Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
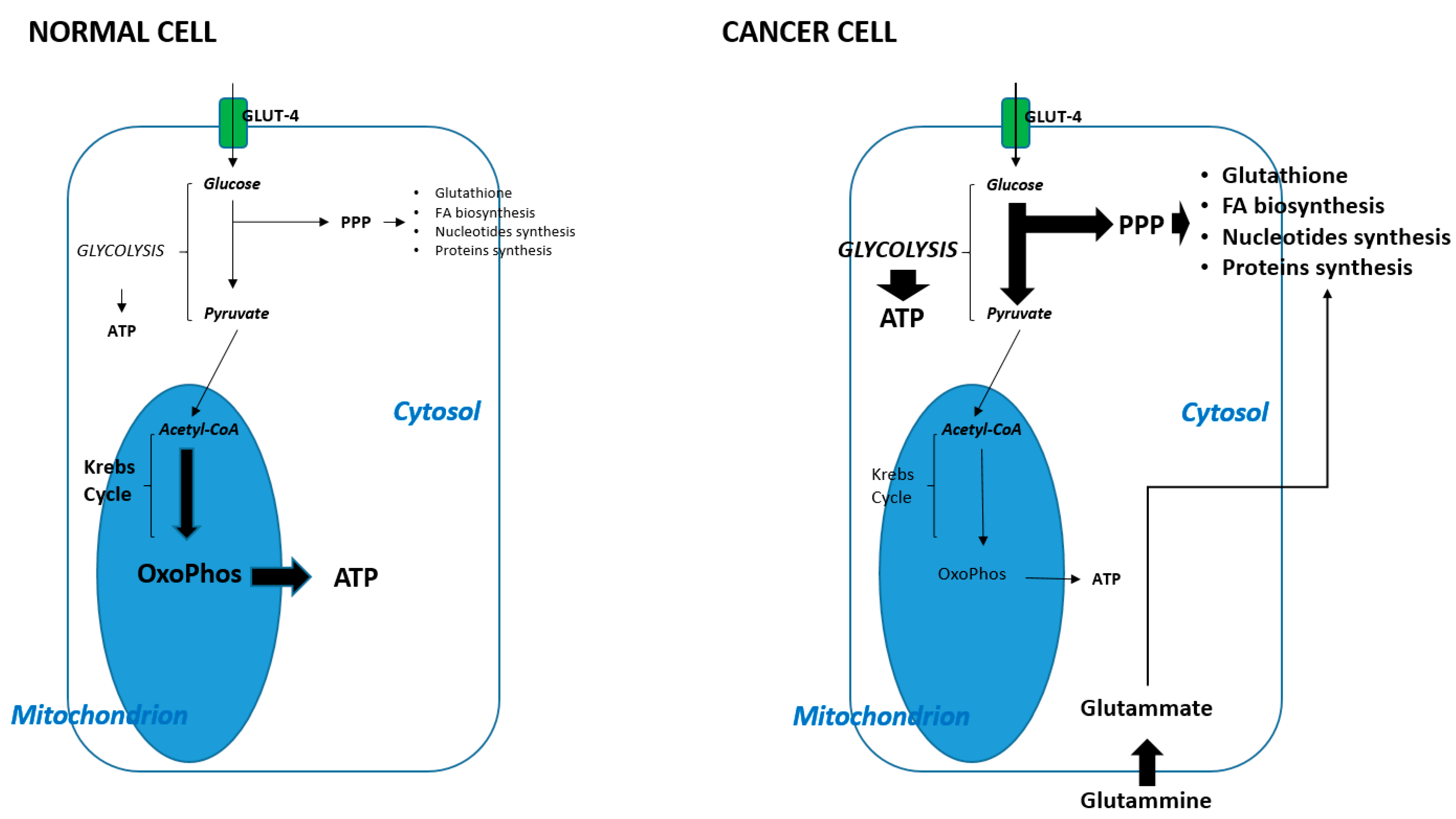
2. Metabolic Shift in Cancer Cells
3. Intracellular Pathways Involved in Metabolic Reprogramming
3.1. PI3K-Akt-mTOR Pathway
3.2. TP53
3.3. HIF-1 Alpha
3.4. PTEN
3.5. c-Myc
4. Interplay between Metabolic Shift and Immune Response
4.1. Immune Surveillance
4.2. Metabolic Reprogramming and Immune Suppression
5. How Can We Target the Immune Metabolism in SCCHNs
5.1. HPV Related SCCHN and PI3K Pathway
5.2. Mutagens Related SCCHN (Alcohol and Tobacco)
5.3. Drugs with Direct Effect on Metabolism
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
References
- Karakosta, A.; Golias, C.; Charalabopoulos, A.; Peschos, D.; Batistatou, A.; Charalabopoulos, K. Genetic models of human cancer as a multistep process. Paradigm models of colorectal cancer, breast cancer, and chronic myelogenous and acute lymphoblastic leukaemia. J. Exp. Clin. Cancer Res. 2005, 24, 505–514. [Google Scholar] [PubMed]
- Bièche, I.; Lidereau, R. Biology of solid cancers: Breast cancer as an example. First part: Genetic systems implicated in carcinogenesis. J. Gynecol. Obstet. Biol. Reprod. 1996, 25, 131–141. [Google Scholar]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; Báez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor cell metabolism: An integral view. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Ionna, F.; Longo, F.; Della Vittoria Scarpati, G.; De Angelis, C.; Ottaiano, A.; Botti, G.; Caponigro, F. Immune Response Against Head and Neck Cancer: Biological Mechanisms and Implication on Therapy. Transl. Oncol. 2020, 13, 262–274. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Marín Hernández, A.; Saavedra, E. Energy metabolism in tumor cells. FEBS J. 2007, 274, 1393–1418. [Google Scholar] [CrossRef]
- Callao, V.; Montoya, E. Toxohormone-like factor from microorganisms with impaired respiration. Science 1961, 134, 2041–2042. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Du, H. Improving Cancer Immunotherapy: Exploring and Targeting Metabolism in Hypoxia Microenvironment. Front. Immunol. 2022, 13, 845923. [Google Scholar] [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell. 2008, 13, 472–482. [Google Scholar] [CrossRef]
- Dai, G.; Ou, J.; Wu, B. A predictive study of metabolism reprogramming in cervical carcinoma. Ann. Transl. Med. 2022, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Best, S.A.; Gubser, P.M.; Sethumadhavan, S.; Kersbergen, A.; Negrón Abril, Y.L.; Goldford, J.; Sellers, K.; Abeysekera, W.; Garnham, A.L.; McDonald, J.A.; et al. Glutaminase inhibition impairs CD8 T cell activation in STK11-/Lkb1-deficient lung cancer. Cell Metab. 2022, 34, 874–887.e6. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; You, D.; Zhu, X.; Cai, L.; Zeng, S.; Hu, X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol. 2021, 46, 102065. [Google Scholar] [CrossRef] [PubMed]
