Skin Surface Dose for Whole Breast Radiotherapy Using Personalized Breast Holder: Comparison with Various Radiotherapy Techniques and Clinical Experiences
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
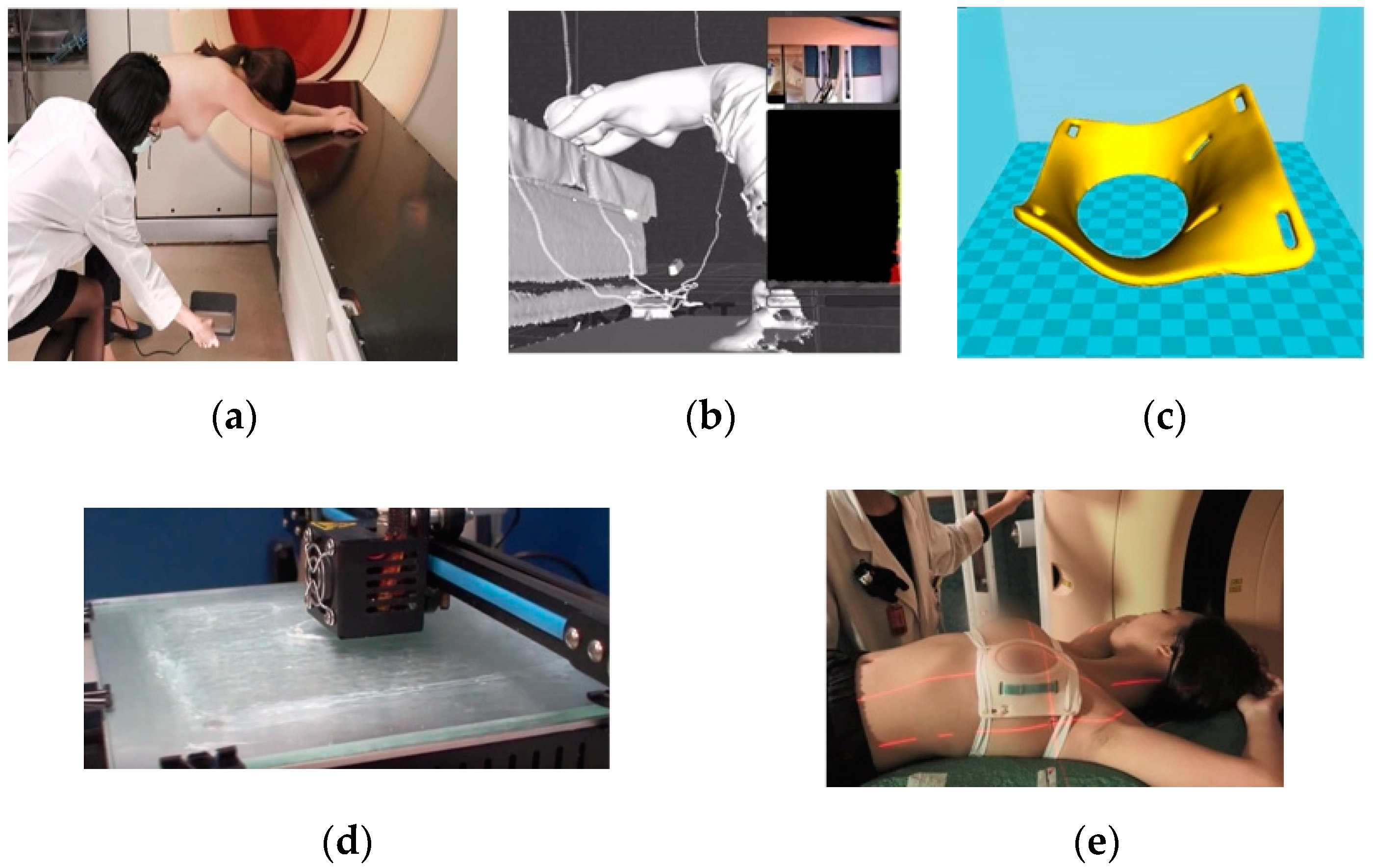
2.1. PERSBRA Design
2.1.1. Rando Phantom
2.1.2. Patient
2.2. Simulation and Planning of Treatment
2.2.1. Rando Phantom
2.2.2. Patient
2.3. Surface Dose Measurement
2.3.1. Rando Phantom
2.3.2. Patient
2.4. Statistics
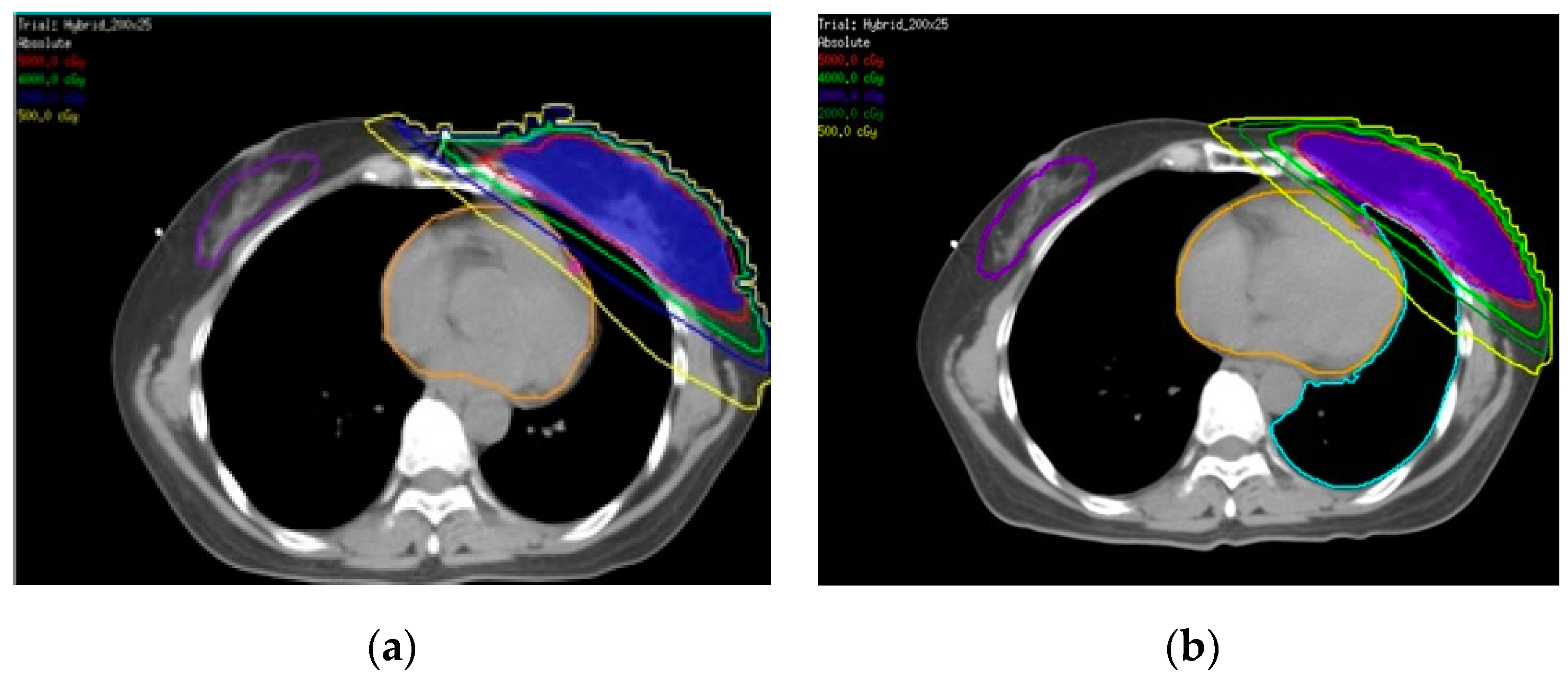
3. Results
3.1. Rando Phantom
3.2. Patient
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.; Ennis, M.; Hood, N.; Graham, M.; Goodwin, P. Quality of Life in Long-Term Breast Cancer Survivors. J. Clin. Oncol. 2013, 31, 3540–3548. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Tyldesley, S.; Foroudi, F.; Barbera, L.; Boyd, C.; Schulze, K.; Walker, H.; Mackillop, W. The Appropriate Rate of Breast Conserving Surgery: An Evidence-based Estimate. Clin. Oncol. 2003, 15, 144–155. [Google Scholar] [CrossRef]
- Eearly Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10 year recurrence and 15 year breast cancer death: Meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, K.; Haddy, N.; Delaloge, S.; Garbay, J.-R.; Garsi, J.-P.; Brindel, P.; Mousannif, A.; Lê, M.G.; Labbe, M.; Arriagada, R.; et al. Long-Term Cardiovascular Mortality after Radiotherapy for Breast Cancer. J. Am. Coll. Cardiol. 2011, 57, 445–452. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Mege, A.; Zioueche, A.; Pourel, N.; Chauvet, B. Radiation-related heart toxicity. Cancer Radiother 2011, 15, 495–503. [Google Scholar]
- Stranzl, H.; Zurl, B. Postoperative irradiation of left-sided breast cancer patients and cardiac toxicity. Does deep inspiration breath-hold (dibh) technique protect the heart? Strahlenther. Onkol. 2008, 184, 354–358. [Google Scholar] [CrossRef]
- Nemoto, K.; Oguchi, M.; Nakajima, M.; Kozuka, T.; Nose, T.; Yamashita, T. Cardiac-sparing radiotherapy for the left breast cancer with deep breath-holding. Jpn. J. Radiol. 2009, 27, 259–263. [Google Scholar] [CrossRef]
- Ferdinand, S.; Mondal, M.; Mallik, S.; Goswami, J.; Das, S.; Manir, K.S.; Sen, A.; Palit, S.; Sarkar, P.; Mondal, S.; et al. Dosimetric analysis of Deep Inspiratory Breath-hold technique (DIBH) in left-sided breast cancer radiotherapy and evaluation of pre-treatment predictors of cardiac doses for guiding patient selection for DIBH. Tech. Innov. Patient Support Radiat. Oncol. 