Individualized Selection Criteria Based on Tumor Burden in Future Remnant Liver for Staged Hepatectomy of Advanced CRLM: Conventional TSH or ALPPS
Abstract
:Simple Summary
Abstract
1. Introduction
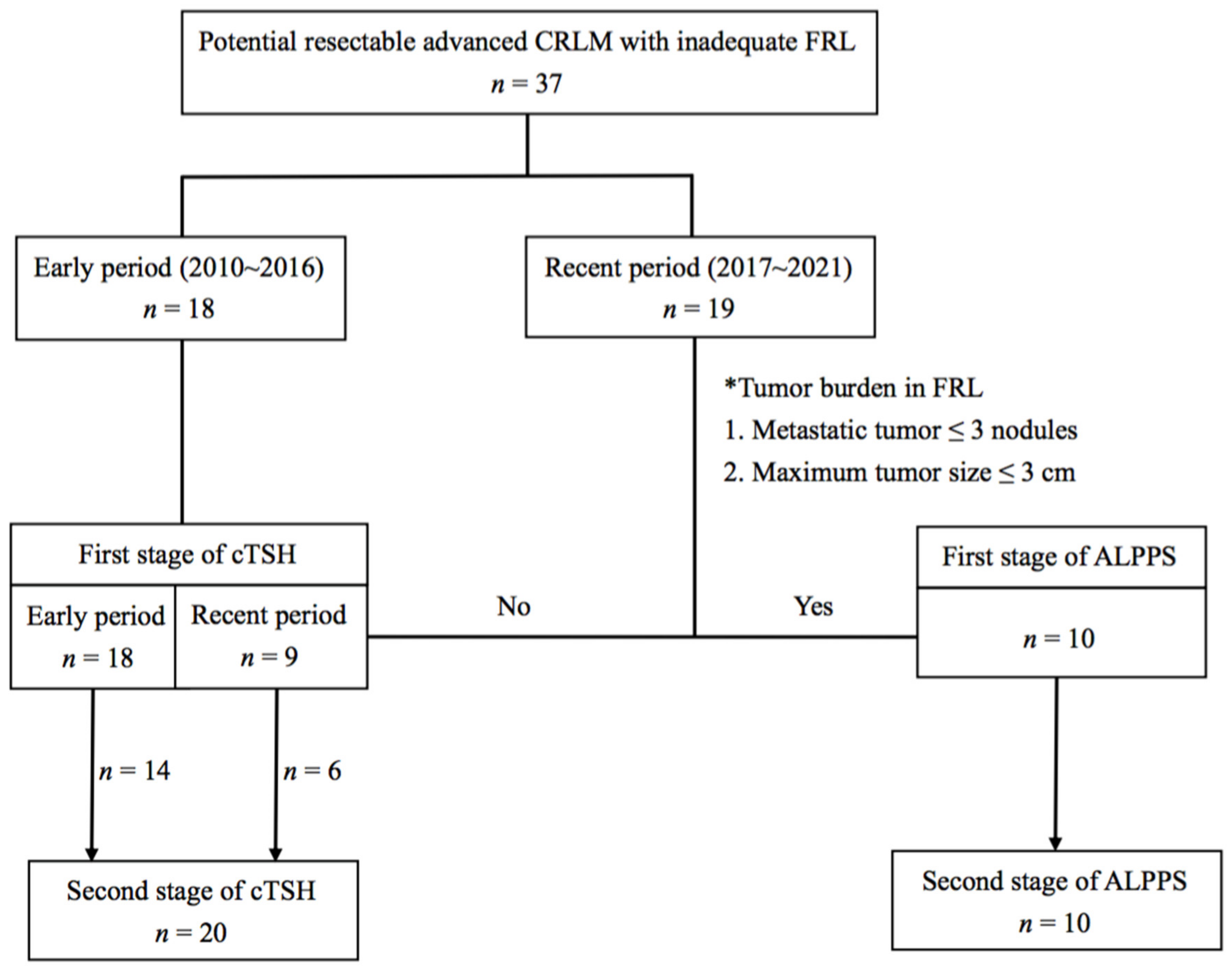
2. Materials and Methods
2.1. Patients
2.2. Staged Hepatectomy
2.3. Postoperative Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Conventional TSH
3.2. ALPPS
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALPPS | Associating liver partitioning and portal vein ligation for staged hepatectomy |
| CRC | Colorectal cancer |
| CRLM | Colorectal liver metastasis |
| cTSH | Conventional two-stage hepatectomy |
| FRL | Future remnant liver |
| LR | Liver resection |
| OS | Overall survival |
| PVL | Portal vein ligation |
| RFS | Recurrence-free survival |
References
- Nordlinger, B.; Van Cutsem, E.; Gruenberger, T.; Glimelius, B.; Poston, G.; Rougier, P.; Sobrero, A.; Ychou, M.; on behalf of the European Colorectal Metastases Treatment Group. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: Recommendations from an expert panel. Ann. Oncol. 2009, 20, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D.; Group EGW. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, E.K.; Vauthey, J.N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann. Surg. 2005, 241, 715–724. [Google Scholar] [CrossRef]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef]
- Mitry, E.; Fields, A.L.; Bleiberg, H.; Labianca, R.; Portier, G.; Tu, D.; Nitti, D.; Torri, V.; Elias, D.; O’Callaghan, C.; et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: A pooled analysis of two randomized trials. J. Clin. Oncol. 2008, 26, 4906–4911. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet. Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Sorbye, H.; Mauer, M.; Gruenberger, T.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; et al. Predictive factors for the benefit of perioperative FOLFOX for resectable liver metastasis in colorectal cancer patients (EORTC Intergroup Trial 40983). Ann. Surg. 2012, 255, 534–539. [Google Scholar] [CrossRef]
- Schnitzbauer, A.A.; Lang, S.A.; Goessmann, H.; Nadalin, S.; Baumgart, J.; Farkas, S.A.; Fichtner-Feigl, S.; Lorf, T.; Goralcyk, A.; Horbelt, R.; et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012, 255, 405–414. [Google Scholar] [CrossRef]
- Adam, R.; Laurent, A.; Azoulay, D.; Castaing, D.; Bismuth, H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann. Surg. 2000, 232, 777–785. [Google Scholar] [CrossRef]
