Chemotherapy Shows a Better Efficacy Than Endocrine Therapy in Metastatic Breast Cancer Patients with a Heterogeneous Estrogen Receptor Expression Assessed by 18F-FES PET
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. 18F-FES PET/CT Imaging
2.3. Image Interpretation
2.4. Outcome Measurements
2.5. Statistical Analysis
3. Results
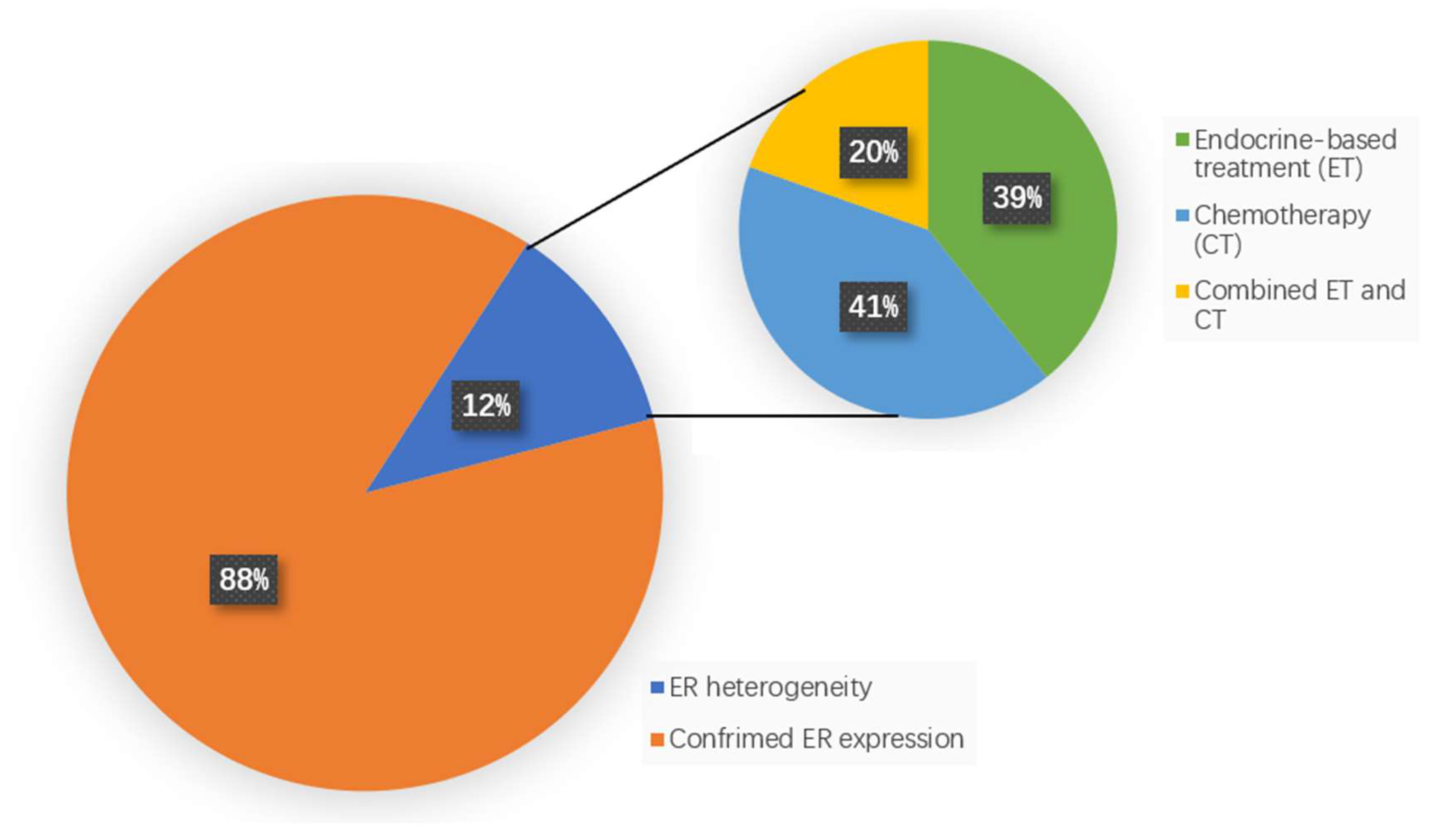
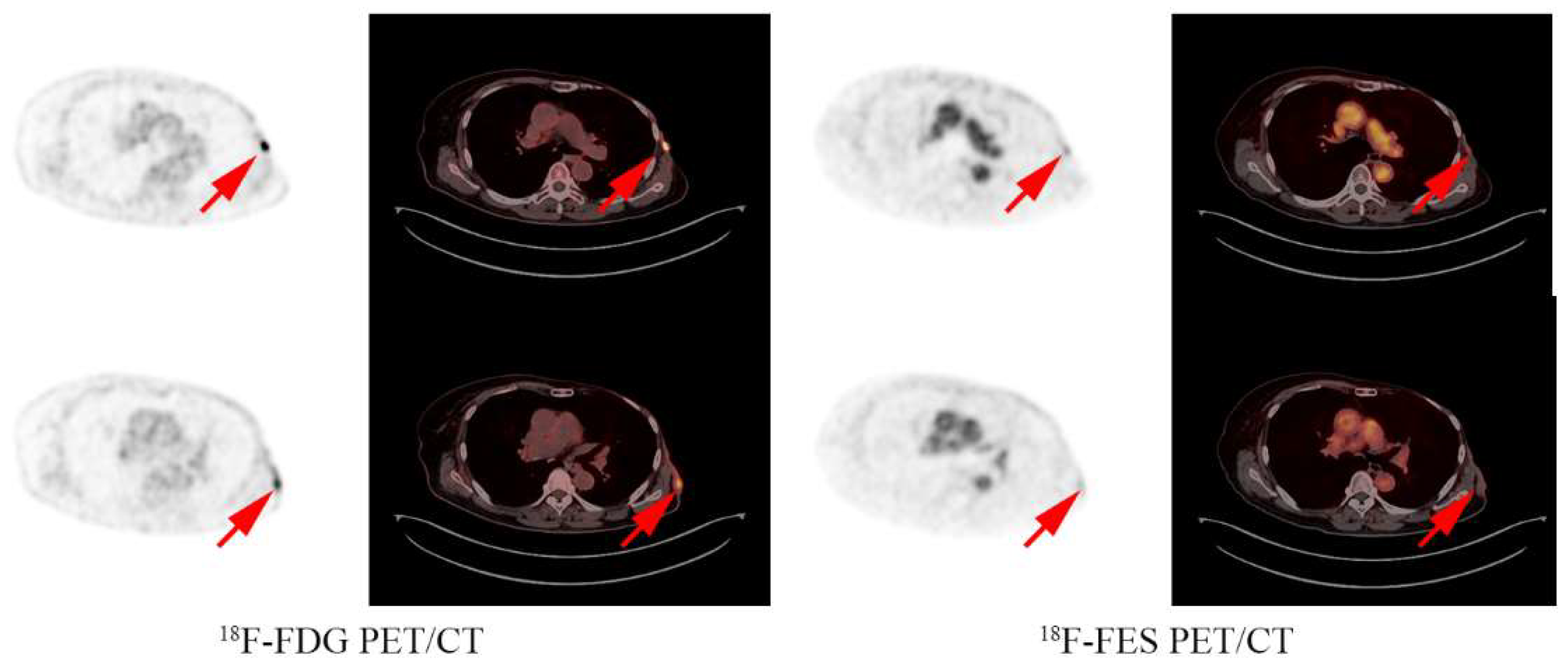
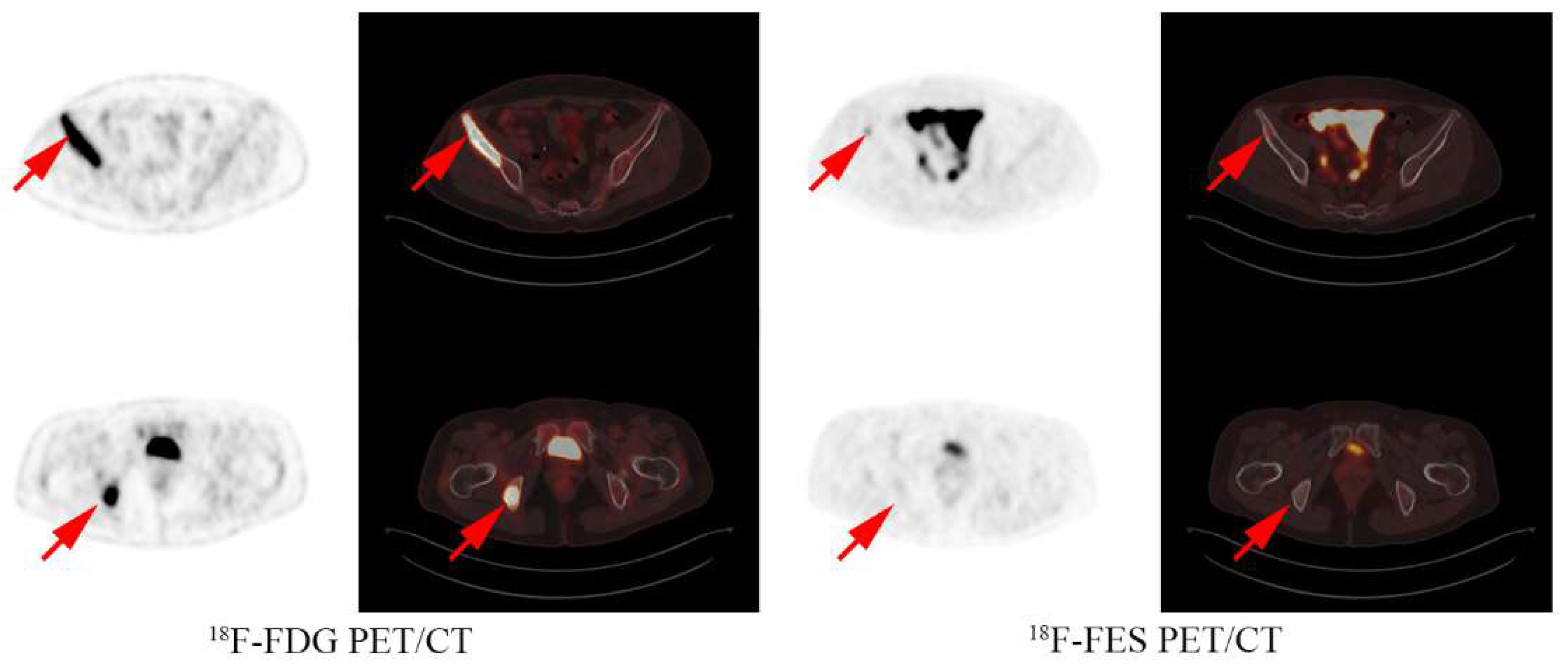
3.1. FES Results and Treatment Options
3.2. Patient Characteristics
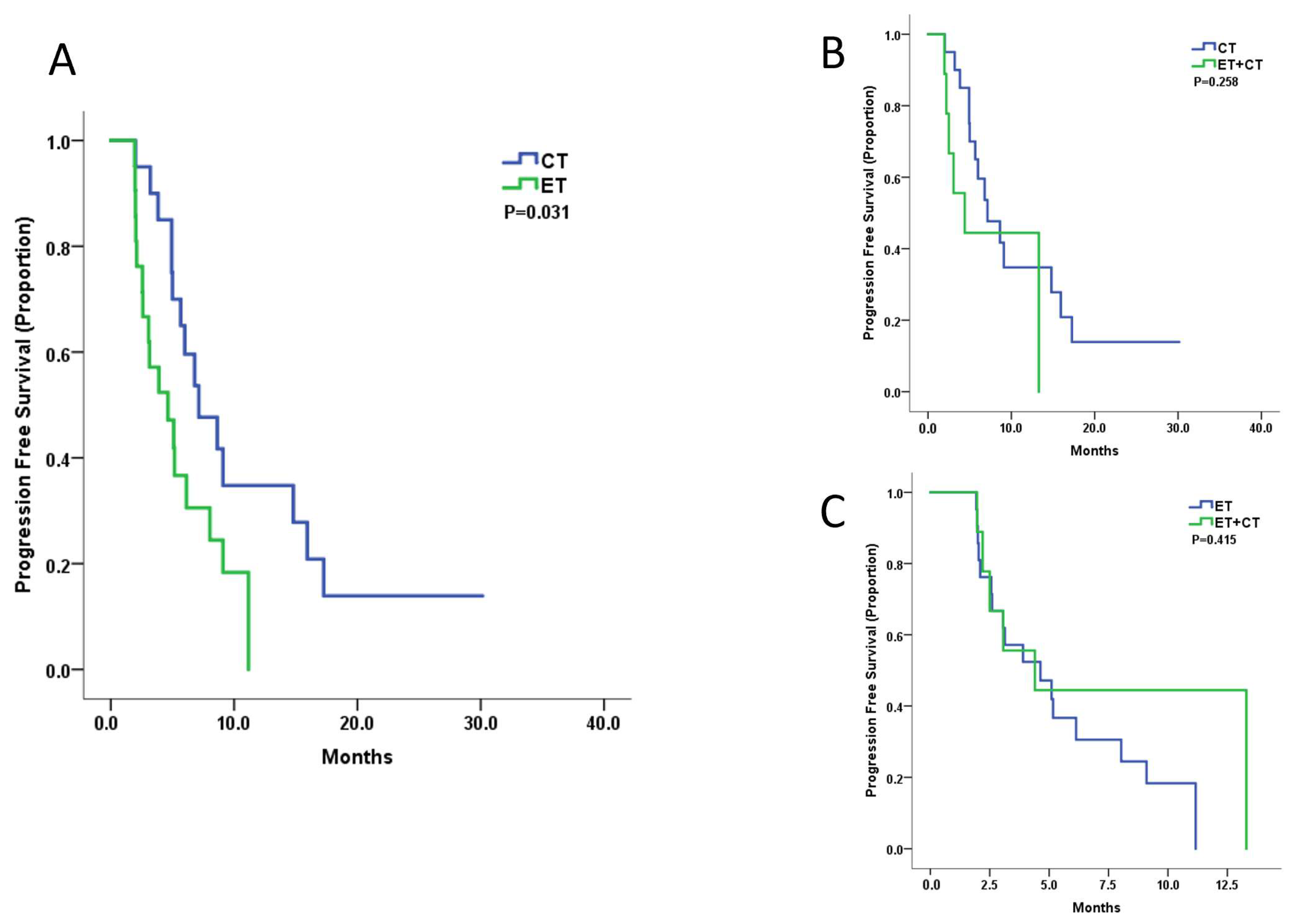
3.3. Treatment Efficacy
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Khongthong, P.; Roseweir, A.K.; Edwards, J. The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocr.-Relat. Cancer 2019, 26, R369–R380. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zattarin, E.; Leporati, R.; Ligorio, F.; Lobefaro, R.; Vingiani, A.; Pruneri, G.; Vernieri, C. Hormone Receptor Loss in Breast Cancer: Molecular Mechanisms, Clinical Settings, and Therapeutic Implications. Cells 2020, 9, 2644. [Google Scholar] [CrossRef]
- Lindström, L.S.; Yau, C.; Czene, K.; Thompson, C.K.; Hoadley, K.; Veer, L.J.V.; Balassanian, R.; Bishop, J.W.; Carpenter, P.M.; Chen, Y.-Y.; et al. Intratumor Heterogeneity of the Estrogen Receptor and the Long-term Risk of Fatal Breast Cancer. JNCI J. Natl. Cancer Inst. 2018, 110, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Guan, J. Breast Cancer: Multiple Subtypes within a Tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef]