2021, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, R.; Mizuno, N.; Itazawa, T.; Saitoh, H.; Kawamori, J. Dosimetric evaluation of deep inspiration breath hold for left-sided breast cancer: Analysis of patient-specific parameters related to heart dose reduction. J. Radiat. Res. 2020, 61, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Hu, S.; Luo, Y.; Zheng, R.; Zhu, Q.; Chen, P.; Chi, B.; Zhang, Y.; Zhong, F.; Long, X. Meta-analysis of deep inspiration breath hold (DIBH) versus free breathing (FB) in postoperative radiotherapy for left-side breast cancer. Breast Cancer 2020, 27, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sixel, K.E.; Aznar, M.C.; Ung, Y.C. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int. J. Radiat. Oncol. 2001, 49, 199–204. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Zhao, F.; Hu, W.; Yao, G.; Lu, Z.; Yan, S. The benefits evaluation of abdominal deep inspiration breath hold based on knowledge-based radiotherapy treatment planning for left-sided breast cancer. J. Appl. Clin. Med. Phys. 2020, 21, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Remouchamps, V.M.; Vicini, F.A.; Sharpe, M.B.; Kestin, L.L.; Martinez, A.A.; Wong, J.W. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 392–406. [Google Scholar] [CrossRef]
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. Onkol. 2019, 195, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Zhang, Y.; Nie, K.; Liu, B.; Haffty, B.G.; Ohri, N.; Yue, N.J. Setup uncertainties and the optimal imaging schedule in the prone position whole breast radiotherapy. Radiat. Oncol. 2019, 14, 76. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fargier-Bochaton, O.; Dipasquale, G.; Laouiti, M.; Kountouri, M.; Gorobets, O.; Nguyen, N.P.; Miralbell, R.; Vinh-Hung, V. Is prone free breathing better than supine deep inspiration breath-hold for left whole-breast radiotherapy? A dosimetric analysis. Strahlenther. Onkol. 2021, 197, 317–331. [Google Scholar] [CrossRef]
- Huppert, N.; Jozsef, G.; De Wyngaert, K.; Formenti, S.C. The role of a prone setup in breast radiation therapy. Front. Oncol. 2011, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Krengli, M.; Masini, L.; Caltavuturo, T.; Pisani, C.; Apicella, G.; Negri, E.; DeAntonio, L.; Brambilla, M.; Gambaro, G. Prone versus supine position for adjuvant breast radiotherapy: A prospective study in patients with pendulous breasts. Radiat. Oncol. 2013, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing during Breast Cancer Irradiation. Front. Oncol. 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurkmans, C.W.; Borger, J.H.; Bos, L.J.; van der Horst, A.; Pieters, B.R.; Lebesque, J.V.; Mijnheer, B.J. Cardiac and lung complication probabilities after breast cancer irradiation. Radiother. Oncol. 2000, 55, 145–151. [Google Scholar] [CrossRef]