- Adam, R.; Imai, K.; Castro Benitez, C.; Allard, M.A.; Vibert, E.; Sa Cunha, A.; Cherqui, D.; Baba, H.; Castaing, D. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br. J. Surg. 2016, 103, 1521–1529. [Google Scholar] [CrossRef]
- Belghiti, J.; Dokmak, S.; Schadde, E. ALPPS: Innovation for innovation’s sake. Surgery 2016, 159, 1287–1288. [Google Scholar] [CrossRef]
- Oldhafer, K.J.; Donati, M.; Jenner, R.M.; Stang, A.; Stavrou, G.A. ALPPS for patients with colorectal liver metastases: Effective liver hypertrophy, but early tumor recurrence. World J. Surg. 2014, 38, 1504–1509. [Google Scholar] [CrossRef]
- Ratti, F.; Schadde, E.; Masetti, M.; Massani, M.; Zanello, M.; Serenari, M.; Cipriani, F.; Bonariol, L.; Bassi, N.; Aldrighetti, L.; et al. Strategies to Increase the Resectability of Patients with Colorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann. Surg. Oncol. 2015, 22, 1933–1942. [Google Scholar] [CrossRef]
- Sandstrom, P.; Rosok, B.I.; Sparrelid, E.; Larsen, P.N.; Larsson, A.L.; Lindell, G.; Schultz, N.A.; Bjornbeth, B.A.; Isaksson, B.; Rizell, M.; et al. ALPPS Improves Resectability Compared with Conventional Two-stage Hepatectomy in Patients with Advanced Colorectal Liver Metastasis: Results from a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann. Surg. 2018, 267, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Hasselgren, K.; Rosok, B.I.; Larsen, P.N.; Sparrelid, E.; Lindell, G.; Schultz, N.A.; Bjornbeth, B.A.; Isaksson, B.; Larsson, A.L.; Rizell, M.; et al. ALPPS Improves Survival Compared with TSH in Patients Affected of CRLM: Survival Analysis from the Randomized Controlled Trial LIGRO. Ann. Surg. 2021, 273, 442–448. [Google Scholar] [CrossRef]
- Chan, K.M.; Wu, T.H.; Wang, Y.C.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Lee, W.C.; Chiang, J.M.; Chen, J.S. Clinical relevance of oncologic prognostic factors in the decision-making of pre-hepatectomy chemotherapy for colorectal cancer hepatic metastasis: The priority of hepatectomy. World J. Surg. Oncol. 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.M.; Wu, T.H.; Cheng, C.H.; Lee, W.C.; Chiang, J.M.; Chen, J.S.; Wang, J.Y. Prognostic significance of the number of tumors and aggressive surgical approach in colorectal cancer hepatic metastasis. World J. Surg. Oncol. 