Vitamin D, Th17 Lymphocytes, and Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Vitamin D and Breast Cancer Prevention and Treatment
2.1. Role of Vitamin D in Development of Breast Cancer
2.1.1. The 25-Hydroxyvitamin D Level and Cancer Correlations
2.1.2. Studies on VDR and Other Vitamin D-Related Molecules
2.1.3. Importance of Vitamin D Supplementation in Breast Cancer Treatment
| Vitamin D Dosage Used for Treatment | Patient Characteristics; Trial Type | Results | References |
|---|---|---|---|
| Healthy people | |||
| 2000 IU/d of vitamin D3 and 1500 mg/d of calcium for 4 years | 2303 healthy postmenopausal women, 55 years or older; 4-year, double-blind, placebo-controlled, population-based randomized clinical trial | Among healthy postmenopausal older women a mean baseline serum 25(OH)D level of 32.8 ng/mL, vitamin D3 and calcium supplementation didn’t decrease significantly the risk of all-type cancer in a 4 years study | [97] |
| 2000 IU/d for 5 years | 1617 participants (793 in the vitamin D group and 824 in the placebo group); randomized, double-blind, placebo-controlled trial, with a two-by-two factorial design | Treatment did not reduce the incidence of breast cancer | [118] |
| Breast cancer patients | |||
| 4000 IU vitamin D3 daily for 12 weeks | 168 breast cancer survivors; single-arm nonrandomized before-and-after trial has been registered in the Iranian Registry of Clinical Trials (IRCT) under the identification code: IRCT2017091736244N1 | The association between the VDR SNPs (ApaI, TaqI, FokI, BsmI, and Cdx2) and changes in response was assessed. Cdx2 genotypes AA and GA, compared to GG, showed higher plasma levels of MMP9; BsmI bb genotype showed a greater decrease in circulating TNF-α levels after vitamin D3 supplementation; VDR genetic polymorphisms were not associated with longitudinal changes in the remaining cancer biomarkers. In breast cancer survivors with low 25(OH)D plasma levels and vitamin D3 supplementation changes in certain inflammatory biomarkers may be affected by VDR SNPs and haplotypes | [121] |
| 4000 IU vitamin D3 daily for 12 weeks | 176 breast cancer survivors who had completed treatment protocol, including surgery, radiotherapy, and chemotherapy; trial has been registered on the IRCT under the identification code: IRCT2017091736244N1 | 85% of women had insufficient and inadequate levels of plasma 25(OH)D at baseline; aa genotype of ApaI showed a greater increase in muscle mass and higher decrease in low-density lipoprotein cholesterol levels; Bb genotype of the BsmI VDR showed a higher increase in waist circumference following vitamin D3 supplementation; haplotype score analyses showed a significant association between inferred haplotypes from BsmI, ApaI, TaqI, and FokI, BsmI and Cdx2 VDR polymorphisms, and on-study visceral fat changes | [122] |
| 50,000 IU/week for 8 weeks | 56 breast cancer patients; 2 treatment arms: placebo and vitamin D3 through a 2-month intervention period; double-blind, placebo-controlled trial | Supplementation with vitamin D3 increased the total antioxidant capacity (TAC) in breast cancer women; no effect was found on inflammatory markers. Serum TAC in the TT/Tt and Ff genotypes was more responsive to vitamin D supplement compared with FF/ff and tt genotypes | [123] |
| 40,000 IU/d of vitamin D3 or placebo for 2–6 weeks prior to breast surgery | 120 newly diagnosed breast cancer patients; prospective, randomized, phase 2, double-blinded presurgical window of opportunity trial; trial registration: NCT01948128. | Significantly higher levels of serum 25(OH)D in the vitamin D-treated group were not associated with any significant effects on tumor proliferation and apoptosis | [124] |
| 10,000 IU daily in the interval between biopsy and surgery | 29 breast cancer patients; controlled, and blinded trial in women with core needle biopsies positive for breast cancer, but without the presence of metastatic lesions; ancillary study of a breast cancer trial (NCT01472445) | Vitamin D supplementation can decrease circulating 27-hydroxycholesterol in breast cancer patients, likely by CYP27A1 inhibition. This suggests a new and additional modality by which vitamin D can inhibit ER+ breast cancer growth; a larger study is needed for verification | [125] |
| 10,000 IU vitamin D3 and 1000 mg calcium each day for 4 months | 40 patients with bone metastases treated with bisphosphonates; single-arm, phase 2 study | Treatment was safe, and reduced inappropriately elevated PTH levels caused by long-term bisphosphonate use; no significant palliative benefit or any significant change in bone resorption was observed | [126] |
