Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Overview of Neoadjuvant Therapy
3. Non-Operative Management and Patient Selection
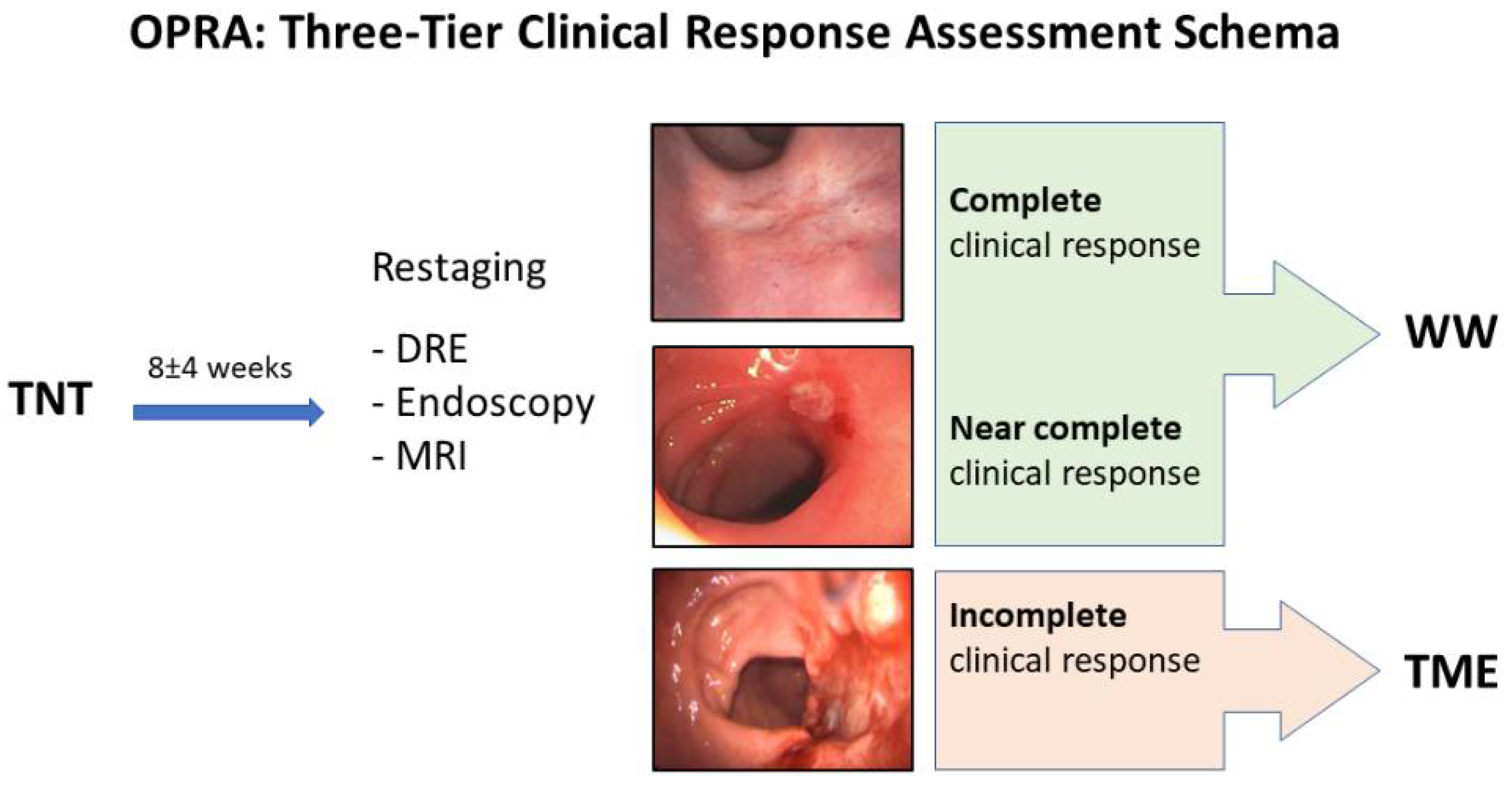
4. Assessment of Response
5. Optimizing Tumor Response: The OPRA Trial
- Patients with near-complete response at restaging can still be offered watch and wait with a close surveillance protocol;
- The rate of rectal cancer response to neoadjuvant therapy is much higher than previously thought and takes time to be achieved;
- Organ preservation is achievable in half of the patients with rectal cancer treated with TNT, particularly when consolidation chemotherapy is employed.