- Quirico, L.; Orso, F.; Cucinelli, S.; Paradzik, M.; Natalini, D.; Centonze, G.; Dalmasso, A.; La Vecchia, S.; Coco, M.; Audrito, V.; et al. miRNA-guided reprogramming of glucose and glutamine metabolism and its impact on cell adhesion/migration during solid tumor progression. Cell Mol. Life Sci. 2022, 79, 216. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.A. Glutamine and cancer. J. Nutr. 2001, 131, 2539S–2542S. [Google Scholar] [CrossRef]
- Szeliga, M.; Obara-Michlewska, M. Glutamine in neo plastic cells: Focus on the expression and roles of glutaminases. Neurochem. Int. 2009, 55, 71–75. [Google Scholar] [CrossRef]
- Ohashi, T.; Inoue, N.; Aoki, M. The Warburg Effect and M2 Macrophage Polarization in Head and Neck Cancer. Gan Kagaku Ryoho Cancer Chemother. 2020, 47, 6–10. [Google Scholar]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Christalle, C.; Chow, T.; Harada, H. HIF-1 dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S. Molecular intricacies of aerobic glycolysis in cancer: Current insights into the classic metabolic phenotype. Crit. Rev. Biochem. Mol. Biol. 2019, 53, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Tameemi, W.A.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-modified cancer cell metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, Q.; Ma, H.; Zhang, L.; Liu, F.; Han, Y. Relationship of PI3K-Akt/mTOR/AMPK signaling pathway genetic mutation with efficacy and prognosis in nasopharyngeal carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 165–173. [Google Scholar] [PubMed]
- Levina, A.; Fleming, K.D.; Burke, J.E.; Leonard, T.A. Activation of the essential kinase PDK1 by phosphoinositide-driven trans-autophosphorylation. Nat. Commun. 2022, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Manglani, K.; Dey, C.S. Tankyrase inhibition augments neuronal insulin sensitivity and glucose uptake via AMPK-AS160 mediated pathway. Neurochem. Int. 2020, 141, 104854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, P.; Liu, J.; Chen, Z.; Ma, W.; Yuan, Y. ATIC inhibits autophagy in hepatocellular cancer through the AKT/FOXO3 pathway and serves as a prognostic signature for modeling patient survival. Int. J. Biol. Sci. 2021, 17, 4442–4458. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Liu, L.; Yang, H.; Zhou, P.; Song, F.; Dong, G.; Chen, J.; Wang, G.; Dong, X. Effect of Heat Stress on Bovine Mammary Cellular Metabolites and Gene Transcription Related to Amino Acid Metabolism, Amino Acid Transportation and Mammalian Target of Rapamycin (mTOR) Signaling. Animals 2021, 11, 3153. [Google Scholar] [CrossRef]