- Boers, J.; Loudini, N.; Brunsch, C.L.; Koza, S.A.; de Vries, E.F.; Glaudemans, A.W.; Hospers, G.A.; Schröder, C.P. Value of (18)F-FES PET in Solving Clinical Dilemmas in Breast Cancer Patients: A Retrospective Study. J. Nucl. Med. 2021, 62, 1214–1220. [Google Scholar] [CrossRef]
- Brien, S.R.O.; Edmonds, C.E.; Katz, D.; Mankoff, D.A.; Pantel, A.R. 18F-Fluoroestradiol (FES) PET/CT: Review of current practice and future directions. Clin. Transl. Imaging 2022, 10, 1–11. [Google Scholar]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef]
- Gemignani, M.L.; Patil, S.; Seshan, V.E.; Sampson, M.; Humm, J.L.; Lewis, J.S.; Brogi, E.; Larson, S.M.; Morrow, M.; Pandit-Taskar, N. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J. Nucl. Med. 2013, 54, 1697–1702. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Xu, X.; Zhang, Y.; Zhang, J.; Xue, J.; Wang, M.; Yuan, H.; Hu, S.; Shi, W.; et al. The Assessment of Estrogen Receptor Status and Its Intratumoral Heterogeneity in Patients with Breast Cancer by Using 18F-Fluoroestradiol PET/CT. Clin. Nucl. Med. 2017, 42, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, G.; Piccardo, A.; Fiz, F.; Siri, G.; Matteucci, F.; Rocca, A.; Rocca, O.; Monti, M.; Brain, E.; Alberini, J.L.; et al. Heterogeneity of bone metastases as an important prognostic factor in patients affected by oestrogen receptor-positive breast cancer. The role of combined [18F]Fluoroestradiol PET/CT and [18F]Fluorodeoxyglucose PET/CT. Eur. J. Radiol. 2021, 141, 109821. [Google Scholar] [CrossRef] [PubMed]