| 2000 IU/1000 mg and 4000 IU/1000 mg based on baseline serum 25(OH)D for 12 weeks | 82 breast cancer patients treated with letrozole | Vitamin D3 supplementation significantly improved serum 25(OH)D concentrations and decreased letrozole-induced arthralgia | [113] |
| 50,000 IU/week for 12 weeks | 60 breast cancer patients treated with letrozole | Vitamin D3 supplementation is safe, and may reduce disability from AI-induced arthralgias | [112] |
| 30,000 IU oral vitamin D3/week for 24 weeks | 160 women with stage I–III breast cancer starting adjuvant letrozole and 25(OH)D level ≤ 40 ng/mL | Treatment was safe and effective in achieving adequate vitamin D levels, but not associated with a decrease in AI-associated musculoskeletal symptoms | [127] |
| 800 IU/d with calcium but women with baseline 25(OH)D < 30 ng/mL also received 16,000 IU of vitamin D3 every 2 weeks for 3 months | 290 breast cancer patients starting AI, prospective cohort | 40 ng/mL 25(OH)D may prevent development of AI-induced arthralgia but higher loading doses are required to achieve this level in women with deficiency at baseline | [128] |
2.2. Impact of Vitamin D on Animal Models of Breast Cancer (Pre-Clinical Studies and Signal Transduction Data)
3. Role of Th17 Cells in Breast Cancer
Importance of Th17 Cells in Breast Cancer
4. Action of Vitamin D on the Immune System in Cancer
5. Vitamin D and Th17 Cells
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coughlin, S.; Caplan, L.; Stewart, J.; Young, L. Do Breast Cancer Survivorship Care Plans Improve Health Outcomes? J. Cancer Treat. Diagn. 2019, 3, 28–33. [Google Scholar] [CrossRef]
- Kleibl, Z.; Kristensen, V.N. Women at high risk of breast cancer: Molecular characteristics, clinical presentation and management. Breast 2016, 28, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y. Estrogen Receptor β Expression and Its Clinical Implication in Breast Cancers: Favorable or Unfavorable? J. Breast Cancer 2022, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.S.; Kanaya, N.; Lo, C.; Mortimer, J.; Chen, S. From Bench to Bedside: What Do We Know about Hormone Receptor-Positive and Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer? J. Steroid Biochem. Mol. Biol. 2015, 153, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Reese, J.M.; Bruinsma, E.S.; Nelson, A.W.; Chernukhin, I.; Carroll, J.S.; Li, Y.; Subramaniam, M.; Suman, V.J.; Negron, V.; Monroe, D.G.; et al. ERβ-mediated induction of cystatins results in suppression of TGFβ signaling and inhibition of triple-negative breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2018, 115, E9580–E9589. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; McCann, M.; Zhang, Z.; Posner, G.H.; Bingham, V.; El-Tanani, M.; Campbell, F.C. Vitamin D receptor modulates the neoplastic phenotype through antagonistic growth regulatory signals. Mol. Carcinog. 2009, 48, 758–772. [Google Scholar] [CrossRef]
- Song, I.-S.; Jeong, Y.J.; Jeong, S.H.; Kim, J.E.; Han, J.; Kim, T.-H.; Jang, S.-W. Modulation of Mitochondrial ERβ Expression Inhibits Triple-Negative Breast Cancer Tumor Progression by Activating Mitochondrial Function. Cell. Physiol. Biochem. 2019, 52, 468–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, J.W.; Lee, S.M.; Meyer, M.B. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: Exploiting new approaches and defining new mechanisms. BoneKEy Rep. 2014, 3, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, L.; Su, X.; Xu, X.; Li, G.; Lin, B.; Cao, J. ER-α36: A novel biomarker and potential therapeutic target in breast cancer. OncoTargets Ther. 2014, 7, 1525–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.-Y.; Yin, L. Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Mol. Cell. Endocrinol. 2015, 418, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorka, I.; Milan, Z.; Momcilo, I.; Ivan, M.; Zoran, K.; Igor, D.; Ivana, I.; Gordana, P.; Snezana, J. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin. Med. Insights Oncol. 2014, 8, S18006. [Google Scholar] [CrossRef] [Green Version]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen Actions in Triple-Negative Breast Cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Giovannucci, E. Vitamin D and Prevention of Colorectal Adenoma: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2958–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, N.; Sousa, B.; Martins, D.; Gomes, M.; Vieira, D.; Veronese, L.A.; Milanezi, F.; Paredes, J.; Costa, J.L.; Schmitt, F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: A study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions Vitamin D pathways unbalanced in breast lesions. BMC Cancer 2010, 10, 483. [Google Scholar] [CrossRef] [Green Version]