6. Oncologic Outcomes
- Non-responders are at risk of both local and distant relapse, which may be higher than the average LARC patient, but is likely due to more aggressive biology;
- The more aggressive biology of non-responders should be taken into consideration when making surgical decisions;
- The grade of clinical response in patients offered selective WW has similar prognostic value as pathologic response in groups of all treated with TME;
- The grade of clinical response at restaging after TNT predicts both organ preservation and oncologic outcomes.
7. Functional Outcomes
8. Summary and Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf2021 (accessed on 12 January 2021).
- Smith, J.J.; Chow, O.S.; Gollub, M.J.; Nash, G.M.; Temple, L.K.; Weiser, M.R.; Guillem, J.G.; Paty, P.B.; Avila, K.; Garcia-Aguilar, J. Organ Preservation in Rectal Adenocarcinoma: A phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015, 15, 767. [Google Scholar] [CrossRef] [Green Version]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Chow, O.S.; Smith, D.D.; Marcet, J.E.; Cataldo, P.A.; Varma, M.G.; Kumar, A.S.; Oommen, S.; Coutsoftides, T.; Hunt, S.R.; et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Ho, V.P.; Lee, Y.; Stein, S.L.; Temple, L.K. Sexual function after treatment for rectal cancer: A review. Dis. Colon Rectum 2011, 54, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Wiltink, L.M.; Nout, R.A.; Meershoek-Klein Kranenbarg, E.; Laurberg, S.; Marijnen, C.A.; van de Velde, C.J. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: Report of a multicenter randomized trial. Clin. Colorectal Cancer 2015, 14, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Palta, M.; Willett, C.G.; Czito, B.G. Short-course versus long-course chemoradiation in rectal cancer--time to change strategies? Curr. Treat. Options Oncol. 2014, 15, 421–428. [Google Scholar] [CrossRef]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rödel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Roh, M.S.; Colangelo, L.H.; O’Connell, M.J.; Yothers, G.; Deutsch, M.; Allegra, C.J.; Kahlenberg, M.S.; Baez-Diaz, L.; Ursiny, C.S.; Petrelli, N.J.; et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 5124–5130. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, S.M.; Yu, C.S.; Kim, J.H.; Kim, T.W.; Kim, J.C. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 2011, 117, 3703–3712. [Google Scholar] [CrossRef]
- Rödel, C.; Martus, P.; Papadoupolos, T.; Füzesi, L.; Klimpfinger, M.; Fietkau, R.; Liersch, T.; Hohenberger, W.; Raab, R.; Sauer, R.; et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8688–8696. [Google Scholar] [CrossRef]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beissbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: Updated results of the CAO/ARO/AIO-94 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Cedermark, B.; Dahlberg, M.; Glimelius, B.; Påhlman, L.; Rutqvist, L.E.; Wilking, N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N. Engl. J. Med. 1997, 336, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 22, 29–42. [Google Scholar] [CrossRef]
- Chin, R.I.; Roy, A.; Pedersen, K.S.; Huang, Y.; Hunt, S.R.; Glasgow, S.C.; Tan, B.R.; Wise, P.E.; Silviera, M.L.; Smith, R.K.; et al. Clinical Complete Response in Patients With Rectal Adenocarcinoma Treated With Short-Course Radiation Therapy and Nonoperative Management. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 715–725. [Google Scholar] [CrossRef]
- Ma, B.; Gao, P.; Song, Y.; Huang, X.; Wang, H.; Xu, Q.; Zhao, S.; Wang, Z. Short-Course Radiotherapy in Neoadjuvant Treatment for Rectal Cancer: A Systematic Review and Meta-analysis. Clin. Colorectal Cancer 2018, 17, 320–330. [Google Scholar] [CrossRef]
- Schmoll, H.-J.; Haustermans, K.; Price, T.J.; Nordlinger, B.; Hofheinz, R.; Daisne, J.-F.; Janssens, J.; Brenner, B.; Schmidt, P.; Reinel, H.; et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: First results of the PETACC-6 randomized phase III trial. J. Clin. Oncol. 2013, 31, 3531. [Google Scholar] [CrossRef]