- Perri, F.; Pisconti, S.; Della Vittoria Scarpati, G. P53 mutations and cancer: A tight linkage. Ann. Transl. Med. 2016, 24, 522. [Google Scholar] [CrossRef]
- Jacquier, V.; Gitenay, D.; Fritsch, S.; Bonnet, S.; Győrffy, B.; Jalaguier, S.; Linares, L.K.; Cavaillès, V.; Teyssier, C. RIP140 inhibits glycolysis-dependent proliferation of breast cancer cells by regulating GLUT3 expression through transcriptional crosstalk between hypoxia induced factor and p53. Cell Mol. Life Sci. 2022, 79, 270. [Google Scholar] [CrossRef]
- Han, C.Y.; Patten, D.A.; Lee, S.G.; Parks, R.J.; Chan, D.W.; Harper, M.E.; Tsang, B.K. p53 Promotes chemoresponsiveness by regulating hexokinase II gene transcription and metabolic reprogramming in epithelial ovarian cancer. Mol. Carcinog. 2019, 58, 2161–2174. [Google Scholar] [CrossRef]
- Yu, L.; Wu, M.; Zhu, G.; Xu, Y. Emerging Roles of the Tumor Suppressor p53 in Metabolism. Front. Cell Dev. Biol. 2022, 9, 762742. [Google Scholar] [CrossRef] [PubMed]
- Korotchkina, L.G.; Patel, M.S. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J. Biol. Chem. 2001, 276, 37223–37229. [Google Scholar] [CrossRef] [PubMed]
- Korotchkina, L.G.; Sidhu, S.; Patel, M.S. Characterization of testis-specific isoenzyme of human pyruvate dehydrogenase. J. Biol. Chem. 2006, 281, 9688–9696. [Google Scholar] [CrossRef]
- Laconi, E. The evolving concept of tumor microenvironments. Bioessays 2007, 29, 738–744. [Google Scholar] [CrossRef]
- Janecka-Widła, A.; Majchrzyk, K.; Mucha-Małecka, A.; Biesaga, B. EGFR/PI3K/Akt/mTOR pathway in head and neck squamous cell carcinoma patients with different HPV status. Pol. J. Pathol. 2021, 72, 296–314. [Google Scholar] [CrossRef]
- Liu, A.; Borges, P.M.; Tay, Y.S.; Thompson, L.D.R.; Kong, M.X.; Lai, J. Thyroid Follicular Cell-derived Carcinomas in a Background of Multiple Adenomatous Nodules Leading to a Diagnosis of PTEN Hamartoma Tumor Syndrome in an Adult Patient with a Novel RECQL4 Mutation. Anticancer Res. 2022, 42, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Hsiao, J.R.; Jiang, S.S.; Chang, J.Y.; Chu, P.Y.; Liu, K.J.; Fang, H.L.; Lin, L.M.; Chen, H.H.; Huang, Y.W.; et al. c-MYC-directed NRF2 drives malignant progression of head and neck cancer via glucose-6-phosphate dehydrogenase and transketolase activation. Theranostics 2021, 11, 5232–5247. [Google Scholar] [CrossRef]
- Mainguené, J.; Vacher, S.; Kamal, M.; Hamza, A.; Masliah-Planchon, J.; Baulande, S.; Ibadioune, S.; Borcoman, E.; Cacheux, W.; Calugaru, V.; et al. Human papilloma virus integration sites and genomic signatures in head and neck squamous cell carcinoma. Mol. Oncol. 2022. [Google Scholar] [CrossRef]