- Matilainen, J.M.; Malinen, M.; Turunen, M.M.; Carlberg, C.; Väisänen, S. The Number of Vitamin D Receptor Binding Sites Defines the Different Vitamin D Responsiveness of the CYP24 Gene in Malignant and Normal Mammary Cells. J. Biol. Chem. 2010, 285, 24174–24183. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-Analysis of Vitamin D, Calcium and the Prevention of Breast Cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef]
- Lim, S.T.; Jeon, Y.W.; Suh, Y.J. Association Between Alterations in the Serum 25-Hydroxyvitamin D Status During Follow-Up and Breast Cancer Patient Prognosis. Asian Pac. J. Cancer Prev. 2015, 16, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Janbabai, G.; Shekarriz, R.; Hassanzadeh, H.; Aarabi, M.; Borhani, S.S. A survey on the relationship between serum 25-hydroxy vitamin D level and tumor characteristics in patients with breast cancer. Int. J. Hematol. Stem Cell Res. 2016, 10, 30–36. [Google Scholar]
- Palmieri, C.; MacGregor, T.; Girgis, S.; Vigushin, D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J. Clin. Pathol. 2006, 59, 1334–1336. [Google Scholar] [CrossRef] [Green Version]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The Role of Vitamin D in Cancer Prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.E. Vitamin D and Breast Cancer: Past and Present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15–20. [Google Scholar] [CrossRef]
- Shirazi, L.; Almquist, M.; Borgquist, S.; Malm, J.; Manjer, J. Serum vitamin D (25OHD3) levels and the risk of different subtypes of breast cancer: A nested case–control study. Breast 2016, 28, 184–190. [Google Scholar] [CrossRef]
- Thanasitthichai, S.; Chaiwerawattana, A.; Prasitthipayong, A. Association of Vitamin D Level with Clinicopathological Features in Breast Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 4881–4883. [Google Scholar] [CrossRef] [Green Version]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.; McCann, S.E.; Khoury, T.; Morrison, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S. Tumor Expression of Vitamin D Receptor and Breast Cancer Histopathological Characteristics and Prognosis. Clin. Cancer Res. 2017, 23, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hofmann, S.; Rack, B.; Harbeck, N.; Jeschke, U.; Sixou, S. Fluorescence Analysis of Vitamin D Receptor Status of Circulating Tumor Cells (CTCS) in Breast Cancer: From Cell Models to Metastatic Patients. Int. J. Mol. Sci. 2017, 18, 1318. [Google Scholar] [CrossRef] [Green Version]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D exposure and Risk of Breast Cancer: A meta-analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Mena, J.M.; Schöttker, B.; Fedirko, V.; Jenab, M.; Olsen, A.; Halkjær, J.; Kampman, E.; de Groot, L.; Jansen, E.; Bueno-De-Mesquita, H.B.; et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: An analysis of cohorts participating in the CHANCES consortium. Eur. J. Epidemiol. 2015, 31, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kanstrup, C.; Teilum, D.; Rejnmark, L.; Bigaard, J.V.; Eiken, P.; Kroman, N.; Tjønneland, A.; Mejdahl, M.K. 25-Hydroxyvitamin D at time of breast cancer diagnosis and breast cancer survival. Breast Cancer Res. Treat. 2019, 179, 699–708. [Google Scholar] [CrossRef]
- Fuhrman, B.J.; Freedman, D.M.; Bhatti, P.; Doody, M.M.; Fu, Y.-P.; Chang, S.-C.; Linet, M.S.; Sigurdson, A.J. Sunlight, polymorphisms of vitamin D-related genes and risk of breast cancer. Anticancer Res. 2013, 33, 543–551. [Google Scholar] [PubMed]
- Zamoiski, R.D.; Freedman, D.M.; Linet, M.S.; Kitahara, C.M.; Liu, W.; Cahoon, E.K. Prospective study of ultraviolet radiation exposure and risk of breast cancer in the United States. Environ. Res. 2016, 151, 419–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiller, T.W.R.; O’Sullivan, D.E.; Brenner, D.R.; Peters, C.E.; King, W.D. Solar Ultraviolet Radiation and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2020, 128, 016002. [Google Scholar] [CrossRef]
- Oh, E.-Y.; Ansell, C.; Nawaz, H.; Yang, C.-H.; Wood, P.A.; Hrushesky, W.J.M. Global breast cancer seasonality. Breast Cancer Res. Treat. 2010, 123, 233–243. [Google Scholar] [CrossRef]