- Li, J.G.; Williams, S.S.; Goffinet, D.R.; Boyer, A.L.; Xing, L. Breast-conserving radiation therapy using combined electron and intensity-modulated radiotherapy technique. Radiother. Oncol. 2000, 56, 65–71. [Google Scholar] [CrossRef]
- Gulybán, A.; Kovács, P.; Sebestyén, Z.; Farkas, R.; Csere, T.; Karácsonyi, G.; Dérczy, K.; Hideghéty, K.; Esik, O. Multisegmented tangential breast fields: A rational way to treat breast cancer. Strahlenther. Onkol. 2008, 184, 262–269. [Google Scholar] [CrossRef]
- Taylor, C.W.; Povall, J.M.; McGale, P.; Nisbet, A.; Dodwell, D.; Smith, J.T.; Darby, S.C. Cardiac Dose from Tangential Breast Cancer Radiotherapy in the Year 2016. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 501–507. [Google Scholar] [CrossRef]
- Ayata, H.B.; Güden, M.; Ceylan, C.; Kücük, N.; Engin, K. Comparison of dose distributions and organs at risk (OAR) doses in conventional tangential technique (CTT) and IMRT plans with different numbers of beam in left-sided breast cancer. Rep. Pr. Oncol. Radiother. 2011, 16, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Rutqvist, L.E.; Lax, I.; Fornander, T.; Johansson, H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int. J. Radiat. Oncol. 1992, 22, 887–896. [Google Scholar] [CrossRef]
- Jagsi, R.; Moran, J.; Marsh, R.; Masi, K.; Griffith, K.A.; Pierce, L.J. Evaluation of Four Techniques Using Intensity-Modulated Radiation Therapy for Comprehensive Locoregional Irradiation of Breast Cancer. Int. J. Radiat. Oncol. 2010, 78, 1594–1603. [Google Scholar] [CrossRef] [Green Version]
- Popescu, C.C.; Olivotto, I.A.; Beckham, W.A.; Ansbacher, W.; Zavgorodni, S.; Shaffer, R.; Wai, E.S.; Otto, K. Volumetric Modulated Arc Therapy Improves Dosimetry and Reduces Treatment Time Compared to Conventional Intensity-Modulated Radiotherapy for Locoregional Radiotherapy of Left-Sided Breast Cancer and Internal Mammary Nodes. Int. J. Radiat. Oncol. 2010, 76, 287–295. [Google Scholar] [CrossRef]
