Evaluation of the Perioperative and Postoperative Course of Surgery for Pineal Germinoma in the SIOP CNS GCT 96 Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Group 1 (Resection Group)
3.2. Group 2 (Biopsy Group)
3.3. Tumor Size
3.4. Tumor Size and Primary Symptoms
3.5. Trends in Surgical Strategy
3.6. Postoperative Neurological Status and Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Echevarría, M.E.; Fangusaro, J.; Goldman, S. Pediatric Central Nervous System Germ Cell Tumors: A Review. Oncologist 2008, 13, 690–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittleman, H.; Cioffi, G.; Vecchione-Koval, T.; Ostrom, Q.T.; Kruchko, C.; Osorio, D.S.; Finlay, J.L.; Barnholtz-Sloan, J.S. Descriptive epidemiology of germ cell tumors of the central nervous system diagnosed in the United States from 2006 to 2015. J. Neuro-Oncol. 2019, 143, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.T.; Gelman, R.; Hochberg, F. Intracranial germ-cell tumors: Natural history and pathogenesis. J. Neurosurg. 1985, 63, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, H.J.; Otsubo, H.; Hendrick, E.B.; Humphreys, R.P.; Drake, J.M.; Becker, L.E.; Greenberg, M.; Jenkin, D. Intracranial germ-cell tumors in children. J. Neurosurg. 1991, 74, 545. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Yoon, P.H.; Ryu, Y.H.; Jeon, P.; Hwang, G.J. MRI of germinomas arising from the basal ganglia and thalamus. Neuroradiology 1998, 40, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Lin, T.; Shirataki, K.; Hosoda, K.; Kurata, H.; Matsumoto, S.; Ito, H. Germ cell tumors of the thalamus and the basal ganglia. Child’s Nerv. Syst. 1990, 6, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaini, M.; Sultan, I.; Abuirmileh, N.; Jaradat, I.; Qaddoumi, I. Pineal gland tumors: Experience from the SEER database. J. Neuro-Oncol. 2009, 94, 351. [Google Scholar] [CrossRef] [Green Version]
- Kersh, C.R.; Constable, W.C.; Eisert, D.R.; Spaulding, C.A.; Hahn, S.S.; Jenrette, J.M., III; Marks, R.D., Jr. Primary central nervous system germ cell tumors. Effect of histologic confirmation on radiotherapy. Cancer 1988, 61, 2148–2152. [Google Scholar] [CrossRef]
- Packer, R.J.; MacDonald, T.; Vezina, G. Central Nervous System Tumors. Pediatr. Clin. N. Am. 2008, 55, 121–145. [Google Scholar] [CrossRef]
- Hirato, J.; Nakazato, Y. Pathology of pineal region tumors. J. Neuro-Oncol. 2001, 54, 239–249. [Google Scholar] [CrossRef]
- Kumar, P.; Tatke, M.; Sharma, A.; Singh, D. Histological analysis of lesions of the pineal region: A retrospective study of 12 years. Pathol. Res. Pract. 2006, 202, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bamberg, M.; Kortmann, R.-D.; Calaminus, G.; Becker, G.; Meisner, C.; Harms, D.; Göbel, U. Radiation Therapy for Intracranial Germinoma: Results of the German Cooperative Prospective Trials MAKEI 83/86/89. J. Clin. Oncol. 1999, 17, 2585. [Google Scholar] [CrossRef] [PubMed]
- Borg, M. Germ cell tumours of the central nervous system in children—controversies in radiotherapy. Med. Pediatr. Oncol. 2003, 40, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Haddock, M.G.; Schild, S.E.; Scheithauer, B.W.; Schomberg, P.J. Radiation therapy for histologically confirmed primary central nervous system germinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 915–923. [Google Scholar] [CrossRef]
- Hardenbergh, P.H.; Golden, J.; Billet, A.; Scott, R.M.; Shrieve, D.C.; Silver, B.; Loeffler, J.S.; Tarbell, N.J. Intracranial germinoma: The case for lower dose radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 419–426. [Google Scholar] [CrossRef]