- Mutlu, H.; Buyukcelik, A.; Çolak, T.; Ozdogan, M.; Erden, A.; Aslan, T.; Akca, Z. Is Sunlight a Predisposing Factor for Triple Negative Breast Cancer in Turkey? Asian Pac. J. Cancer Prev. 2013, 14, 801–803. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.; Xu, B.; Ji, N.; Yao, S.; Pawlish, K.; Llanos, A.A.M.; Lin, Y.; Demissie, K.; Ambrosone, C.B.; Hong, C.-C.; et al. Intake of vitamin D and calcium, sun exposure, and risk of breast cancer subtypes among black women. Am. J. Clin. Nutr. 2019, 111, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.N.; Cotterchio, M.; Kirsh, V.A.; Knight, J.A. Ultraviolet Sunlight Exposure During Adolescence and Adulthood and Breast Cancer Risk: A Population-based Case-Control Study Among Ontario Women. Am. J. Epidemiol. 2011, 174, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, D.M.; Dosemeci, M.; McGlynn, K. Sunlight and mortality from breast, ovarian, colon, prostate, and non-melanoma skin cancer: A composite death certificate based case-control study. Occup. Environ. Med. 2002, 59, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Manson, J.E.; Lee, I.-M.; Cook, N.R.; Buring, J.E.; Zhang, S.M. Intakes of Calcium and Vitamin D and Breast Cancer Risk in Women. Arch. Intern. Med. 2007, 167, 1050–1059. [Google Scholar] [CrossRef]
- McCullough, M.L.; Rodriguez, C.; Diver, W.R.; Feigelson, H.S.; Stevens, V.L.; Thun, M.J.; Calle, E.E. Dairy, Calcium, and Vitamin D Intake and Postmenopausal Breast Cancer Risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2898–2904. [Google Scholar] [CrossRef] [Green Version]
- Prentice, R.L.; Pettinger, M.B.; Jackson, R.D.; Wactawski-Wende, J.; LaCroix, A.Z.; Anderson, G.L.; Chlebowski, R.T.; Manson, J.E.; Van Horn, L.; Vitolins, M.Z.; et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos. Int. 2012, 24, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Tayyem, R.F.; Mahmoud, R.I.; Shareef, M.H.; Marei, L.T. Nutrient intake patterns and breast cancer risk among Jordanian women: A case-control study. Epidemiol. Health 2019, 41, e2019010. [Google Scholar] [CrossRef]
- Fereidani, S.S.; Eini-Zinab, H.; Heidari, Z.; Jalali, S.; Sedaghat, F.; Rashidkhani, B. Nutrient Patterns and Risk of Breast Cancer among Iranian Women: A Case- Control Study. Asian Pac. J. Cancer Prev. 2018, 19, 2619–2624. [Google Scholar] [CrossRef]
- Krusinska, B.; Wadolowska, L.; Biernacki, M.; Slowinska, M.A.; Drozdowski, M. Serum ‘Vitamin-Mineral’ Profiles: Associations with Postmenopausal Breast Cancer Risk Including Dietary Patterns and Supplementation. A Case-Control Study. Nutrients 2019, 11, 2244. [Google Scholar] [CrossRef] [Green Version]
- Lowe, L.C.; Guy, M.; Mansi, J.L.; Peckitt, C.; Bliss, J.; Wilson, R.G.; Colston, K.W. Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur. J. Cancer 2005, 41, 1164–1169. [Google Scholar] [CrossRef]
- Imtiaz, S.; Siddiqui, N. Vitamin-D status at breast cancer diagnosis: Correlation with social and environmental factors and dietary intake. J. Ayub Med. Coll. Abbottabad JAMC 2015, 26, 186–190. [Google Scholar]
- Eliassen, A.H.; Warner, E.T.; Rosner, B.; Collins, L.C.; Beck, A.H.; Quintana, L.M.; Tamimi, R.M.; Hankinson, S.E. Plasma 25-Hydroxyvitamin D and Risk of Breast Cancer in Women Followed over 20 Years. Cancer Res. 2016, 76, 5423–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemida, M.A.; AbdElmoneim, N.A.; Hewala, T.I.; Rashad, M.M.; Abdaallah, S. Vitamin D Receptor in Breast Cancer Tissues and Its Relation to Estrogen Receptor Alpha (ER-α) Gene Expression and Serum 25-hydroxyvitamin D Levels in Egyptian Breast Cancer Patients: A Case-control Study. Clin. Breast Cancer 2019, 19, e407–e414. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.H.; Makonnen, E.; Fotoohi, A.; Yimer, G.; Seifu, D.; Assefa, M.; Tigeneh, W.; Aseffa, A.; Howe, R.; Aklillu, E. Vitamin D Status and Association of VDR Genetic Polymorphism to Risk of Breast Cancer in Ethiopia. Nutrients 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Eliassen, A.H.; Spiegelman, D.; Willett, W.C.; Hankinson, S.E. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses’ Health Study II. Cancer Causes Control 2014, 25, 819–827. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.; Sandler, D.P.; Taylor, J.; Weinberg, C. Serum Vitamin D and Risk of Breast Cancer within Five Years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef] [PubMed]
- Thanasitthichai, S.; Prasitthipayong, A.; Boonmark, K.; Purisa, W.; Guayraksa, K. Negative Impact of 25-hydroxyvitamin D Deficiency on Breast Cancer Survival. Asian Pac. J. Cancer Prev. 2019, 20, 3101–3106. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Elser, C.; Ennis, M.; Goodwin, P.J. Blood levels of vitamin D and early stage breast cancer prognosis: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 141, 331–339. [Google Scholar] [CrossRef]
- Jiang, X.; Dimou, N.L.; Al-Dabhani, K.; Lewis, S.J.; Martin, R.; Haycock, P.C.; Gunter, M.J.; Key, T.J.; Eeles, R.; Muir, K.; et al. Circulating vitamin D concentrations and risk of breast and prostate cancer: A Mendelian randomization study. Int. J. Epidemiol. 2018, 48, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- Viala, M.; Chiba, A.; Thezenas, S.; Delmond, L.; Lamy, P.-J.; Mott, S.L.; Schroeder, M.C.; Thomas, A.; Jacot, W. Impact of vitamin D on pathological complete response and survival following neoadjuvant chemotherapy for breast cancer: A retrospective study. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Hollis, B.W.; Trump, D.L.; Lappe, J.M. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs. 50 nmol/L): Pooled analysis of two randomized trials and a prospective cohort: Pooled analysis of two randomized trials and a prospective cohort. PLoS ONE 2018, 13, e0199265. [Google Scholar] [CrossRef] [Green Version]