- Jensen, C.A.; Funderud, M.; Lervåg, C. Free breathing VMAT versus deep inspiration breath-hold 3D conformal radiation therapy for early stage left-sided breast cancer. J. Appl. Clin. Med. Phys. 2021, 22, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Diao, P.; Zhang, D.; Wu, J.; Xin, X.; Fontanarosa, D.; Liu, M.; Li, J.; Orlandini, L.C. Impact of Positioning Errors on the Dosimetry of Breath-Hold-Based Volumetric Arc Modulated and Tangential Field-in-Field Left-Sided Breast Treatments. Front. Oncol. 2020, 10, 554131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; You, D.; Su, Y.; Dong, W.; Ma, Z. Dosimetry and Feasibility Studies of Volumetric Modulated Arc Therapy with Deep Inspiration Breath-Hold Using Optical Surface Management System for Left-Sided Breast Cancer Patients. Front. Oncol. 2020, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bourgeois, D.; Guo, B.; Zhang, R. Comparison of conventional and advanced radiotherapy techniques for left-sided breast cancer after breast conserving surgery. Med. Dosim. 2020, 45, e9–e16. [Google Scholar] [CrossRef]
- Xie, Y.; Bourgeois, D.; Guo, B.; Zhang, R. Postmastectomy radiotherapy for left-sided breast cancer patients: Comparison of advanced techniques. Med. Dosim. 2020, 45, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-C.; Chang, H.-M.; Lin, H.-H.; Lu, C.-C.; Lai, L.-H. Dosimetric Comparison of Intensity-Modulated Radiotherapy, Volumetric Modulated Arc Therapy and Hybrid Three-Dimensional Conformal Radiotherapy/Intensity-Modulated Radiotherapy Techniques for Right Breast Cancer. J. Clin. Med. 2020, 9, 3884. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsieh, C.-C.; Chang, C.-S.; Chen, M.-F. A Retrospective Analysis of Dose Distribution and Toxicity in Patients with Left Breast Cancer Treated with Adjuvant Intensity-Modulated Radiotherapy: Comparison with Three-Dimensional Conformal Radiotherapy. Cancer Manag. Res. 2020, 12, 9173–9182. [Google Scholar] [CrossRef]
- Yoon, J.; Xie, Y.; Zhang, R. Evaluation of surface and shallow depth dose reductions using a Superflab bolus during conventional and advanced external beam radiotherapy. J. Appl. Clin. Med. Phys. 2018, 19, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Kumar, R. Nccn clinical practice guidelines in oncology for breast cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [Green Version]
- Rudra, S.; AI-Hallaq, H.A. Effect of rtog breast guidelines on dose volume histogram parameters. J. Appl. Clin. Med. Phys. 2014, 15, 127–137. [Google Scholar] [CrossRef]
- Andreo, P.; Burns, D.T.; Hohlfeld, K.; Huq, M.S.; Kanai, T.; Laitano, F.; Smyth, V.; Vynckier, S. Absorbed Dose Determination External Beam Radiotherapy: An International Code of Practice for dosimetry Based on Absorbed Dose to Water; Technical Report Series No. 398; International Atomic Energy Agency: Vienna, Austria, 2000. [Google Scholar]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D.; Shore, R.E.; Andersson, M. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the wecare study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilge, H.; Cakir, A.; Okutan, M.; Acar, H. Surface dose measurements with GafChromic EBT film for 6 and 18MV photon beams. Phys. Med. 2009, 25, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Almberg, S.S.; Lindmo, T.; Frengen, J. Superficial doses in breast cancer radiotherapy using conventional and IMRT techniques: A film-based phantom study. Radiother. Oncol. 2011, 100, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Roberson, P.L.; Chen, Y.; Marsh, R.B.; Pierce, L.J.; Moran, J.M. Assessment of skin dose for breast chest wall radiotherapy as a function of bolus material. Phys. Med. Biol. 2008, 53, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.; Paxton, A.; Cerviño, L. Dosimetric assessment of brass mesh bolus for postmastectomy photon radiotherapy. J. Appl. Med. Phys. 2016, 17, 86–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seppälä, J.; Voutilainen, A.; Heikkilä, J.; Vauhkonen, M. Surface doses of flattening filter free beams with volumetric modulated arc therapy dose delivery for breast cancer. Phys. Imaging Radiat. Oncol. 2017, 2, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Javedan, K.; Feygelman, V.; Zhang, R.R.; Moros, E.G.; Correa, C.R.; Trotti, A.; Li, W.; Zhang, G.G. Monte carlo comparison of superficial dose between flattening filter free and flattened beams. Phys. Med. 2014, 30, 503–508. [Google Scholar] [CrossRef]
- Panettieri, V.; Barsoum, P.; Westermark, M.; Brualla, L.; Lax, I. Aaa and pbc calculation accuracy in the surface build-up region in tangential beam treatments. Phantom and breast case study with the monte carlo code penelope. Radiother. Oncol. 2009, 93, 94–101. [Google Scholar] [CrossRef]
- Gasteuil, J.; Noblet, C.; Moreau, M.; Meyer, P. A GATE/Geant4 Monte Carlo toolkit for surface dose calculation in VMAT breast cancer radiotherapy. Phys. Med. 2019, 61, 112–117. [Google Scholar]
- Chou, H.-L.; Shueng, P.-W.; Liao, L.-J.; Hsu, C.-X.; Kuo, D.-Y.; Lo, W.-C.; Hou, P.-Y.; Wang, L.-Y.; Chou, S.-F.; Hsieh, C.-H. Prophylactic NS-21 maintains the skin moisture but does not reduce the severity of radiation dermatitis in patients with head and neck cancer: A randomized control trial. Radiat. Oncol. 2019, 14, 90. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Chuang, C.; Quivey, J.M.; Phillips, T.L.; Akazawa, P.; Verhey, L.J.; Xia, P. Skin toxicity due to intensity-modulated radiotherapy for head-and-neck carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 630–637. [Google Scholar] [CrossRef]