- Currin, E.; Peterson, L.M.; Schubert, E.K.; Link, J.M.; Krohn, K.A.; Livingston, R.B.; Mankoff, D.A.; Linden, H.M. Temporal Heterogeneity of Estrogen Receptor Expression in Bone-Dominant Breast Cancer: 18F-Fluoroestradiol PET Imaging Shows Return of ER Expression. J. Natl. Compr. Canc. Netw. 2016, 14, 144–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hao, W.; Li, Y.; Du, B.; Li, X. Heterogeneity of estrogen receptor based on 18F-FES PET imaging in breast cancer patients. Clin. Transl. Imaging 2021, 9, 599–607. [Google Scholar] [CrossRef]
- Yu, K.D.; Cai, Y.; Wu, S.; Shui, R.; Shao, Z. Estrogen receptor-low breast cancer: Biology chaos and treatment paradox. Cancer Commun. 2021, 41, 968–980. [Google Scholar] [CrossRef]
- Mori, T.; Kasamatsu, S.; Mosdzianowski, C.; Welch, M.J.; Yonekura, Y.; Fujibayashi, Y. Automatic synthesis of 16 alpha-[(18)F]fluoro-17beta-estradiol using a cassette-type [(18)F]fluorodeoxyglucose synthesizer. Nucl. Med. Biol. 2006, 33, 281–286. [Google Scholar] [CrossRef]
- Linden, H.M.; Kurland, B.F.; Peterson, L.M.; Schubert, E.K.; Gralow, J.R.; Specht, J.M.; Ellis, G.K.; Lawton, T.J.; Livingston, R.B.; Petra, P.H.; et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin. Cancer Res. 2011, 17, 4799–4805. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Zhang, Y.; Xue, J.; Wang, M.-W.; Shi, W.; Zhu, B.; Hu, S.; Yao, Z.; Pan, H.; et al. The preliminary study of 16α-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS ONE 2015, 10, e0116341. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Zhang, Y.; Xue, J.; Wang, M.; Shi, W.; Zhu, B.; Hu, S.; Yao, Z.; Pan, H.; et al. Can fluorine-18 fluoroestradiol positron emission tomography-computed tomography demonstrate the heterogeneity of breast cancer in vivo? Clin. Breast Cancer 2013, 13, 359–363. [Google Scholar] [CrossRef]
- Lindström, L.S.; Karlsson, E.; Wilking, U.M.; Johansson, U.; Hartman, J.; Lidbrink, E.K.; Hatschek, T.; Skoog, L.; Bergh, J. Clinically Used Breast Cancer Markers Such As Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor Receptor 2 Are Unstable Throughout Tumor Progression. J. Clin. Oncol. 2012, 30, 2601–2608. [Google Scholar] [CrossRef]
- Bado, I.L.; Zhang, W.; Hu, J.; Xu, Z.; Wang, H.; Sarkar, P.; Li, L.; Wan, Y.-W.; Liu, J.; Wu, W.; et al. The bone microenvironment increases phenotypic plasticity of ER(+) breast cancer cells. Dev. Cell 2021, 56, 1100–1117.e9. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Zanotti, G.; Hunger, M.; Perkins, J.J.; Horblyuk, R.; Martin, M. Treatment patterns and real world clinical outcomes in ER+/HER2-post-menopausal metastatic breast cancer patients in the United States. BMC Cancer 2017, 17, 393. [Google Scholar] [CrossRef]
- Osborne, C.K.; Kitten, L.; Arteaga, C.L. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J. Clin. Oncol. 