- de Lyra, E.C.; da Silva, I.A.; Katayama, M.L.H.; Brentani, M.M.; Nonogaki, S.; Góes, J.C.S.; Folgueira, M.A.A.K. 25(OH)D3 and 1,25(OH)2D3 serum concentration and breast tissue expression of 1α-hydroxylase, 24-hydroxylase and Vitamin D receptor in women with and without breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 100, 184–192. [Google Scholar] [CrossRef]
- Ditsch, N.; Toth, B.; Mayr, D.; Lenhard, M.; Gallwas, J.; Weissenbacher, T.; Dannecker, C.; Friese, K.; Jeschke, U. The Association between Vitamin D Receptor Expression and Prolonged Overall Survival in Breast Cancer. J. Histochem. Cytochem. 2011, 60, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcnamara, M.; Rosenberger, K.D. The Significance of Vitamin D Status in Breast Cancer: A State of the Science Review. J. Midwifery Women’s Health 2019, 64, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Zhalehjoo, N.; Shakiba, Y.; Panjehpour, M. Gene expression profiles of CYP24A1 and CYP27B1 in malignant and normal breast tissues. Mol. Med. Rep. 2016, 15, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheible, C.; Thill, M.; Baum, S.; Solomayer, E.; Friedrich, M. Implication of CYP24A1 splicing in breast cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Raimondi, S.; Gandini, S. Meta-analysis of Vitamin D–Binding Protein and Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1758–1765. [Google Scholar] [CrossRef] [Green Version]
- Abbas, S.; Nieters, A.; Linseisen, J.; Slanger, T.; Kropp, S.; Mutschelknauss, E.J.; Flesch-Janys, D.; Chang-Claude, J. Vitamin D receptor gene polymorphisms and haplotypes and postmenopausal breast cancer risk. Breast Cancer Res. 2008, 10, R31. [Google Scholar] [CrossRef] [Green Version]
- Trabert, B.; Malone, K.E.; Daling, J.R.; Doody, D.R.; Bernstein, L.; Ursin, G.; Marchbanks, P.A.; Strom, B.L.; Humphrey, M.C.; Ostrander, E.A. Vitamin D receptor polymorphisms and breast cancer risk in a large population-based case-control study of Caucasian and African-American women. Breast Cancer Res. 2007, 9, R84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.U.N.; Maqbool, S.A.; Khan, T.A. Association of low penetrance vitamin D receptor Tru9I (rs757343) gene polymorphism with risk of premenopausal breast cancer. J. Int. Med. Res. 2018, 46, 1801–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, S.; Dhasmana, A.; Bhatt, M.L.B.; Lohani, M.; Arif, J.M. Molecular Mechanism of Cancer Susceptibility Associated with Fok1 Single Nucleotide Polymorphism of VDR in Relation to Breast Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.U.N.; Khan, T.A.; Maqbool, S.A. Vitamin D Receptor Cdx-2 Polymorphism and Premenopausal Breast Cancer Risk in Southern Pakistani Patients. PLoS ONE 2015, 10, e0122657. [Google Scholar] [CrossRef]
- Abd-Elsalam, E.A.-E.; Ismaeil, N.A.; Abd-Alsalam, H.S. Vitamin D receptor gene polymorphisms and breast cancer risk among postmenopausal Egyptian women. Tumor Biol. 2015, 36, 6425–6431. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Alavi, S.; Majidzadeh-A, K.; GhaffarPour, M.; Soleimani, A.; Mahdian, R. BsmI but not FokI polymorphism of VDR gene is contributed in breast cancer. Med. Oncol. 2013, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gapska, P.; Scott, R.J.; Serrano-Fernandez, P.; Huzarski, T.; Byrski, T.; Kładny, J.; Gronwald, J.; Górski, B.; Cybulski, C.; Lubinski, J.; et al. Vitamin D receptor variants and breast cancer risk in the Polish population. Breast Cancer Res. Treat. 2008, 115, 629–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, M.U.; Muzaffar, M.; Khan, F.A.; Kabisch, M.; Muhammad, N.; Faiz, S.; Loya, A.; Hamann, U. Association between the BsmI Polymorphism in the Vitamin D Receptor Gene and Breast Cancer Risk: Results from a Pakistani Case-Control Study. PLoS ONE 2015, 10, e0141562. [Google Scholar] [CrossRef]