| Median Dose | Hybrid | IMRT | VMAT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cGy) | TPS Baseline | EBT3 Film | p Value | TLD | p Value | TPS Baseline | EBT3 Film | p Value | TLD | p Value | TPS Baseline | EBT3 Film | p Value | TLD | p Value |
| (IQR) | |||||||||||||||
| Without PERSBRA | 210.84 | 212.14 | 0.75 | 215.54 | 0.5 | 213.12 | 218.75 | 0.5 | 219.54 | 0.25 | 212.52 | 217.51 | 0.25 | 211.23 | 1 |
| (3.00) | (10.61) | (4.70) | (3.00) | (2.34) | (3.01) | ||||||||||
| Large Mesh PERSBRA | 211.33 | 213.07 | 0.75 | 218.46 | 0.5 | 211.86 | 208.55 | 0.25 | 217.71 | 0.75 | 210.84 | 206.8 | 0.25 | 203.58 | 0.75 |
| (3.99) | (13.61) | (0.91) | (15.85) | (0.42) | (17.27) | ||||||||||
| Fine Mesh PERSBRA | 212.31 | 216.2 | 1 | 214.09 | 0.75 | 215.13 | 224.49 | 0.25 | 220.02 | 0.25 | 220.55 | 225.82 | 0.75 | 217.23 | 1 |
| (6.52) | (5.27) | 1.54) | (6.81) | (7.00) | (11.75) | ||||||||||
| Solid PERSBRA | 210.2 | 212.59 | 0.25 | 224 | 0.25 | 210.96 | 215.52 | 0.25 | 205.75 | 0.25 | 215.21 | 209.80 | 0.5 | 220.91 | 0.25 |
| (1.29) | (3.21) | (2.67) | (4.44) | (5.37) | (6.50) | ||||||||||
| Hybrid | IMRT | VMAT | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median Dose | P1 (Medial Field) | P2 (Lateral Field) | P1 (Medial Field) | P2 (Lateral Field) | P1 (Medial Field) | P2 (Lateral Field) | ||||||||||||||||||
| (cGy) | TPS | EBT3 Film | TLD | p Value | TPS | EBT3 Film | TLD | p Value | TPS | EBT3 Film | TLD | p Value | TPS | EBT3 Film | TLD | p Value | TPS | EBT3 Film | TLD | p Value | TPS | EBT3 Film | TLD | p Value |
| (IQR) | ||||||||||||||||||||||||
| Without PERSBRA | 25.88 | 100.34 | 89.92 | 0.25 | 41.44 | 132.83 | 112.90 | 0.25 | 25.21 | 106.49 | 88.29 | 0.25 | 40.56 | 147.20 | 119.87 | 0.25 | 44.93 | 130.36 | 100.40 | 0.25 | 64.25 | 149.26 | 99.86 | 0.25 |
| (2.65) | (3.70) | (1.74) | (3.50) | (5.54) | (1.11) | (1.02) | (5.14) | (1.16) | (4.57) | (3.44) | (6.10) | |||||||||||||
| Large Mesh PERSBRA | 92.18 | 172.08 | 157.73 | 0.25 | 136.55 | 172.80 | 171.05 | 0.25 | 89.36 | 163.98 | 169.94 | 0.50 | 131.98 | 170.58 | 165.28 | 0.5 | 91.51 | 160.96 | 148.47 | 0.25 | 96.60 | 159.95 | 156.16 | 0.5 |
| (5.03) | (11.12) | (2.79) | (3.28) | (1.59) | (8.00) | (1.48) | (4.96) | (0.67) | (3.55) | (5.20) | (2.06) | |||||||||||||
| Fine Mesh PERSBRA | 133.56 | 184.06 | 179.29 | 0.75 | 160.57 | 194.12 | 196.76 | 1 | 151.84 | 180.50 | 183.12 | 0.25 | 153.13 | 202.50 | 201.66 | 0.25 | 157.18 | 166.08 | 156.07 | 0.5 | 132.74 | 202.42 | 194.65 | 0.25 |
