The “Balloon-Like” Sign: Differential Diagnosis between Postoperative Air Leak and Residual Pleural Space: Radiological Findings and Clinical Implications of the Young–Laplace Equation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Physical Considerations
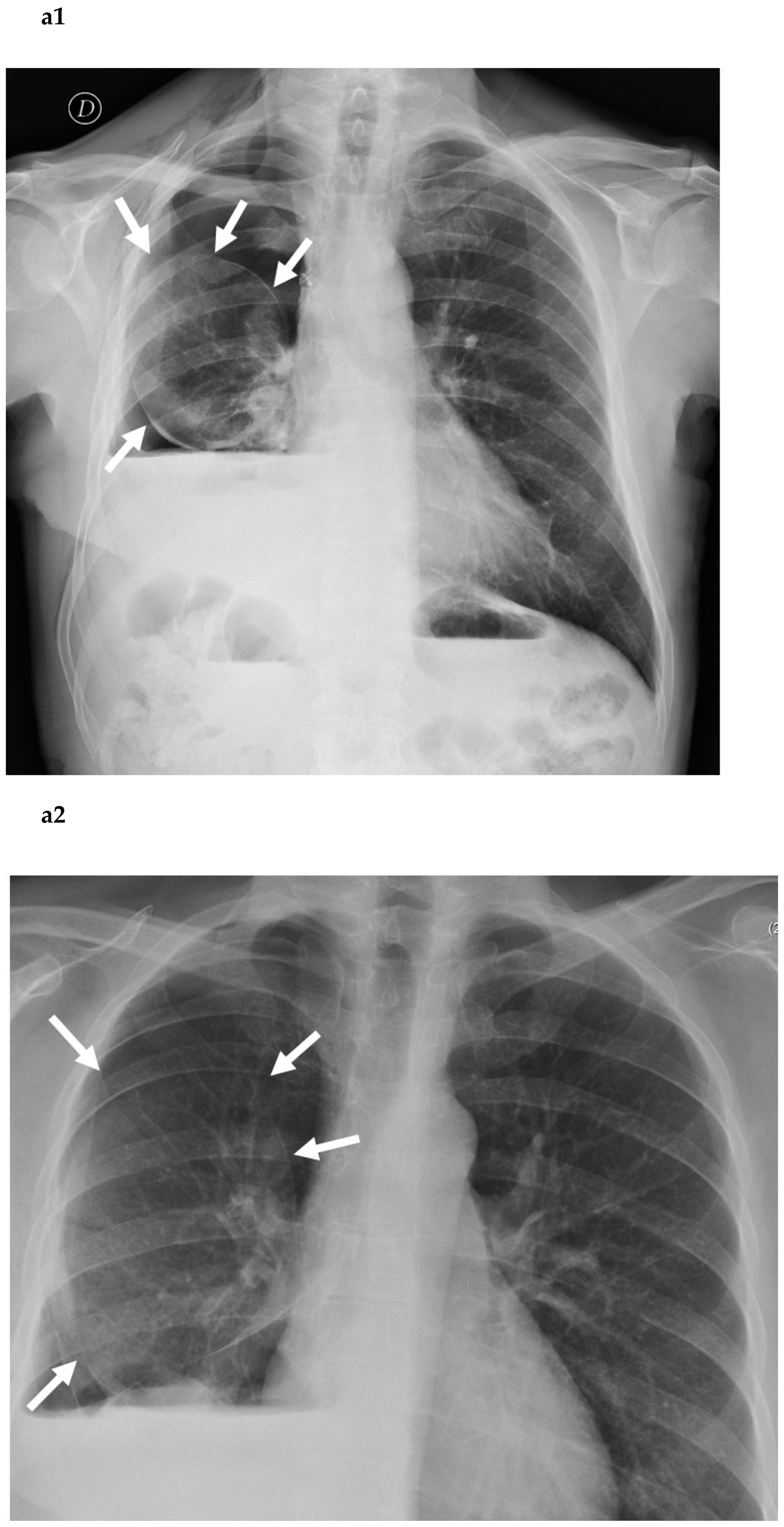
3. Clinical Considerations (Prolonged Air Leak)
4. Clinical Consideration (Residual Pleural Space)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, W.L. Natural history of residual air spaces after pulmonary resection. Chest. Surg. Clin. N. Am. 1996, 6, 585–613. [Google Scholar]
- Solak, O.; Sayar, A.; Metin, M.; Turna, A.; Erdogu, V.; Pekçolaklar, A.; Gürses, A. Definition of postresectional residual pleural space. Can. J. Surg. 2007, 50, 39–42. [Google Scholar]
- Misthos, P.; Kokotsakis, J.; Konstantinou, M.; Skottis, I.; Lioulias, A. Postoperative residual pleural spaces: Characteristics and natural history. Asian Cardiovasc. Thorac. Ann. 2007, 15, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Venuta, F.; Rendina, E.A.; De Giacomo, T.; Coloni, G.F. Postoperative strategies to treat permanent air leaks. Thorac. Surg. Clin. 2010, 20, 391–397. [Google Scholar]
- Mueller, M.R.; Marzluf, B.A. The anticipation and management of air leaks and residual spaces post lung resection. J. Thorac. Dis. 2014, 6, 271–284. [Google Scholar] [PubMed]
- Petrella, F.; Rizzo, S.; Radice, D.; Borri, A.; Galetta, D.; Gasparri, R.; Solli, P.; Veronesi, G.; Bellomi, M.; Spaggiari, L. Predicting prolonged air leak after standard pulmonary lobectomy: Computed tomography assessment and risk factors stratification. Surgeon 2011, 9, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.D.; Giudice, G.; Lequaglie, C. How to distinguish an active air leak from a pleural space effect. Asian Cardiovasc. Thorac. Ann. 2012, 20, 682–688. [Google Scholar] [CrossRef]
- Kuhn, K.F.; Noschese, F. Basic Physics: A Self-Teaching Guide; Jossey–Bass: San Francisco, CA, USA, 2020. [Google Scholar]
- Casha, A.R.; Bertolaccini, L.; Camilleri, L.; Manche, A.; Gauci, M.; Melikyan, G.; Gatt, R.; Dudek, K.; Solli, P.; Grima, J.N. Pathophysiological mechanism of post-lobectomy air leaks. J. Thorac. Dis. 2018, 10, 3689–3700. [Google Scholar] [CrossRef]
- College of Intensive Care Medicine of Australia and New Zealand. Syllabus for the basic sciences in intensive care medicine. In A Guide to the CICM First Part Examination, 3rd ed.; College of Intensive Care Medicine of Australia and New Zealand: Wellington, New Zealand, 2017. [Google Scholar]
- Basford, J.R. The Law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch. Phys. Med. Rehabil. 2002, 83, 1165–1170. [Google Scholar] [CrossRef]
- Sugiura, S.; Nakajima, M.; Kumazawa, N.; Iwamoto, S.; Seki, M. Characterization of spontaneous transformation-based droplet formation during microchannel emulsification. J. Phys. Chem. 2002, 106, 9405–9409. [Google Scholar] [CrossRef]