- Vaněk, O.; Kalousková, B.; Abreu, C.; Nejadebrahim, S.; Skořepa, O. Natural killer cell-based strategies for immunotherapy of cancer. Adv. Protein Chem. Struct. Biol. 2022, 129, 91–133. [Google Scholar]
- Fiuza-Luces, C.; Valenzuela, P.L.; Castillo-García, A.; Lucia, A. Exercise Benefits Meet Cancer Immunosurveillance: Implications for Immunotherapy. Trends Cancer 2021, 7, 91–93. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Elkord, E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019, 457, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhan, H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. 2019, 457, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.J.; Cheong, J.H. New Immunometabolic Strategy Based on Cell Type-Specific Metabolic Reprogramming in the Tumor Immune Microenvironment. Cells 2022, 11, 768. [Google Scholar] [CrossRef]
- Niu, Y.; Mayr, T.; Muders, M.H. Competition for nutrients or cell intrinsic programming?—Metabolic mechanisms behind the tumor promoting immune microenvironment in cancer. Signal Transduct. Target. Ther. 2021, 6, 279. [Google Scholar] [CrossRef]
- Worsley, C.M.; Veale, R.B.; Mayne, E.S. The acidic tumour microenvironment: Manipulating the immune response to elicit escape. Hum. Immunol. 2022, 83, 399–408. [Google Scholar] [CrossRef]
- Damgaci, S.; Ibrahim-Hashim, A.; Enriquez-Navas, P.M.; Pilon-Thomas, S.; Guvenis, A.; Gillies, R.J. Hypoxia and acidosis: Immune suppressors and therapeutic targets. Immunology 2018, 154, 354–362. [Google Scholar] [CrossRef]
- Noman, M.Z.; Hasmim, M.; Messai, Y.; Terry, S.; Kieda, C.; Janji, B.; Chouaib, S. Hypoxia: A key player in antitumor immune response. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C569–C579. [Google Scholar] [CrossRef]
- Hsu, T.S.; Lin, Y.L.; Wang, Y.A.; Mo, S.T.; Chi, P.Y.; Lai, A.C.; Pan, H.Y.; Chang, Y.J.; Lai, M.Z. HIF-2α is indispensable for regulatory T cell function. Nat. Commun. 2020, 11, 5005. [Google Scholar] [CrossRef]
- Chung, C.H.; Parker, J.S.; Karaca, G.; Wu, J.; Funkhouser, W.K.; Moore, D.; Butterfoss, D.; Xiang, D.; Zanation, A.; Yin, X.; et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 2004, 5, 489–500. [Google Scholar] [CrossRef]
- Walter, V.; Yin, X.; Wilkerson, M.D.; Cabanski, C.R.; Zhao, N.; Du, Y.; Ang, M.K.; Hayward, M.C.; Salazar, A.H.; Hoadley, K.A.; et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS ONE 2013, 8, e56823. [Google Scholar] [CrossRef]
- The BURAN Study of Buparlisib (AN2025) in Combination with Paclitaxel Compared to Paclitaxel Alone, in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT04338399 (accessed on 5 May 2022).