- Banwell, V.C.; Phillips, H.A.; Duff, M.J.; Speake, D.; McLean, C.; Williams, L.J.; He, Y.; Paterson, H.M. Five-year oncological outcomes after selective neoadjuvant radiotherapy for resectable rectal cancer. Acta Oncol. 2019, 58, 1267–1272. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Büchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martos, C.; Garcia-Albeniz, X.; Pericay, C.; Maurel, J.; Aparicio, J.; Montagut, C.; Safont, M.J.; Salud, A.; Vera, R.; Massuti, B.; et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: Long-term results of the Spanish GCR-3 phase II randomized trial†. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Yothers, G.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Colangelo, L.H.; Lucas, P.C.; Gollub, M.J.; et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results From the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1225–1230. [Google Scholar] [CrossRef]
- Goffredo, P.; Khan, A.; Mott, S.L.; Jensen, C.; Madoff, R.; Gaertner, W.; You, N.; Hassan, I. Total Neoadjuvant Therapy versus Standard Neoadjuvant Chemoradiation in Patients with Locally Advanced Rectal Cancer: A Comparison of Short- and Long-term Oncologic Outcomes. Ann. Surg. 2021. [Google Scholar] [CrossRef]
- Garcia-Aguilar, J.; Patil, S.; Gollub, M.J.; Kim, J.K.; Yuval, J.B.; Thompson, H.M.; Verheij, F.S.; Omer, D.M.; Lee, M.; Dunne, R.F.; et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022, JCO-22. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Sabbaga, J.; Nadalin, W.; São Julião, G.P.; Gama-Rodrigues, J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: Results of a prospective study using additional chemotherapy during the resting period. Dis. Colon Rectum 2009, 52, 1927–1934. [Google Scholar] [CrossRef]
- Caycedo-Marulanda, A.; Patel, S.V.; Verschoor, C.P.; Uscategui, J.P.; Chadi, S.A.; Moeslein, G.; Chand, M.; Maeda, Y.; Monson, J.R.T.; Wexner, S.D.; et al. A Snapshot of the International Views of the Treatment of Rectal Cancer Patients, a Multi-regional Survey: International Tendencies in Rectal Cancer. World J. Surg. 2021, 45, 302–312. [Google Scholar] [CrossRef]
- Lynn, P.B.; Strombom, P.; Garcia-Aguilar, J. Organ-Preserving Strategies for the Management of Near-Complete Responses in Rectal Cancer after Neoadjuvant Chemoradiation. Clin. Colon Rectal Surg. 2017, 30, 395–403. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; Silva e Sousa, A.H., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711–717; discussion 717–718. [Google Scholar] [CrossRef]
- Maas, M.; Lambregts, D.M.; Nelemans, P.J.; Heijnen, L.A.; Martens, M.H.; Leijtens, J.W.; Sosef, M.; Hulsewé, K.W.; Hoff, C.; Breukink, S.O.; et al. Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment. Ann. Surg. Oncol. 2015, 22, 3873–3880. [Google Scholar] [CrossRef] [Green Version]
- Emmertsen, K.J.; Laurberg, S. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br. J. Surg. 2013, 100, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Brown, G. Preoperative staging of rectal cancer. Acta Oncol. 2008, 47, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Jang, J.K.; Park, I.J.; Park, S.H.; Kim, J.B.; Park, J.H.; Kim, T.W.; Ro, J.S.; Lim, S.B.; Yu, C.S.; et al. Lateral lymph node and its association with distant recurrence in rectal cancer: A clue of systemic disease. Surg. Oncol. 2020, 35, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Wu, X.Y.; Yu, R.; Li, C.; Wang, L.Q.; Shen, W.; Lu, P.H. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: A meta-analysis in rectal cancer. PLoS ONE 2012, 7, e45388. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Aguilar, J.; Chen, Z.; Smith, D.D.; Li, W.; Madoff, R.D.; Cataldo, P.; Marcet, J.; Pastor, C. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann. Surg. 2011, 254, 486–492; discussion 492–493. [Google Scholar] [CrossRef] [Green Version]