2014, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Schadde, E.; Raptis, D.A.; Schnitzbauer, A.A.; Ardiles, V.; Tschuor, C.; Lesurtel, M.; Abdalla, E.K.; Hernandez-Alejandro, R.; Jovine, E.; Machado, M.; et al. Prediction of Mortality after ALPPS Stage-1: An Analysis of 320 Patients from the International ALPPS Registry. Ann. Surg. 2015, 262, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Linecker, M.; Bjornsson, B.; Stavrou, G.A.; Oldhafer, K.J.; Lurje, G.; Neumann, U.; Adam, R.; Pruvot, F.R.; Topp, S.A.; Li, J.; et al. Risk Adjustment in ALPPS Is Associated with a Dramatic Decrease in Early Mortality and Morbidity. Ann. Surg. 2017, 266, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Peng, Y.; Hu, J.; Wang, X.; Sun, H.; Sun, J.; Shi, Y.; Xiao, Y.; Ding, Z.; Yang, X.; et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-Related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann. Surg. 2020, 271, 534–541. [Google Scholar] [CrossRef]
- Chan, A.; Zhang, W.Y.; Chok, K.; Dai, J.; Ji, R.; Kwan, C.; Man, N.; Poon, R.; Lo, C.M. ALPPS Versus Portal Vein Embolization for Hepatitis-Related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant before Major Hepatectomy. Ann. Surg. 2021, 273, 957–965. [Google Scholar] [CrossRef]
- Chan, K.M.; Wang, Y.C.; Wu, T.H.; Cheng, C.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Lee, W.C. Comment on “ALPPS Improves Survival Compared with TSH in Patients Affected of CRLM Survival Analysis from the Randomized Controlled Trial LIGRO”: Metastatic Tumor Burden in the Future Liver Remnant for Decision-making of Staged Hepatectomy. Ann. Surg. 2021, 274, e749–e750. [Google Scholar] [CrossRef]
- Tong, Y. Comment on “ALPPS Improves Survival Compared with TSH in Patients Affected of CRLM”—Is It Time to Entry the IDEAL Stage 4? Ann. Surg. 2021, 274, e731. [Google Scholar] [CrossRef]
- Vigano, L.; Capussotti, L.; Lapointe, R.; Barroso, E.; Hubert, C.; Giuliante, F.; Ijzermans, J.N.; Mirza, D.F.; Elias, D.; Adam, R. Early recurrence after liver resection for colorectal metastases: Risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6025 patients. Ann. Surg. Oncol. 2014, 21, 1276–1286. [Google Scholar] [CrossRef]
- Sadot, E.; Groot Koerkamp, B.; Leal, J.N.; Shia, J.; Gonen, M.; Allen, P.J.; DeMatteo, R.P.; Kingham, T.P.; Kemeny, N.; Blumgart, L.H.; et al. Resection margin and survival in 2368 patients undergoing hepatic resection for metastatic colorectal cancer: Surgical technique or biologic surrogate? Ann. Surg. 2015, 262, 476–485. [Google Scholar] [CrossRef] [Green Version]