| (7.67) | (13.24) | (5.28) | (3.98) | (2.38) | (1.55) | (0.90) | (3.90) | (2.77) | (7.13) | (0.75) | (6.47) | |||||||||||||
| Solid PERSBRA | 157.04 | 184.52 | 182.15 | 1 | 160.24 | 195.34 | 199.78 | 0.25 | 158.77 | 181.07 | 184.51 | 0.25 | 159.06 | 203.06 | 199.55 | 1 | 187.00 | 178.90 | 182.90 | 1 | 141.18 | 198.03 | 199.27 | 1 |
| (1.81) | (4.88) | (0.93) | (1.07) | (0.44) | (4.33) | (0.56) | (6.01) | (4.19) | (15.67) | (2.76) | (3.01) | |||||||||||||
| OARs | without PERSBRA-Hybrid Median (IQR) | with PERSBRA-Hybrid Median (IQR) | p Value |
|---|---|---|---|
| Lt Lung V20 (%) | 15.0 (4.0) | 105.0 (54.0) | <0.0001 |
| Lt Lung V10 (%) | 20.0 (5.0) | 15.5 (4.0) | <0.0001 |
| Heart Dmean (cGy) | 473.8 (1684.0) | 335 (144.1) | 0.0019 |
| LAD Dmean (cGy) | 2021.1 (918.5) | 1433.8 (868.8) | 0.0022 |
| Rt breast Dmean (cGy) | 58.8 (24.2) | 64.5 (38.5.2) | 0.1116 |
| All data (N = 25) are presented as median (IQR) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-P.; Lin, C.-Y.; Kuo, C.-C.; Chen, T.-H.; Lin, S.-C.; Tseng, K.-H.; Cheng, H.-W.; Chao, H.-L.; Yen, S.-H.; Lin, R.-Y.; et al. Skin Surface Dose for Whole Breast Radiotherapy Using Personalized Breast Holder: Comparison with Various Radiotherapy Techniques and Clinical Experiences. Cancers 2022, 14, 3205. https://doi.org/10.3390/cancers14133205
Chen C-P, Lin C-Y, Kuo C-C, Chen T-H, Lin S-C, Tseng K-H, Cheng H-W, Chao H-L, Yen S-H, Lin R-Y, et al. Skin Surface Dose for Whole Breast Radiotherapy Using Personalized Breast Holder: Comparison with Various Radiotherapy Techniques and Clinical Experiences. Cancers. 2022; 14(13):3205. https://doi.org/10.3390/cancers14133205
Chicago/Turabian StyleChen, Chiu-Ping, Chi-Yeh Lin, Chia-Chun Kuo, Tung-Ho Chen, Shao-Chen Lin, Kuo-Hsiung Tseng, Hao-Wen Cheng, Hsing-Lung Chao, Sang-Hue Yen, Ruo-Yu Lin, and et al. 2022. "Skin Surface Dose for Whole Breast Radiotherapy Using Personalized Breast Holder: Comparison with Various Radiotherapy Techniques and Clinical Experiences" Cancers 14, no. 13: 3205. https://doi.org/10.3390/cancers14133205
APA StyleChen, C. -P., Lin, C. -Y., Kuo, C. -C., Chen, T. -H., Lin, S. -C., Tseng, K. -H., Cheng, H. -W., Chao, H. -L., Yen, S. -H., Lin, R. -Y., Feng, C. -J., Lu, L. -S., Chiou, J. -F., & Hsu, S. -M. (2022). Skin Surface Dose for Whole Breast Radiotherapy Using Personalized Breast Holder: Comparison with Various Radiotherapy Techniques and Clinical Experiences. Cancers, 14(13), 3205. https://doi.org/10.3390/cancers14133205