- Okereke, I.; Murthy, S.C.; Alster, J.M.; Blackstone, E.H.; Rice, T.W. Characterization and importance of air leak after lobectomy. Ann. Thorac. Surg 2005, 79, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Ferraris, V.A.; Bridges, C.R.; Clough, E.R.; Mitchell, J.D.; Fernando, H.C.; Shrager, J.B. Management of alveolar air leaksafter pulmonary resection. Ann. Thorac. Surg. 2010, 89, 1327–1335. [Google Scholar] [CrossRef]
- Steéphan, F.; Boucheseiche, S.; Hollande, J.; Flahault, A.; Cheffi, A.; Bazelly, B.; Bonnet, F. Pulmonary complications following lung resection: A comprehensive analysis of incidence and possible risk factors. Chest 2000, 118, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccone, A.M.; Meyers, B.F.; Guthrie, T.J.; Davis, G.E.; Yusen, R.D.; Lefrak, S.S.; Patterson, G.A.; Cooper, G.D. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J. Thorac. Cardiovasc. Surg. 2003, 125, 513–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Caro, A.; Calvo MJ, R.; Lanzas, J.T.; Chau, R.; Cascales, P.; Parrilla, P. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur. J. Cardiothorac. Surg. 2007, 31, 203–208. [Google Scholar] [CrossRef] [PubMed]
- DeCamp, M.M.; Blackstone, E.H.; Naunheim, K.S.; Krasna, M.J.; Wood, D.E.; Meli, Y.M.; McKenna, R.J.; NETT Research Group. Patient and surgical factors influencing air leak after lung volume reduction surgery: Lessons learned from the National Emphysema Treatment Trial. Ann. Thorac. Surg. 2006, 82, 197–206. [Google Scholar] [CrossRef]
- Amin, R.; Noone, P.G.; Ratjen, F. Chemical pleurodesis versus surgical intervention for persistent and recurrent pneumothoraces in cystic fibrosis. Cochrane Database Syst. Rev. 2009, 2, CD007481. [Google Scholar]
- Csekeo, A.; Agócs, L.; Egerváry, M.; Zeiler, H. Surgery for pulmonary aspergillosis. Eur. J. Cardiothorac. Surg. 1997, 12, 876–879. [Google Scholar] [CrossRef] [Green Version]
- Carbognani, P.; Spaggiari, L.; Solli, P.; Rusca, M. Pneumoperitoneum for prolonged air leaks after lower lobectomies. Ann. Thorac. Surg 1998, 66, 604–605. [Google Scholar]
- Toker, A.; Dilege, S.; Tanju, S.; Kiyan, A.; Kalayci, G. Perioperative pneumoperitoneum after lobectomy—bilobectomy operations for lung cancer: A prospective study. Thorac. Cardiovasc. Surg. 2003, 51, 93–96. [Google Scholar] [CrossRef]
- Lang-Lazdunski, L.; Coonar, A.S. A prospective study of autologous ‘blood patch’ pleurodesis for persistent air leak after pulmonary resection. Eur. J. Cardiothorac. Surg. 2004, 26, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Droghetti, A.; Schiavini, A.; Muriana, P.; Comel, A.; De Donno, G.; Beccaria, M.; Canneto, B.; Sturani, C.; Muriana, G. Autologous blood patch in persistent air leaks after pulmonary resection. J. Thorac. Cardiovasc. Surg. 2006, 132, 556–559. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.E.; Cerfolio, R.J.; Gonzalez, X.; Springmeyer, S.C. Bronchoscopic management of prolonged air leak. Clin. Chest. Med. 2010, 31, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Firlinger, I.; Stubenberger, E.; Müller, M.R.; Burghuber, O.; Valipour, A. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann. Thorac. Surg. 2013, 95, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Borri, A.; Casiraghi, M.; Cavaliere, S.; Donghi, S.; Galetta, D.; Gasparri, R.; Guarize, J.; Pardolesi, A.; Solli, P.; et al. Operative rigid bronchoscopy: Indications, basic techniques and results. Multimed. Man. Cardiothorac. Surg. 2014, 2014, mmu006. [Google Scholar] [CrossRef]