1989, 7, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.S.; Wang, G.; Somer, B.G.; Blakely, L.J.; Wheeler, B.M.; Walker, M.S.; Stepanski, E.J.; Houts, A.C. Phase II trial of fulvestrant with metronomic capecitabine for postmenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer. Clin. Breast. Cancer 2014, 14, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.A.; van Kampen, R.; Voogd, A.; Dercksen, M.; Berkmortel, F.V.D.; Smilde, T.; van de Wouw, A.; Peters, F.; van Riel, J.; Peters, N.; et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: A study of the Southeast Netherlands Breast Cancer Consortium. Ann. Oncol. 2016, 27, 256–262. [Google Scholar] [CrossRef]
- Bonotto, M.; Gerratana, L.; Di Maio, M.; DE Angelis, C.; Cinausero, M.; Moroso, S.; Milano, M.; Stanzione, B.; Gargiulo, P.; Iacono, D.; et al. Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: A propensity score analysis. Breast 2017, 31, 114–120. [Google Scholar] [CrossRef]
- Albain, K.S.; Barlow, W.E.; Ravdin, P.M.; Farrar, W.B.; Burton, G.V.; Ketchel, S.J.; Cobau, C.D.; Levine, E.G.; Ingle, J.N.; Pritchard, K.I.; et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet 2009, 374, 2055–2063. [Google Scholar] [CrossRef]
- Regan, M.M.; Walley, B.; Francis, P.A.; Fleming, G.F.; Gomez, H.; Colleoni, M.; Tondini, C.; Pinotti, G.; Salim, M.; Spazzapan, S.; et al. Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: An exploratory analysis of TEXT and SOFT. Ann. Oncol. 2017, 28, 2225–2232. [Google Scholar] [CrossRef]