- McKay, J.D.; McCullough, M.L.; Ziegler, R.G.; Kraft, P.; Saltzman, B.S.; Riboli, E.; Barricarte, A.; Berg, C.D.; Bergland, G.; Bingham, S.; et al. Vitamin D Receptor Polymorphisms and Breast Cancer Risk: Results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Niravath, P.; Chen, B.; Chapman, J.-A.W.; Agarwal, S.K.; Welschhans, R.L.; Bongartz, T.; Kalari, K.; Shepherd, L.E.; Bartlett, J.; Pritchard, K.; et al. Vitamin D Levels, Vitamin D Receptor Polymorphisms, and Inflammatory Cytokines in Aromatase Inhibitor-Induced Arthralgias: An Analysis of CCTG MA.27. Clin. Breast Cancer 2017, 18, 78–87. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Jiang, Q.; Zhang, Y.; Liu, A.; Wang, H.; Zhang, J.; Qin, Q.; Hong, Z. Do genetic polymorphisms of the vitamin D receptor contribute to breast/ovarian cancer? A systematic review and network meta-analysis. Gene 2018, 677, 211–227. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.A.; Bellerba, F.; Corso, F.; Gandini, S. Vitamin d receptor polymorphisms and cancer. Adv. Exp. Med. Biol. 2020, 1268, 53–114. [Google Scholar]
- Tyler Miller, R. Control of renal calcium, phosphate, electrolyte, and water excretion by the calcium-sensing receptor. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 345–358. [Google Scholar] [CrossRef]
- Fudge, N.J.; Kovacs, C.S. Physiological studies in heterozygous calcium sensing receptor (CaSR) gene-ablated mice confirm that the CaSR regulates calcitonin release in vivo. BMC Physiol. 2004, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Peterlik, M.; Kállay, E.; Cross, H.S. Calcium Nutrition and Extracellular Calcium Sensing: Relevance for the Pathogenesis of Osteoporosis, Cancer and Cardiovascular Diseases. Nutrients 2013, 5, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Hendy, G.N.; Canaff, L. Calcium-Sensing Receptor Gene: Regulation of Expression. Front. Physiol. 2016, 7, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Promkan, M.; Liu, G.; Varani, J.; Chakrabarty, S. Role of Calcium Sensing Receptor (CaSR) in tumorigenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Takyar, F.M.; Swan, K.; Jeong, J.; VanHouten, J.; Sullivan, C.; Dann, P.; Yu, H.; Fiaschi-Taesch, N.; Chang, W.; et al. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone–Related Protein. Cancer Res. 2016, 76, 5348–5360. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Wysolmerski, J.J. Calcium-Sensing Receptor in Breast Physiology and Cancer. Front. Physiol. 2016, 7, 440. [Google Scholar] [CrossRef] [Green Version]
- Saidak, Z.; Boudot, C.; Abdoune, R.; Petit, L.; Brazier, M.; Mentaverri, R.; Kamel, S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009, 315, 2072–2080. [Google Scholar] [CrossRef]
- Mamillapalli, R.; VanHouten, J.; Zawalich, W.; Wysolmerski, J. Switching of G-protein Usage by the Calcium-sensing Receptor Reverses Its Effect on Parathyroid Hormone-related Protein Secretion in Normal Versus Malignant Breast Cells. J. Biol. Chem. 2008, 283, 24435–24447. [Google Scholar] [CrossRef] [Green Version]
- Tennakoon, S.; Aggarwal, A.; Kállay, E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta 2016, 1863, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- Mihai, R.; Stevens, J.; McKinney, C.; Ibrahim, N. Expression of the calcium receptor in human breast cancer—A potential new marker predicting the risk of bone metastases. Eur. J. Surg. Oncol. (EJSO) 2006, 32, 511–515. [Google Scholar] [CrossRef]
- Huang, C.; Hydo, L.M.; Liu, S.; Miller, R.T. Activation of choline kinase by extracellular Ca2+ is Ca2+-sensing receptor, Gα12 and Rho-dependent in breast cancer cells. Cell. Signal. 2009, 21, 1894–1900. [Google Scholar] [CrossRef]
- MacLeod, R.J.; Yano, S.; Chattopadhyay, N.; Brown, E. Extracellular calcium-sensing receptor transactivates the epidermal growth factor receptor by a triple-membrane-spanning signaling mechanism. Biochem. Biophys. Res. Commun. 2004, 320, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bedolla, M.A.; González-Domínguez, E.; Zavala-Barrera, C.; Gutiérrez-López, T.Y.; Hidalgo-Moyle, J.J.; Vázquez-Prado, J.; Sánchez-Torres, C.; Reyes-Cruz, G. Calcium-sensing-receptor (CaSR) controls IL-6 secretion in metastatic breast cancer MDA-MB-231 cells by a dual mechanism revealed by agonist and inverse-agonist modulators. Mol. Cell. Endocrinol. 2016, 436, 159–168. [Google Scholar] [CrossRef]
- Peterlik, M.; Grant, W.B.; Cross, H.S. Calcium, Vitamin D and Cancer. Anticancer Res. 2009, 29, 3687–3698. [Google Scholar] [PubMed]
- El Hiani, Y.; Ahidouch, A.; Roudbaraki, M.; Guenin, S.; Brûlé, G.; Ouadid-Ahidouch, H. Calcium-Sensing Receptor Stimulation Induces Nonselective Cation Channel Activation in Breast Cancer Cells. J. Membr. Biol. 2006, 211, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, I.S.; Sergeev, I.N.; Bastholm, L.; Elling, F.; Norman, A.W.; Jäättelä, M. Calcium and Calpain as Key Mediators of Apoptosis-like Death Induced by Vitamin D Compounds in Breast Cancer Cells. J. Biol. Chem. 2002, 277, 30738–30745. [Google Scholar] [CrossRef] [Green Version]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef]