- Wolden, S.L.; Wara, W.M.; Larson, D.A.; Prados, M.D.; Edwards, M.S.B.; Sneed, P.K. Radiation therapy for primary intracranial germ-cell tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 943–949. [Google Scholar] [CrossRef]
- Sawamura, Y.; Ikeda, J.; Shirato, H.; Tada, M.; Abe, H. Germ cell tumours of the central nervous system: Treatment consideration based on 111 cases and their long-term clinical outcomes. Eur. J. Cancer 1998, 34, 104–110. [Google Scholar] [CrossRef]
- Jensen, A.W.; Issa Laack, N.N.; Buckner, J.C.; Schomberg, P.J.; Wetmore, C.J.; Brown, P.D. Long-Term Follow-Up of Dose-Adapted and Reduced-Field Radiotherapy with or without Chemotherapy for Central Nervous System Germinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1449–1456. [Google Scholar] [CrossRef]
- Haas-Kogan, D.A.; Missett, B.T.; Wara, W.M.; Donaldson, S.S.; Lamborn, K.R.; Prados, M.D.; Fisher, P.G.; Huhn, S.L.; Fisch, B.M.; Berger, M.S.; et al. Radiation therapy for intracranial germ cell tumors. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 511–518. [Google Scholar] [CrossRef]
- Allen, J.C.; Darosso, R.C.; Donahue, B.; Nirenberg, A. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer 1994, 74, 940–944. [Google Scholar] [CrossRef]
- Allen, J.C.; Kim, J.H.; Packer, R.J. Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J. Neurosurg. 1987, 67, 65. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.C.; Peethambaram, P.P.; Smithson, W.A.; Groover, R.V.; Schomberg, P.J.; Kimmel, D.W.; Raffel, C.; O’Fallon, J.R.; Neglia, J.; Shaw, E.G. Phase II Trial of Primary Chemotherapy Followed by Reduced-Dose Radiation for CNS Germ Cell Tumors. J. Clin. Oncol. 1999, 17, 933. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Grant, R.; Baruchel, S.; Chan, H.; Malkin, D.; Weitzman, S.; Greenberg, M.L. Comparison of survival outcomes in patients with intracranial germinomas treated with radiation alone versus reduced-dose radiation and chemotherapy. Child’s Nerv. Syst. 1998, 14, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.G.; Rockhill, J.K.; Olson, J.M.; Ellenbogen, R.G.; Geyer, J.R. Cisplatin-Based Chemotherapy Followed By Focal, Reduced-Dose Irradiation For Pediatric Primary Central Nervous System Germinomas. J. Pediatr. Hematol. Oncol. 2006, 28, 36–39. [Google Scholar]
- Kretschmar, C.; Kleinberg, L.; Greenberg, M.; Burger, P.; Holmes, E.; Wharam, M. Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2007, 48, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Khatua, S.; Dhall, G.; O’Neil, S.; Jubran, R.; Villablanca, J.G.; Marachelian, A.; Nastia, A.; Lavey, R.; Olch, A.J.; Gonzalez, I.; et al. Treatment of primary CNS germinomatous germ cell tumors with chemotherapy prior to reduced dose whole ventricular and local boost irradiation. Pediatr. Blood Cancer 2010, 55, 42–46. [Google Scholar] [CrossRef]
- Kreth, F.W.; Schätz, C.R.; Pagenstecher, A.; Faist, M.; Volk, B.; Ostertag, C.B. Stereotactic management of lesions of the pineal region. Neurosurgery 1996, 39, 280–289. [Google Scholar] [CrossRef]
- Regis, J.; Bouillot, P.; Rouby-Volot, F.; Figarella-Branger, D.; Dufour, H.; Peragut, J.C. Pineal region tumors and the role of stereotactic biopsy: Review of the mortality, morbidity, and diagnostic rates in 370 cases. Neurosurgery 1996, 39, 907–912. [Google Scholar]
- Popovic, E.A.; Kelly, P.J. Stereotactic procedures for lesions of the pineal region. Mayo Clin. Proc. 1993, 68, 965–970. [Google Scholar] [CrossRef]
- Dempsey, P.K.; Lunsford, L.D. Stereotactic radiosurgery for pineal region tumors. Neurosurg. Clin. N. Am. 1992, 3, 245–253. [Google Scholar] [CrossRef]
- Clark, K. The Lumbar Spine and Back Pain. Neurosurgery 1988, 22, 965–966. [Google Scholar] [CrossRef]