- Brisson, R.J.; Kochanny, S.; Arshad, S.; Dekker, A.; DeSouza, J.A.; Saloura, V.; Vokes, E.E.; Seiwert, T.Y. A pilot study of the pan-class I PI3K inhibitor buparlisib in combination with cetuximab in patients with recurrent or metastatic head and neck cancer. Head Neck 2019, 41, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Soulières, D.; Faivre, S.; Mesía, R.; Remenár, E.; Li, S.H.; Karpenko, S.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.; et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef]
- Marret, G.; Isambert, N.; Rezai, K.; Gal, J.; SaadaBouzid, G.; Rolland, F.; Chausson, M.; Borcoman, E.; Alt, G.; Klijanienko, J.; et al. Phase I trial of copanlisib, a selective PI3K inhibitor, in combination with cetuximab in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Investig. New Drugs 2021, 39, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Swiecicki, P.L.; Durm, G.; Bellile, E.; Bhangale, A.; Brenner, J.C.; Worden, F.P. A multi-center phase II trial evaluating the efficacy of palbociclib in combination with carboplatin for the treatment of unresectable recurrent or metastatic head and neck squamous cell carcinoma. Investig. New Drugs 2020, 38, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Oppelt, P.; Ley, J.C.; Worden, F.; Palka, K.; Maggiore, R.; Liu, J.; Adkins, D. Palbociclib and cetuximab in cetuximab-resistant human papillomavirus-related oropharynx squamous-cell carcinoma: A multicenter phase 2 trial. Oral Oncol. 2021, 114, 105164. [Google Scholar] [CrossRef]
- Adkins, D.R.; Lin, J.C.; Sacco, A.; Ley, J.; Oppelt, P.; Vanchenko, V.; Komashko, N.; Yen, C.J.; Wise-Draper, T.; Lopez-Picazo Gonzalez, J.; et al. Palbociclib and cetuximab compared with placebo and cetuximab in platinum-resistant, cetuximab-naïve, human papillomavirus-unrelated recurrent or metastatic head and neck squamous cell carcinoma: A double-blind, randomized, phase 2 trial. Oral Oncol. 2021, 115, 105192. [Google Scholar] [CrossRef]
- Adkins, D.; Ley, J.; Neupane, P.; Worden, F.; Sacco, A.G.; Palka, K.; Grilley-Olson, J.E.; Maggiore, R.; Salama, N.N.; Trinkaus, K.; et al. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: A multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019, 20, 1295–1305. [Google Scholar] [CrossRef]
- Michel, L.; Ley, J.; Wildes, T.M.; Schaffer, A.; Robinson, A.; Chun, S.; Lee, W.; Lewis, J., Jr.; Trinkaus, K.; Adkins, D. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2016, 58, 41–48. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y. Cancer metabolism and intervention therapy. Mol. Biomed. 2021, 2, 5. [Google Scholar] [CrossRef]
- Asensio, C.; Levoin, N.; Guillaume, C.; Guerquin, M.J.; Rouguieg, K.; Chrétien, F.; Chapleur, Y.; Netter, P.; Minn, A.; Lapicque, F. Irreversible inhibition of glucose-6-phosphate dehydrogenase by the coenzyme A conjugate of ketoprofen: A key to oxidative stress induced by non-steroidal anti-inflammatory drugs? Biochem. Pharmacol. 2007, 73, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Ho, H.Y.; Cheng, M.L.; You, T.H.; Yu, J.S.; Chiu, D.T. Impaired dephosphorylation renders G6PD-knockdown HepG2 cells more susceptible to H2O2-induced apoptosis. Free Radic. Biol. Med. 2010, 49, 361–373. [Google Scholar] [CrossRef]