- de Rosa, N.; Rodriguez-Bigas, M.A.; Chang, G.J.; Veerapong, J.; Borras, E.; Krishnan, S.; Bednarski, B.; Messick, C.A.; Skibber, J.M.; Feig, B.W.; et al. DNA Mismatch Repair Deficiency in Rectal Cancer: Benchmarking Its Impact on Prognosis, Neoadjuvant Response Prediction, and Clinical Cancer Genetics. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3039–3046. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Wynn, G.; Marks, J.; Kessler, H.; Gama-Rodrigues, J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis. Colon Rectum 2010, 53, 1692–1698. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Peeters, K.; Beets, G.L.; Figueiredo, N.L.; Bastiaannet, E.; Vahrmeijer, A.; Temmink, S.J.D.; Meershoek-Klein Kranenbarg, W.M.E.; Roodvoets, A.G.H.; Habr-Gama, A.; et al. Watch and wait after a clinical complete response in rectal cancer patients younger than 50 years. Br. J. Surg. 2021, 109, 114–120. [Google Scholar] [CrossRef]
- Gani, C.; Gani, N.; Zschaeck, S.; Eberle, F.; Schaeffeler, N.; Hehr, T.; Berger, B.; Fischer, S.G.; Claßen, J.; Zipfel, S.; et al. Organ Preservation in Rectal Cancer: The Patients’ Perspective. Front. Oncol. 2019, 9, 318. [Google Scholar] [CrossRef]
- Kennedy, E.D.; Borowiec, A.M.; Schmocker, S.; Cho, C.; Brierley, J.; Li, S.; Victor, J.C.; Baxter, N.N. Patient and Physician Preferences for Nonoperative Management for Low Rectal Cancer: Is It a Reasonable Treatment Option? Dis. Colon Rectum 2018, 61, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Dijkhoff, R.A.P.; Beets-Tan, R.G.H.; Lambregts, D.M.J.; Beets, G.L.; Maas, M. Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review. Eur. J. Radiol. 2017, 95, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, X.Y.; Shi, Y.J.; Wang, L.; Zhu, H.T.; Tang, Z.; Wang, S.; Li, X.T.; Tian, J.; Sun, Y.S. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 7253–7262. [Google Scholar] [CrossRef] [Green Version]
- Koyama, F.C.; Lopes Ramos, C.M.; Ledesma, F.; Alves, V.A.F.; Fernandes, J.M.; Vailati, B.B.; São Julião, G.P.; Habr-Gama, A.; Gama-Rodrigues, J.; Perez, R.O.; et al. Effect of Akt activation and experimental pharmacological inhibition on responses to neoadjuvant chemoradiotherapy in rectal cancer. Br. J. Surg. 2018, 105, e192–e203. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Weiser, M.R.; Goodman, K.A.; Gonen, M.; Hollywood, E.; Cercek, A.; Reidy-Lagunes, D.L.; Gollub, M.J.; Shia, J.; Guillem, J.G.; et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: A pilot trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Conroy, T.; Lamfichekh, N.; Etienne, P.L.; Rio, E.; Francois, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2020, 38, 4007. [Google Scholar] [CrossRef]
- Probst, C.P.; Becerra, A.Z.; Aquina, C.T.; Tejani, M.A.; Wexner, S.D.; Garcia-Aguilar, J.; Remzi, F.H.; Dietz, D.W.; Monson, J.R.; Fleming, F.J. Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J. Am. Coll Surg. 2015, 221, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Fokas, E.; Allgäuer, M.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 3212–3222. [Google Scholar] [CrossRef]
- Fokas, E.; Schlenska-Lange, A.; Polat, B.; Klautke, G.; Grabenbauer, G.G.; Fietkau, R.; Kuhnt, T.; Staib, L.; Brunner, T.; Grosu, A.L.; et al. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022, 8, e215445. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; You, Y.N.; Agarwal, A.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Eng, C.; Feig, B.W.; Das, P.; Krishnan, S.; Crane, C.H.; et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1770–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Valk, M.J.M.; Hilling, D.E.; Bastiaannet, E.; Meershoek-Klein Kranenbarg, E.; Beets, G.L.; Figueiredo, N.L.; Habr-Gama, A.; Perez, R.O.; Renehan, A.G.; van de Velde, C.J.H. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. Lancet 2018, 391, 2537–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habr-Gama, A.; Gama-Rodrigues, J.; São Julião, G.P.; Proscurshim, I.; Sabbagh, C.; Lynn, P.B.; Perez, R.O. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 822–828. [Google Scholar] [CrossRef]