- Yela, D.A.; Ikejiri, T.A.; Machado, C.R.; Mutta, D.; Benetti-Pinto, C.L. Tamoxifen use as a malignancy risk factor in postmenopausal women with endometrial polyps. Menopause 2019, 26, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Piao, J.; Jeon, M.J. Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen. Yonsei Med. J. 2020, 61, 317–322. [Google Scholar] [CrossRef]
- Wong, C.; Chen, S. The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance. J. Steroid Biochem. Mol. Biol. 2012, 131, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Desai, K.V. Pathways to Endocrine Therapy Resistance in Breast Cancer. Front. Endocrinol. 2019, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Forbes, J.F.; Bradley, R.; Ingle, J.N.; Aihara, T.; Bliss, J.M.; Boccardo, F.; Coates, A.S.; Coombes, R.C.; Cuzick, J.; et al. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Miller, W.R.; Larionov, A.A. Understanding the mechanisms of aromatase inhibitor resistance. Breast Cancer Res. 2012, 14, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.E.; McCloskey, E.V. Bisphosphonates in oncology. Bone 2011, 49, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, C.; Tzoracoleftherakis, E.; Polychronis, A.; Venizelos, B.; Dafni, U.; Xepapadakis, G.; Papadiamantis, J.; Zobolas, V.; Misitzis, J.; Kalogerakos, K.; et al. Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: Results from the ARBI prospective clinical trial. Breast Cancer Res. 2010, 12, R24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagha, P.P.; Sen, S. The Efficacy of Bisphosphonates in Preventing Aromatase Inhibitor Induced Bone Loss for Postmenopausal Women with Early Breast Cancer: A Systematic Review and Meta-Analysis. J. Oncol. 2014, 2014, 625060. [Google Scholar] [CrossRef] [PubMed]
- Wang-Gillam, A.; Miles, D.A.; Hutchins, L.F. Evaluation of Vitamin D Deficiency in Breast Cancer Patients on Bisphosphonates. J. Oncol. 2008, 13, 821–827. [Google Scholar] [CrossRef]
- Tanaka, M.; Itoh, S.; Takeuchi, Y. Effectiveness of bisphosphonate combined with activated vitamin D in patients with aromatase inhibitor-induced osteoporosis after breast cancer operation. Osteoporos. Sarcopenia 2018, 4, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Aapro, M.S.; Body, J.-J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.-C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO, IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Grossmann, M.; Ramchand, S.K.; Milat, F.; Vincent, A.; Lim, E.; Kotowicz, M.A.; Hicks, J.; Teede, H.J. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: Position statement summary. Med. J. Aust. 2019, 211, 224–229. [Google Scholar] [CrossRef]
- Bouvard, B.; Chatelais, J.; Soulié, P.; Hoppé, E.; Saulnier, P.; Capitain, O.; Mege, M.; Mesgouez-Nebout, N.; Jadaud, E.; Abadie-Lacourtoisie, S.; et al. Osteoporosis treatment and 10 years’ oestrogen receptor+ breast cancer outcome in postmenopausal women treated with aromatase inhibitors. Eur. J. Cancer 2018, 101, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2009, 119, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vani, S.A.V.; Ananthanarayanan, P.; Kadambari, D.; Harichandrakumar, K.; Niranjjan, R.; Nandeesha, H. Effects of vitamin D and calcium supplementation on side effects profile in patients of breast cancer treated with letrozole. Clin. Chim. Acta 2016, 459, 53–56. [Google Scholar] [CrossRef]
- Nogues, X.; Servitja, S.; Peña, M.J.; Prieto-Alhambra, D.; Nadal, R.; Mellibovsky, L.; Albanell, J.; Diez-Perez, A.; Tusquets, I. Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 2010, 66, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.M.; Murphy, L.; Zgaga, L.; Bennett, K. De novo vitamin D supplement use post-diagnosis is associated with breast cancer survival. Breast Cancer Res. Treat. 2018, 172, 179–190. [Google Scholar] [CrossRef]
- Madden, J.M.; Leacy, F.P.; Zgaga, L.; Bennett, K. Fitting Marginal Structural and G-Estimation Models under Complex Treatment Patterns: Investigating the Association between de Novo Vitamin D Supplement Use after Breast Cancer Diagnosis and All-Cause Mortality Using Linked Pharmacy Claim and Registry Data. Am. J. Epidemiol. 2019, 189, 224–234. [Google Scholar] [CrossRef]
- Zeichner, S.B.; Koru-Sengul, T.; Shah, N.; Liu, Q.; Markward, N.J.; Montero, A.J.; Glück, S.; Silva, O.; Ahn, E.R. Improved Clinical Outcomes Associated with Vitamin D Supplementation During Adjuvant Chemotherapy in Patients with HER2+ Nonmetastatic Breast Cancer. Clin. Breast Cancer 2015, 15, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Peila, R.; Xue, X.; Cauley, J.A.; Chlebowski, R.; Manson, J.E.; Nassir, R.; Saquib, N.; Shadyab, A.H.; Zhang, Z.; Wassertheil-Smoller, S.; et al. A Randomized Trial of Calcium Plus Vitamin D Supplementation and Risk of Ductal Carcinoma In Situ of the Breast. JNCI Cancer Spectr. 2021, 5, pkab072. [Google Scholar] [CrossRef]