- De Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Nordlinger, B.; Guiguet, M.; Vaillant, J.C.; Balladur, P.; Boudjema, K.; Bachellier, P.; Jaeck, D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996, 77, 1254–1262. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hiroshima, Y.; Matsuo, K.; Murakami, T.; Kawaguchi, D.; Endo, I.; Yamazaki, K.; Ishida, Y.; Tanaka, K. Remnant Liver Tumor Growth Activity during Treatment Associating Liver Partition and Portal Vein Occlusion for Staged Hepatectomy (ALPPS). J. Gastrointest. Surg. 2017, 21, 1851–1858. [Google Scholar] [CrossRef]
- Robles-Campos, R.; Brusadin, R.; Lopez-Conesa, A.; Lopez-Lopez, V.; Navarro-Barrios, A.; Lopez-Espin, J.J.; Arevalo-Perez, J.; Parrilla, P. Long-Term Outcome after Conventional Two-Stage Hepatectomy versus Tourniquet-ALPPS in Colorectal Liver Metastases: A Propensity Score Matching Analysis. World J. Surg. 2019, 43, 2281–2289. [Google Scholar] [CrossRef]
- Chiang, J.M.; Hung, H.Y.; You, J.F.; Chiang, S.F.; Lee, C.F.; Chou, H.S.; Lee, W.C.; Chan, K.M. Applicability of postoperative carcinoembryonic antigen levels in determining post-liver-resection adjuvant chemotherapy regimens for colorectal cancer hepatic metastasis. Medicine 2019, 98, e17696. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.F.; Cherqui, D.; Geller, D.A.; O’Rourke, N.; Iannitti, D.; Dagher, I.; Koffron, A.J.; Thomas, M.; Gayet, B.; Han, H.S.; et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann. Surg. 2009, 250, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’Rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Gall, T.M.; Sodergren, M.H.; Frampton, A.E.; Fan, R.; Spalding, D.R.; Habib, N.A.; Pai, M.; Jackson, J.E.; Tait, P.; Jiao, L.R. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann. Surg. 2015, 261, e45–e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melandro, F.; Giovanardi, F.; Hassan, R.; Larghi Laureiro, Z.; Ferri, F.; Rossi, M.; Mennini, G.; Pawlik, T.M.; Lai, Q. Minimally Invasive Approach in the Setting of ALPPS Procedure: A Systematic Review of the Literature. J. Gastrointest. Surg. 2019, 23, 1917–1924. [Google Scholar] [CrossRef]
- Dupre, A.; Lefranc, A.; Buc, E.; Delpero, J.R.; Quenet, F.; Passot, G.; Evrard, S.; Rivoire, M. Use of bioresorbable membranes to reduce abdominal and perihepatic adhesions in 2-stage hepatectomy of liver metastases from colorectal cancer: Results of a prospective, randomized controlled phase II trial. Ann. Surg. 2013, 258, 30–36. [Google Scholar] [CrossRef]




| Characteristics | cTSH n = 27 (%) | ALPPS n = 10 (%) | p Value |
|---|---|---|---|
| Age (years), median (range) | 61.4 (29.6–79.0) | 50.8 (23.5–66.1) | 0.104 |
| Gender | |||
| Male | 17 (63.0) | 2 (20.0) | 0.029 |
| Female | 10 (37.0) | 8 (80.0) | |
| BMI | 24.9 (17.9–40.9) | 23.5 (20.4–28.0) | 0.489 |
| Comorbidity | 0.716 | ||