- Martin, M.; Campone, M.; Bondarenko, I.; Sakaeva, D.; Krishnamurthy, S.; Roman, L.; Lebedeva, L.; Vedovato, J.C.; Aapro, M. Randomised phase III trial of vinflunine plus capecitabine versus capecitabine alone in patients with advanced breast cancer previously treated with an anthracycline and resistant to taxane. Ann. Oncol. 2018, 29, 1195–1202. [Google Scholar] [CrossRef]
| Characteristics | Endocrine-Based Therapy (ET) N = 21 n (%) | Chemotherapy (CT) N = 20 n (%) | ET + CT N = 10 n (%) | p Values |
|---|---|---|---|---|
| Median age | 55 | 55 | 48 | 0.39 |
| (range) | (29–73) | (39–70) | (32–68) | |
| Age > 48 | 16 | 15 | 7 | 0.93 |
| Median disease-free interval | 3 | 3 | 2 | 0.76 |
| (range) | (0–13) | (0–12) | (0–15) | |
| De novo stage IV | 3 (14) | 2 (10) | 2 (20) | |
| ECOG score | ||||
| 0–1 | 19 (90) | 19 (95) | 9 (90) | 0.84 |
| ≥2 | 2 (10) | 1 (5) | 1 (10) | |
| Number of metastatic sites | ||||
| 1 | 10 (48) | 11 (55) | 6 (60) | 0.78 |
| 2 | 8 (38) | 7 (35) | 4 (40) | |
| ≥3 | 3 (14) | 2 (10) | 0 (0) | |
| Metastatic sites | ||||
| Visceral | 11 (52) | 10 (50) | 4 (40) | 0.81 |
| Liver | 3 (14) | 3 (15) | 1 (10) | 0.92 |
| Lung | 10 (48) | 6 (30) | 2 (20) | 0.26 |
| Bone | 18 (85) | 16 (80) | 10 (100) | 0.32 |
| Median treatment lines | 1 | 1 | 2 | 0.12 |
| (range) | (1–4) | (1–6) | (1–5) |
| Adverse Events (Grade 3/4) | ET N = 21 n (%) | CT N = 20 n (%) | ET + CT N = 10 N (%) |
|---|---|---|---|
| Diarrhea | 1 (4.8) | 0 | 1 (10) |
| Leukopenia | 1 (4.8) | 4 (20) | 0 |
| Anemia | 0 | 1 (5) | 0 |
| Thrombocytopenia | 0 | 1 (5) | |
| Palmar-plantar erythrodysesthesia syndrome | 0 | 1 (5) | 1 (10) |