- Haegelen, C.; Touzet, G.; Reyns, N.; Maurage, C.A.; Ayachi, M.; Blond, S. Stereotactic robot-guided biopsies of brain stem lesions: Experience with 15 cases. Neurochirurgie 2010, 56, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.N.; Stein, B.M. Surgical management of pineal region tumors. Acta Neurochir. 1995, 134, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Hudgins, R.J.; Wilson, C.B.; Levin, V.A.; Wara, W.M. Pineal region tumors in children. J. Neurosurg. 1988, 68, 689–697. [Google Scholar] [CrossRef]
- Matsutani, M.; Sano, K.; Takakura, K.; Fujimaki, T.; Nakamura, O.; Funata, N.; Seto, T. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J. Neurosurg. 1997, 86, 446–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamura, Y.; de Tribolet, N.; Ishii, N.; Abe, H. Management of primary intracranial germinomas: Diagnostic surgery or radical resection? J. Neurosurg. 1997, 87, 262–266. [Google Scholar] [CrossRef]
- Balmaceda, C.; Modak, S.; Finlay, J. Central nervous system germ cell tumors. Semin. Oncol. 1998, 25, 243–250. [Google Scholar]
- Herrmann, H.D.; Westphal, M.; Winkler, K.; Laas, R.W.; Schulte, F.J. Treatment of nongerminomatous germ-cell tumors of the pineal region. Neurosurgery 1994, 34, 524–529. [Google Scholar] [CrossRef]
- Reddy, A.T.; Wellons, J.C., 3rd; Allen, J.C.; Fiveash, J.B.; Abdullatif, H.; Braune, K.W.; Grabb, P.A. Refining the staging evaluation of pineal region germinoma using neuroendoscopy and the presence of preoperative diabetes insipidus. Neuro Oncol. 2004, 6, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Schulz, M.; Afshar-Bakshloo, M.; Koch, A.; Capper, D.; Driever, P.H.; Tietze, A.; Grün, A.; Thomale, U.W. Management of pineal region tumors in a pediatric case series. Neurosurg. Rev. 2020, 44, 1417–1427. [Google Scholar] [CrossRef]
- Pettorini, B.L.; Al-Mahfoud, R.; Jenkinson, M.D.; Avula, S.; Pizer, B.; Mallucci, C. Surgical pathway and management of pineal region tumours in children. Childs Nerv. Syst. 2013, 29, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, P.F.; Souweidane, M.M. Pineal region tumors: Simultaneous endoscopic third ventriculostomy and tumor biopsy. World Neurosurg. 2013, 79 (Suppl. 2), S18.e9–S18.e13. [Google Scholar] [CrossRef] [PubMed]
- Calaminus, G.; Kortmann, R.; Worch, J.; Nicholson, J.C.; Alapetite, C.; Garre, M.L.; Patte, C.; Ricardi, U.; Saran, F.; Frappaz, D. SIOP CNS GCT 96: Final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013, 15, 788–796. [Google Scholar] [PubMed]
- Marsh, W.R.; Laws, E.R., Jr. Shunting and irradiation of pineal tumors. Clin. Neurosurg. 1985, 32, 384–396. [Google Scholar] [PubMed]
- Stein, B.M.; Bruce, J.N. Surgical management of pineal region tumors (honored guest lecture). Clin. Neurosurg. 1992, 39, 509–532. [Google Scholar]
- Lapras, C.; Patet, J.D.; Mottolese, C.; Lapras, C., Jr. Direct surgery for pineal tumors: Occipital-transtentorial approach. Prog. Exp. Tumor Res. 1987, 30, 268–280. [Google Scholar]
- Pendl, G.; Vorkapic, P. Surgery of pineal region lesions in childhood. Acta Neurochir. Suppl. 1985, 35, 114–118. [Google Scholar]
- Herrmann, H.D.; Winkler, D.; Westphal, M. Treatment of tumours of the pineal region and posterior part of the third ventricle. Acta Neurochir. 1992, 116, 137–146. [Google Scholar] [CrossRef]
- Konovalov, A.N.; Pitskhelauri, D.I. Principles of treatment of the pineal region tumors. Surg. Neurol. 2003, 59, 250–268. [Google Scholar] [CrossRef]
- Balossier, A.; Blond, S.; Reyns, N. Endoscopic Versus Stereotactic Procedure for Pineal Tumor Biopsies: Focus on Overall Efficacy Rate. World Neurosurg. 2016, 92, 223–228. [Google Scholar] [CrossRef]