- Moldasheva, A.; Surov, V.; Aljofan, M. Editorial: New lights Through Old Windows: Metformin and Derivatives as Anti-Cancer Treatments. Front. Pharmacol. 2022, 13, 889642. [Google Scholar] [CrossRef] [PubMed]
- Nalweyiso, J.; Okechukwu, P.N.; Sie Ting, L.N.; Hui, T.Y.; Balachandran, A.; Siew Ling, C.L.; Ghadeer, S.; Anisa Fromming, G.R.; Johnson, S. Glycosylated Sulfonylurea (2DGs) Modulates Insulin—Dependent and Insulin—Independent Signaling Pathways via PI3K and P38 MAPK in L6 Skeletal Muscle Cell Line. FASEB J. 2022, 36 (Suppl. 1). [Google Scholar] [CrossRef]
- Wu, Z.; Wu, L.; Zou, L.; Wang, M.; Liu, X. Metformin induces myeloma cells necrosis and apoptosis and it is considered for therapeutic use. J. Chemother. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Desrichard, A.; Kuo, F.; Chowell, D.; Lee, K.W.; Riaz, N.; Wong, R.J.; Chan, T.A.; Morris, L.G.T. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. J. Natl. Cancer Inst. 2018, 110, 1386–1392. [Google Scholar] [CrossRef] [PubMed]


| STUDY | Phase | Design (Number of p.ts) | Results | Type of Drug |
|---|---|---|---|---|
| Head Neck. 2019 Nov.;41(11):3842–3849 [53]. | Phase I | Buparlisib + Cetuximab (12) | Safe at a dose of 100 mg/daily | Anti-PI3K |
| Lancet Oncol. 2017 Mar.;18(3):323–335 [54]. | Phase II | Buparlisib + Paclitaxel (158) | Median progression-free survival was 4.6 months (endpoint met) | Anti PI3K |
| Invest New Drugs. 2021 Dec.;39(6):1641–1648 [55]. | Phase I | Copanlisib + Cetuximab (11) | Safe at a dose of 30 mg/daily | Anti PI3K |
| Oral Oncol. 2021 Apr.;115:105192 [56]. | Phase II | Palbociclib +/− Cetuximab (125) | No differences in OS | Anti CDK 4/6 |
| Oral Oncol. 2021 Mar.;114:105164 [57]. | Phase II | Palbociclib + Cetuximab (24) | ORR: 4% (endpoint not met) | Anti CDK 4/6 |
| Invest New Drugs. 2020 Oct.;38(5):1550–1558 [58]. | Phase II | CBDCA + Palbociclib (21) | DCR: 23% (endpoint not met) | Anti CDK 4/6 |
| Lancet Oncol. 2019 Sep.;20(9):1295–1305 [59]. | Phase II | Palbociclib + Cetuximab (62) | ORR 39% (endpoint met) | Anti CDK 4/6 |
| Oral Oncol. 2016 Jul.;58:41–48 [60]. | Phase I | Palbociclib + Cetuximab (9) | Safe at a dose of 125 mg/daily | Anti CDK 4/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perri, F.; Della Vittoria Scarpati, G.; Pontone, M.; Marciano, M.L.; Ottaiano, A.; Cascella, M.; Sabbatino, F.; Guida, A.; Santorsola, M.; Maiolino, P.; et al. Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review. Cancers 2022, 14, 3560. https://doi.org/10.3390/cancers14153560
Perri F, Della Vittoria Scarpati G, Pontone M, Marciano ML, Ottaiano A, Cascella M, Sabbatino F, Guida A, Santorsola M, Maiolino P, et al. Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review. Cancers. 2022; 14(15):3560. https://doi.org/10.3390/cancers14153560
Chicago/Turabian StylePerri, Francesco, Giuseppina Della Vittoria Scarpati, Monica Pontone, Maria Luisa Marciano, Alessandro Ottaiano, Marco Cascella, Francesco Sabbatino, Agostino Guida, Mariachiara Santorsola, Piera Maiolino, and et al. 2022. "Cancer Cell Metabolism Reprogramming and Its Potential Implications on Therapy in Squamous Cell Carcinoma of the Head and Neck: A Review" Cancers 14, no. 15: 3560. https://doi.org/10.3390/cancers14153560








