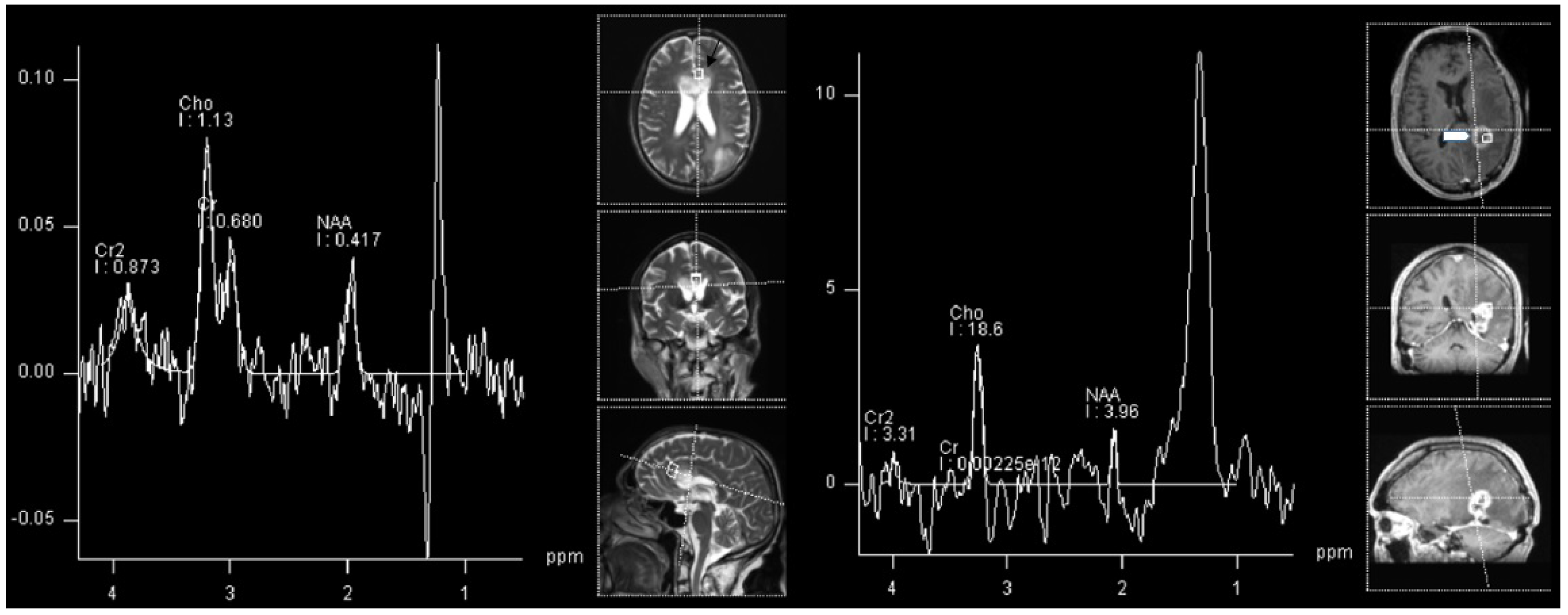
| WHO Grade | Most Common Molecular Features | |
|---|---|---|
| Circumstribed gliomas | ||
| Pilocytic astrocytoma | 1 | KIAA1549-BRAF [11] |
| High-grade astrocytoma with piloid features | new subtype | specific DNA-methylation profile [12] |
| Pleomorphic xanthoastrocytoma | 2, 3 | BRAF [13] |
| Subependymal giant cell astrocytoma | 1 | TSC1, TSC2 [14] |
| Chordoid glioma | 2 | PRKCA [15] |
| Astroblastoma, MN1-altered | new subtype | MN1 [16] |
| Pediatric diffuse low grade gliomas | ||
| Diffuse astrocytoma, MYB- or MYBL1-altered | 1 | MYB, MYBL1 [17] |
| Angiocentric glioma | 1 | MYB [10] |
| Polymorphous low-grade neuroepithelial tumor of the young | 1 | PLNTYs, BRAF, FGFR [18] |
| Diffuse low-grade glioma, MAPK pathway-altered | new subtype | FGFR1, BRAF [19] |
| Pediatric-type diffuse high-grade gliomas | ||
| Diffuse midline glioma, H3 K27-altered | 4 | H3 K27 [20] |
| Diffuse hemispheric glioma, H3 G34-mutant | 4 | H3F3A (G34R/V) [21], GFAP [22], p53 [23] |
| Diffuse pediatric-type high-grade glioma, H3- and IDH-wt | 4 | IDH-wt, H3-wt, MYCN, PDGFRA [24] |
| Infant-type hemispheric glioma | new subtype | NTRK, ALK, ROS1, MET [25] |
| Adult-type diffuse gliomas | ||
| Astrocytoma, IDH-mutant | 2, 3, 4 | IDH1, IDH2 [26], ATRX [27] |
| Oligodendroglioma, IDH-mutant, and 1p/19q-codeleted | 2, 3 | IDH [28], 1p19q-codeletion, ATRX, p53 [29] |
| Glioblastoma, IDH-wt | 4 | no IDH mutation (IDH-wt), ATRX, TERT [30] |
| Matrix | 8 × 8 × 8 |
| Field of view | 240 × 240 × 200 mm |
| Voxel size | 30 × 30 mm |
| Slice thickness | 25 mm |
| Repetition time | 2000 ms |
| Echo time | 2.3 ms |
| Flip angle | 90 |
| Pros | Cons |
|---|---|
| Important information about the nature of the lesions | Hardware, software and know-how considerations-cost |
| More accurate follow-up | Relatively time costly and artifact-prone sequence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galijasevic, M.; Steiger, R.; Mangesius, S.; Mangesius, J.; Kerschbaumer, J.; Freyschlag, C.F.; Gruber, N.; Janjic, T.; Gizewski, E.R.; Grams, A.E. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers 2022, 14, 3197. https://doi.org/10.3390/cancers14133197
Galijasevic M, Steiger R, Mangesius S, Mangesius J, Kerschbaumer J, Freyschlag CF, Gruber N, Janjic T, Gizewski ER, Grams AE. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers. 2022; 14(13):3197. https://doi.org/10.3390/cancers14133197
Chicago/Turabian StyleGalijasevic, Malik, Ruth Steiger, Stephanie Mangesius, Julian Mangesius, Johannes Kerschbaumer, Christian Franz Freyschlag, Nadja Gruber, Tanja Janjic, Elke Ruth Gizewski, and Astrid Ellen Grams. 2022. "Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art" Cancers 14, no. 13: 3197. https://doi.org/10.3390/cancers14133197
APA StyleGalijasevic, M., Steiger, R., Mangesius, S., Mangesius, J., Kerschbaumer, J., Freyschlag, C. F., Gruber, N., Janjic, T., Gizewski, E. R., & Grams, A. E. (2022). Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers, 14(13), 3197. https://doi.org/10.3390/cancers14133197