- Pecker, J.; Scarabin, J.M.; Brucher, J.M.; Vallee, B. Contribution of stereotactic methods to diagnosis and treatment of tumors of the pineal region (author’s transl). Rev. Neurol. 1978, 134, 287–294. [Google Scholar] [PubMed]
- Balossier, A.; Blond, S.; Touzet, G.; Lefranc, M.; de Saint-Denis, T.; Maurage, C.A.; Reyns, N. Endoscopic versus stereotactic procedure for pineal tumour biopsies: Comparative review of the literature and learning from a 25-year experience. Neurochirurgie 2015, 61, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Pople, I.K.; Athanasiou, T.C.; Sandeman, D.R.; Coakham, H.B. The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br. J. Neurosurg. 2001, 15, 305–311. [Google Scholar]
- Oi, S.; Shibata, M.; Tominaga, J.; Honda, Y.; Shinoda, M.; Takei, F.; Tsugane, R.; Matsuzawa, K.; Sato, O. Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: A prospective study. J. Neurosurg. 2000, 93, 245–253. [Google Scholar] [CrossRef]
- Constantini, S.; Mohanty, A.; Zymberg, S.; Cavalheiro, S.; Mallucci, C.; Hellwig, D.; Ersahin, Y.; Mori, H.; Mascari, C.; Val, J.A.; et al. Safety and diagnostic accuracy of neuroendoscopic biopsies: An international multicenter study. J. Neurosurg. Pediatr. 2013, 11, 704–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, N.; Murai, H.; Ishihara, S.; Kitamura, T.; Miki, T.; Miwa, T.; Miyajima, M.; Nishiyama, K.; Ohira, T.; Ono, S.; et al. Nationwide investigation of the current status of therapeutic neuroendoscopy for ventricular and paraventricular tumors in Japan. J. Neurosurg. 2011, 115, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Ishijima, B.; Hirakawa, K.; Nakamura, N.; Sano, K. Ventriculofiberscope: A new technique for endoscopic diagnosis and operation. Technical note. J. Neurosurg. 1973, 38, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T. Endoscopic biopsy of intraventricular tumors with the use of a ventriculofiberscope. Neurosurgery 1978, 2, 110–113. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Zaben, M.J.; Mathad, N.V.; Sparrow, O.C. Endoscopic biopsy and third ventriculostomy for the management of pineal region tumors. World Neurosurg. 2015, 83, 543–547. [Google Scholar] [CrossRef]
- Fedorko, S.; Zweckberger, K.; Unterberg, A.W. Quality of life following surgical treatment of lesions within the pineal region. J. Neurosurg. 2018, 130, 28–37. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Bowden, S.; Bruce, J.N. Microsurgical resection of pineal region tumors. J. Neurooncol. 2016, 130, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Mottolese, C.; Szathmari, A.; Ricci-Franchi, A.C.; Gallo, P.; Beuriat, P.A.; Capone, G. Supracerebellar infratentorial approach for pineal region tumors: Our surgical and technical considerations. Neurochirurgie 2015, 61, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Abecassis, I.J.; Hanak, B.; Barber, J.; Mortazavi, M.; Ellenbogen, R.G. A Single-Institution Experience with Pineal Region Tumors: 50 Tumors Over 1 Decade. Oper. Neurosurg. 2017, 13, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.M.; Geever, B.W.; Patel, A.S.; Chaker, A.N.; Bhimani, A.D.; Kheirkhah, P.; Hobbs, J.G.; Esfahani, D.R.; Mehta, A.I. Supratentorial and Infratentorial Approaches to Pineal Surgery: A Database Analysis. J. Neurol. Surg. B Skull Base 2019, 80, 364–370. [Google Scholar] [CrossRef]
- Qi, S.; Fan, J.; Zhang, X.A.; Zhang, H.; Qiu, B.; Fang, L. Radical resection of nongerminomatous pineal region tumors via the occipital transtentorial approach based on arachnoidal consideration: Experience on a series of 143 patients. Acta Neurochir. 2014, 156, 2253–2262, Erratum in: Acta Neurochir. 2015, 157, 349. [Google Scholar] [CrossRef]