| Yes | 11 (40.7) | 5 (50.0) | |
| No | 16 (59.3) | 5 (50.0) | |
| Primary tumor location | 1.000 | ||
| Colon | 22 (81.4) | 8 (80.0) | |
| Rectum | 5 (18.6) | 2 (20.0) | |
| Metastatic types | |||
| Synchronous | 27 (100) | 10 (100) | 1.000 |
| Metachronous | 0 (0) | 0 (0) | |
| Metastases in whole liver | |||
| Maximum tumor size (cm) | 5.6 (1.3–19.3) | 6.4 (2.5–8.2) | 0.678 |
| Total tumor number | 7 (1–24) | 7 (2–15) | 0.710 |
| Metastases in FRL | |||
| Maximum tumor size (cm) | 2.0 (0–10.2) | 2.5 (0–3.0) | 0.226 |
| Tumor number | 3 (0–8) | 1 (0–3) | 0.003 |
| Serum CEA | 25.8 (2.2–3197) | 6.7 (0.7–40.7) | 0.419 |
| Pre-operative liver function test | |||
| AST (U/L) | 26.0 (14.0–53.0) | 26.0 (16.0–30.0) | 0.674 |
| ALT (U/L) | 22.0 (6.0–60.0) | 27.5 (16.0–38.0) | 0.389 |
| Alk-P (U/L) | 83.0 (55.0–400.0) | 72.0 (48.0–181.0) | 0.271 |
| Total bilirubin (mg/dL) | 0.5 (0.2–0.9) | 0.6 (0.3–1.1) | 0.169 |
| Albumin (g/dL) | 4.2 (3.5–4.9) | 4.5 (4.2–5.0) | 0.004 |
| Prothrombin time (INR) | 1.0 (0.9–1.3) | 1.0 (0.9–1.1) | 0.353 |
| Platelet count (1000/μL) | 254.0 (163.0–543.0) | 214.5 (164.0–432.0) | 0.448 |
| Pre-operative chemotherapy | 0.313 | ||
| FOLFIRI + Bevacizumab | 7 (25.9) | 5 (50.0) | |
| FOLFOX + Bevacizumab | 1 (3.7) | 0 | |
| FOLFIRI + Cetuximab | 9 (33.3) | 4 (40.0) | |
| No | 10 (37.0) | 1 (10.0) | |
| Number of pre-operative chemotherapy | 7 (0–17) | 7 (0–12) | 0.670 |
| 1st stage liver resection | 0.359 | ||
| Laparoscopic approach | 4 (14.8) | 3 (30.0) | |
| Traditional laparotomy approach | 23 (85.2) | 7 (70.0) | |
| 2nd stage liver resection | 0.057 | ||
| Laparoscopic approach | |||
| Extended right hemihepatectomy | 0 (0) | 2 (20.0) | |
| Right hemihepatectomy | 0 (0) | 1 (10.0) | |
| Traditional laparotomy approach | |||
| Extended right hemihepatectomy | 10 (37.0) | 5 (50.0) | |
| Right hemihepatectomy | 10 (37.0) | 2 (20.0) | |
| Failed to second staged hepatectomy | 7 (25.9) | 0 (0) | 0.155 |
| Patients final status | 0.001 | ||
| Alive with CRC free | 4 (14.8) | 7 (70.0) | |
| Alive with recurrent CRC | 7 (25.9) | 3 (30.0) | |
| Dead of CRC | 16 (59.3) | 0 |
| Characteristics | cTSH n = 20 | ALPPS n = 10 | p Value |
|---|---|---|---|
| Clavien complication grade | 1.000 | ||
| I | 3 | 2 | |
| II | 1 | 0 | |
| III | 1 | 0 | |
| IV | 0 | 0 | |
| V | 1 | 0 | |
| Postoperative chemotherapy | 0.947 | ||
| FOLFIRI + Bevacizumab | 7 | 4 | |
| FOLFOX + Bevacizumab | 1 | 0 | |
| FOLFIRI + Cetuximab | 4 | 2 | |
| FOLFOX + Cetuximab | 1 | 2 | |
| FOlFIRI | 2 | 1 | |
| FOLFOX | 4 | 1 | |