| Peripheral neurotoxicity | 0 | 2 (10) | 0 |
| All | 2 (9.5) | 7 (35) | 2 (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Du, X.; Zhao, Y.; Gong, C.; Hu, S.; You, S.; Song, S.; Hu, X.; Yang, Z.; Wang, B. Chemotherapy Shows a Better Efficacy Than Endocrine Therapy in Metastatic Breast Cancer Patients with a Heterogeneous Estrogen Receptor Expression Assessed by 18F-FES PET. Cancers 2022, 14, 3531. https://doi.org/10.3390/cancers14143531
Xie Y, Du X, Zhao Y, Gong C, Hu S, You S, Song S, Hu X, Yang Z, Wang B. Chemotherapy Shows a Better Efficacy Than Endocrine Therapy in Metastatic Breast Cancer Patients with a Heterogeneous Estrogen Receptor Expression Assessed by 18F-FES PET. Cancers. 2022; 14(14):3531. https://doi.org/10.3390/cancers14143531
Chicago/Turabian StyleXie, Yizhao, Xinyue Du, Yannan Zhao, Chengcheng Gong, Shihui Hu, Shuhui You, Shaoli Song, Xichun Hu, Zhongyi Yang, and Biyun Wang. 2022. "Chemotherapy Shows a Better Efficacy Than Endocrine Therapy in Metastatic Breast Cancer Patients with a Heterogeneous Estrogen Receptor Expression Assessed by 18F-FES PET" Cancers 14, no. 14: 3531. https://doi.org/10.3390/cancers14143531
APA StyleXie, Y., Du, X., Zhao, Y., Gong, C., Hu, S., You, S., Song, S., Hu, X., Yang, Z., & Wang, B. (2022). Chemotherapy Shows a Better Efficacy Than Endocrine Therapy in Metastatic Breast Cancer Patients with a Heterogeneous Estrogen Receptor Expression Assessed by 18F-FES PET. Cancers, 14(14), 3531. https://doi.org/10.3390/cancers14143531