- Cho, B.K.; Wang, K.C.; Nam, D.H.; Kim, D.G.; Jung, H.W.; Kim, H.J.; Han, D.H.; Choi, K.S. Pineal tumors: Experience with 48 cases over 10 years. Childs Nerv. Syst. 1998, 14, 53–58. [Google Scholar] [CrossRef]
- Hernesniemi, J.; Romani, R.; Albayrak, B.S.; Lehto, H.; Dashti, R.; Ramsey, C., 3rd; Karatas, A.; Cardia, A.; Navratil, O.; Piippo, A.; et al. Microsurgical management of pineal region lesions: Personal experience with 119 patients. Surg. Neurol. 2008, 70, 576–583. [Google Scholar] [CrossRef]
- Shepard, M.J.; Haider, A.S.; Prabhu, S.S.; Sawaya, R.; DeMonte, F.; McCutcheon, I.E.; Weinberg, J.S.; Ferguson, S.D.; Suki, D.; Fuller, G.N.; et al. Long term outcomes following surgery for pineal region tumors. J. Neurooncol. 2022, 156, 491–498. [Google Scholar] [CrossRef]
- Takami, H.; Fukuoka, K.; Fukushima, S.; Nakamura, T.; Mukasa, A.; Saito, N.; Yanagisawa, T.; Nakamura, H.; Sugiyama, K.; Kanamori, M.; et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol. 2019, 21, 1565–1577. [Google Scholar] [CrossRef]

| Biopsy/Resection | Total | ||||
|---|---|---|---|---|---|
| Resection | Biopsy | p-Value (Chi-Square Test) | |||
| Tumor size | <2 cm | 7 | 7 | 14 | |
| 2–3 cm | 26 | 9 | 35 | ||
| >3 cm | 10 | 22 | 32 | ||
| Total | 43 | 38 | 81 | 0.002 | |
| Tumor Size < 2 cm | Tumor Size 2–3 cm | Tumor Size > 3 cm | p-Value (Chi Square Test) | ||||
|---|---|---|---|---|---|---|---|
| Biopsy | Resection | Biopsy | Resection | Biopsy | Resection | ||
| Headache | 6 | 5 | 9 | 22 | 20 | 8 | 0.090 |
| Hydrocephalus | 5 | 6 | 8 | 23 | 18 | 9 | 0.068 |
| Parinaud syndrome | 2 | 6 | 4 | 11 | 11 | 7 | 0.064 |
| Papilledema | 5 | 0 | 3 | 9 | 8 | 5 | 0.057 |
| Double vision | 4 | 6 | 5 | 17 | 11 | 3 | 0.050 |
| Biopsy/Resection | Total | |||||
|---|---|---|---|---|---|---|
| Resection | Biopsy | p-Value (Fisher Exact Test) | ||||
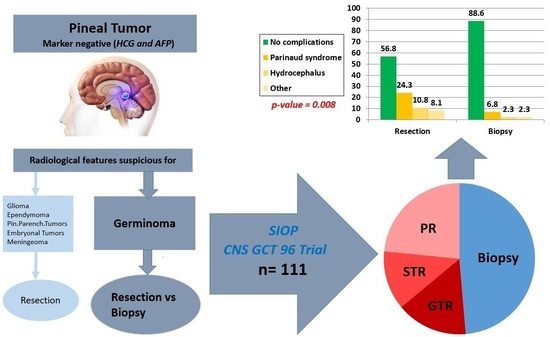
| Postoperative complications | No complications | 21 | 39 | 60 | ||
| Postop complications | 16 | 5 | 21 | p = 0.008 | ||
| Parinaud syndrome/double vision | 9 | 3 | 12 | |||
| Hydrocephalus | 4 | 1 | 5 | |||
| Others | 3 | 1 | 4 | |||
| Total | 37 | 44 | 81 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabo, E.; Czech, T.; Nicholson, J.C.; Mallucci, C.; Mottolese, C.; Piatelli, G.; Frappaz, D.; Murray, M.J.; Faure-Conter, C.; Garrè, M.L.; et al. Evaluation of the Perioperative and Postoperative Course of Surgery for Pineal Germinoma in the SIOP CNS GCT 96 Trial. Cancers 2022, 14, 3555. https://doi.org/10.3390/cancers14143555
Shabo E, Czech T, Nicholson JC, Mallucci C, Mottolese C, Piatelli G, Frappaz D, Murray MJ, Faure-Conter C, Garrè ML, et al. Evaluation of the Perioperative and Postoperative Course of Surgery for Pineal Germinoma in the SIOP CNS GCT 96 Trial. Cancers. 2022; 14(14):3555. https://doi.org/10.3390/cancers14143555
Chicago/Turabian StyleShabo, Ehab, Thomas Czech, James C. Nicholson, Conor Mallucci, Carmine Mottolese, Gianluca Piatelli, Didier Frappaz, Matthew Jonathan Murray, Cecile Faure-Conter, Maria Luisa Garrè, and et al. 2022. "Evaluation of the Perioperative and Postoperative Course of Surgery for Pineal Germinoma in the SIOP CNS GCT 96 Trial" Cancers 14, no. 14: 3555. https://doi.org/10.3390/cancers14143555
APA StyleShabo, E., Czech, T., Nicholson, J. C., Mallucci, C., Mottolese, C., Piatelli, G., Frappaz, D., Murray, M. J., Faure-Conter, C., Garrè, M. L., Sarikaya-Seiwert, S., Weinhold, L., Haberl, H., & Calaminus, G. (2022). Evaluation of the Perioperative and Postoperative Course of Surgery for Pineal Germinoma in the SIOP CNS GCT 96 Trial. Cancers, 14(14), 3555. https://doi.org/10.3390/cancers14143555