| No | 1 | 0 | |
| CRC recurrence after liver resection | 0.807 | ||
| Single anatomic site | |||
| Liver | 3 | 2 | |
| Lung | 7 | 1 | |
| bone | 1 | 0 | |
| Multiple anatomic sites | |||
| Liver and lung | 2 | 1 | |
| Systemic spreading | 3 | 1 |
| Case No. | Age/Sex (yr) | ALPPS | Total Liver Metastases | CRC Recurrence | Outcomes | |
|---|---|---|---|---|---|---|
| First Stage | Second Stage | |||||
| Tumors in FRL (Number/Maximum Size, cm) | Extend of LR | Number/Maximum Size (cm) | Months/Status | |||
| 1 | 66/M | None | S4–S8 | 2/6.9 | None | 59.7/NED |
| 2 | 24/F | 1/3.0 | S5-S8 | 5/8.2 | Lympho nodes | 39.2/AD |
| 3 | 32/F | None | S1, S5–S8 | 7/2.5 | Liver † | 36.0/NED |
| 4 | 50/F | 1/1.4 | S4–S8 | 11/5.1 | Liver † | 25.7/NED |
| 5 | 64/F | 2/1.0 | S4–S8 | 6/7.9 | Liver, lung | 24.5/AD |
| 6 * | 50/F | 1/2.5 | S4–S8 | 6/6.0 | lung | 23.8/AD |
| 7 | 55/M | 2/2.6 | S4–S8 | 11/7.4 | None | 23.4/NED |
| 8 * | 52/F | 2/3.0 | S4–S8 | 15/3.7 | None | 13.7/NED |
| 9 | 65/F | 3/2.0 | S5–S8 | 9/5.1 | None | 6.0/NED |
| 10 * | 40/F | 2/3.0 | S4–S8 | 5/6.7 | None | 5.7/NED |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.-M.; Hung, H.-C.; Lee, J.-C.; Wu, T.-H.; Wang, Y.-C.; Cheng, C.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Lee, W.-C. Individualized Selection Criteria Based on Tumor Burden in Future Remnant Liver for Staged Hepatectomy of Advanced CRLM: Conventional TSH or ALPPS. Cancers 2022, 14, 3553. https://doi.org/10.3390/cancers14143553
Chan K-M, Hung H-C, Lee J-C, Wu T-H, Wang Y-C, Cheng C-H, Lee C-F, Wu T-J, Chou H-S, Lee W-C. Individualized Selection Criteria Based on Tumor Burden in Future Remnant Liver for Staged Hepatectomy of Advanced CRLM: Conventional TSH or ALPPS. Cancers. 2022; 14(14):3553. https://doi.org/10.3390/cancers14143553
Chicago/Turabian StyleChan, Kun-Ming, Hao-Chien Hung, Jin-Chiao Lee, Tsung-Han Wu, Yu-Chao Wang, Chih-Hsien Cheng, Chen-Fang Lee, Ting-Jung Wu, Hong-Shiue Chou, and Wei-Chen Lee. 2022. "Individualized Selection Criteria Based on Tumor Burden in Future Remnant Liver for Staged Hepatectomy of Advanced CRLM: Conventional TSH or ALPPS" Cancers 14, no. 14: 3553. https://doi.org/10.3390/cancers14143553
APA StyleChan, K.-M., Hung, H.-C., Lee, J.-C., Wu, T.-H., Wang, Y.-C., Cheng, C.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., & Lee, W.-C. (2022). Individualized Selection Criteria Based on Tumor Burden in Future Remnant Liver for Staged Hepatectomy of Advanced CRLM: Conventional TSH or ALPPS. Cancers, 14(14), 3553. https://doi.org/10.3390/cancers14143553