- Dossa, F.; Chesney, T.R.; Acuna, S.A.; Baxter, N.N. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 501–513. [Google Scholar] [CrossRef]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg. 2012, 99, 918–928. [Google Scholar] [CrossRef]
- Smith, J.J.; Strombom, P.; Chow, O.S.; Roxburgh, C.S.; Lynn, P.; Eaton, A.; Widmar, M.; Ganesh, K.; Yaeger, R.; Cercek, A.; et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019, 5, e185896. [Google Scholar] [CrossRef]
- Jimenez-Rodriguez, R.M.; Quezada-Diaz, F.; Hameed, I.; Kalabin, A.; Patil, S.; Smith, J.J.; Garcia-Aguilar, J. Organ Preservation in Patients with Rectal Cancer Treated with Total Neoadjuvant Therapy. Dis. Colon Rectum 2021, 64, 1463–1470. [Google Scholar] [CrossRef]
- Thompson, H.; Kim, J.K.; Yuval, J.B.; Verheij, F.; Patil, S.; Gollub, M.J.; Wu, A.J.-C.; Lee, M.; Hezel, A.F.; Marcet, J.; et al. Survival and organ preservation according to clinical response after total neoadjuvant therapy in locally advanced rectal cancer patients: A secondary analysis from the organ preservation in rectal adenocarcinoma (OPRA) trial. J. Clin. Oncol. 2021, 39, 3509. [Google Scholar] [CrossRef]
- Quezada-Diaz, F.F.; Smith, J.J.; Jimenez-Rodriguez, R.M.; Wasserman, I.; Pappou, E.P.; Patil, S.; Wei, I.H.; Nash, G.M.; Guillem, J.G.; Weiser, M.R.; et al. Patient-Reported Bowel Function in Patients With Rectal Cancer Managed by a Watch-and-Wait Strategy After Neoadjuvant Therapy: A Case-Control Study. Dis. Colon Rectum 2020, 63, 897–902. [Google Scholar] [CrossRef]
- Hupkens, B.J.P.; Martens, M.H.; Stoot, J.H.; Berbee, M.; Melenhorst, J.; Beets-Tan, R.G.; Beets, G.L.; Breukink, S.O. Quality of Life in Rectal Cancer Patients After Chemoradiation: Watch-and-Wait Policy Versus Standard Resection—A Matched-Controlled Study. Dis. Colon Rectum 2017, 60, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aguilar, J.; Smith, D.D.; Avila, K.; Bergsland, E.K.; Chu, P.; Krieg, R.M. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase II prospective trial. Ann. Surg. 2011, 254, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rödel, P. Short-Course Radiotherapy Versus Chemoradiotherapy, Followed by Consolidation Chemotherapy, and Selective Organ Preservation for MRI-Defined Intermediate and High-Risk Rectal Cancer Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT042466842021 (accessed on 5 May 2022).
- Cercek, A.; Stadler, Z.K.; Cohen, J.L.; Weiss, J.A.; Lamendola-Essel, M.F.; Krishnan, A.; Yaeger, R.; Segal, N.H.; Connell, L.C.; Dika, I.H.E.; et al. A phase II study of induction PD-1 blockade in subjects with locally advanced mismatch repair-deficient rectal adenocarcinoma. J. Clin. Oncol. 2020, 38, TPS4123. [Google Scholar] [CrossRef]
- Ciombor, K.K. Testing Nivolumab and Ipilimumab With Short-Course Radiation in Locally Advanced Rectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT047513702021 (accessed on 14 May 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goffredo, P.; Quezada-Diaz, F.F.; Garcia-Aguilar, J.; Smith, J.J. Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers 2022, 14, 3204. https://doi.org/10.3390/cancers14133204
Goffredo P, Quezada-Diaz FF, Garcia-Aguilar J, Smith JJ. Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers. 2022; 14(13):3204. https://doi.org/10.3390/cancers14133204
Chicago/Turabian StyleGoffredo, Paolo, Felipe F. Quezada-Diaz, Julio Garcia-Aguilar, and J. Joshua Smith. 2022. "Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial" Cancers 14, no. 13: 3204. https://doi.org/10.3390/cancers14133204
APA StyleGoffredo, P., Quezada-Diaz, F. F., Garcia-Aguilar, J., & Smith, J. J. (2022). Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers, 14(13), 3204. https://doi.org/10.3390/cancers14133204