- Brims, F.J.; Maskell, N.A. Ambulatory treatment in the management of pneumothorax: A systematic review of the literature. Thorax 2013, 68, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.I., Jr.; Landreneau, R.J.; Wright, C.E.; Santucci, T.S.; Sammons, B.H. A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann. Thorac. Surg. 2001, 71, 319–322. [Google Scholar] [CrossRef]
- Allama, A.M. Pleural tent for decreasing air leak following upper lobectomy: A prospective randomised trial. Eur. J. Cardiothorac. Surg. 2010, 38, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Al Refai, M.; Monteverde, M.; Borri, A.; Salati, M.; Sabbatini, A.; Fianchini, A. Pleural tent after upper lobectomy: A randomized study of efficacy and duration of effect. Ann. Thorac. Surg. 2002, 74, 1958–1962. [Google Scholar] [CrossRef]
- Varela, G.; Jimenez, M.F.; Novoa, N.; Aranda, J.L. Estimating hospital costs attributable to prolonged air leak in pulmonary obectomy. Eur. J. Cardiothorac. Surg. 2005, 27, 329e33. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.W. Management of the postresection space in tuberculosis. I. Following segmental and wedge resection. J. Thorac. Surg. 1955, 29, 649–657. [Google Scholar] [CrossRef]
- Bell, J.W. Management of the postresection space in tuberculosis. II. Following lobectomy. J. Thorac. Surg. 1956, 31, 442–451. [Google Scholar] [CrossRef]
- Langston, H.T.; Barker, W.L. Pleural effusions and infections of the pleura. In General Thoracic Surgery, 2nd ed.; Shields, T.W., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, F.; Rizzo, S.; Bertolaccini, L.; Casiraghi, M.; Girelli, L.; Lo Iacono, G.; Mazzella, A.; Spaggiari, L. The “Balloon-Like” Sign: Differential Diagnosis between Postoperative Air Leak and Residual Pleural Space: Radiological Findings and Clinical Implications of the Young–Laplace Equation. Cancers 2022, 14, 3533. https://doi.org/10.3390/cancers14143533
Petrella F, Rizzo S, Bertolaccini L, Casiraghi M, Girelli L, Lo Iacono G, Mazzella A, Spaggiari L. The “Balloon-Like” Sign: Differential Diagnosis between Postoperative Air Leak and Residual Pleural Space: Radiological Findings and Clinical Implications of the Young–Laplace Equation. Cancers. 2022; 14(14):3533. https://doi.org/10.3390/cancers14143533
Chicago/Turabian StylePetrella, Francesco, Stefania Rizzo, Luca Bertolaccini, Monica Casiraghi, Lara Girelli, Giorgio Lo Iacono, Antonio Mazzella, and Lorenzo Spaggiari. 2022. "The “Balloon-Like” Sign: Differential Diagnosis between Postoperative Air Leak and Residual Pleural Space: Radiological Findings and Clinical Implications of the Young–Laplace Equation" Cancers 14, no. 14: 3533. https://doi.org/10.3390/cancers14143533