- Cadeau, C.; Fournier, A.; Mesrine, S.; Clavel-Chapelon, F.; Fagherazzi, G.; Boutron-Ruault, M.-C. Interaction between current vitamin D supplementation and menopausal hormone therapy use on breast cancer risk: Evidence from the E3N cohort. Am. J. Clin. Nutr. 2015, 102, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Kazemian, E.; Akbari, M.E.; Moradi, N.; Gharibzadeh, S.; Mondul, A.M.; Jamshidi-Naeini, Y.; Khademolmele, M.; Zarins, K.R.; Ghodoosi, N.; Amouzegar, A.; et al. Vitamin D Receptor Genetic Variation and Cancer Biomarkers among Breast Cancer Patients Supplemented with Vitamin D3: A Single-Arm Non-Randomized Before and After Trial. Nutrients 2019, 11, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemian, E.; Amouzegar, A.; Akbari, M.E.; Moradi, N.; Gharibzadeh, S.; Jamshidinaeini, Y.; Khademolmele, M.; As’Habi, A.; Davoodi, S.H. Vitamin D receptor gene polymorphisms affecting changes in visceral fat, waist circumference and lipid profile in breast cancer survivors supplemented with vitamin D3. Lipids Health Dis. 2019, 18, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, H.; Amani, R.; Hosseini, S.A.; Ekrami, A.; Ahmadzadeh, A.; Latifi, S.M. Genetic Variations in VDR could Modulate the Efficacy of Vitamin D3 Supplementation on Inflammatory Markers and Total Antioxidant Capacity among Breast Cancer Women: A Randomized Double Blind Controlled Trial. Asian Pac. J. Cancer Prev. 2019, 20, 2065–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaout, A.; Robertson, S.; Pond, G.R.; Vieth, R.; Jeong, A.; Hilton, J.; Ramsey, T.; Clemons, M. Randomized window of opportunity trial evaluating high-dose vitamin D in breast cancer patients. Breast Cancer Res. Treat. 2019, 178, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Going, C.C.; Alexandrova, L.; Lau, K.; Yeh, C.Y.; Feldman, D.; Pitteri, S.J. Vitamin D supplementation decreases serum 27-hydroxycholesterol in a pilot breast cancer trial. Breast Cancer Res. Treat. 2017, 167, 797–802. [Google Scholar] [CrossRef]
- Amir, E.; Simmons, C.E.; Freedman, O.C.; Dranitsaris, G.; Cole, D.E.C.; Vieth, R.; Ooi, W.S.; Clemons, M. A phase 2 trial exploring the effects of high-dose (10,000 IU/day) vitamin D3in breast cancer patients with bone metastases. Cancer 2009, 116, 284–291. [Google Scholar] [CrossRef]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.-W.; Fabian, C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Javaid, M.; Servitja, S.; Arden, N.K.; Garcia, M.M.; Diez-Perez, A.; Albanell, J.; Tusquets, I.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: A prospective cohort study. Breast Cancer Res. Treat. 2010, 125, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Rossdeutscher, L.; Li, J.; Luco, A.-L.; Fadhil, I.; Ochietti, B.; Camirand, A.; Huang, D.C.; Reinhardt, T.A.; Muller, W.; Kremer, R. Chemoprevention Activity of 25-Hydroxyvitamin D in the MMTV-PyMT Mouse Model of Breast Cancer. Cancer Prev. Res. 2015, 8, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Ooi, L.L.; Zhou, H.; Kalak, R.; Zheng, Y.; Conigrave, A.D.; Seibel, M.J.; Dunstan, C.R. Vitamin D Deficiency Promotes Human Breast Cancer Growth in a Murine Model of Bone Metastasis. Cancer Res. 2010, 70, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.D.; Aggarwal, A.; Swami, S.; Krishnan, A.V.; Ji, L.; Albertelli, M.; Feldman, B.J. Tumor Autonomous Effects of Vitamin D Deficiency Promote Breast Cancer Metastasis. Endocrinology 2016, 157, 1341–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Li, J.; Chu, J.; Jin, S.; Fu, Z.; Miao, D.; Yin, Y. 1,25(OH)2D3 deficiency increases TM40D tumor growth in bone and accelerates tumor-induced bone destruction in a breast cancer bone metastasis model. Biomed. Pharmacother. 2017, 95, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, R.; Qiao, W.; Yuan, X.; Wang, S.; Goltzman, D.; Miao, D. 1,25-Dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int. J. Cancer 2018, 143, 368–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.A.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of Mouse Breast Tumor-Initiating Cells by Calcitriol and Dietary Vitamin D. Mol. Cancer Ther. 2015, 14, 1951–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Luco, A.-L.; Ochietti, B.; Fadhil, I.; Camirand, A.; Reinhardt, T.A.; St-Arnaud, R.; Muller, W.; Kremer, R. Tumoral Vitamin D Synthesis by CYP27B1 1-α-Hydroxylase Delays Mammary Tumor Progression in the PyMT-MMTV Mouse Model and Its Action Involves NF-κB Modulation. Endocrinology 2016, 157, 2204–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milczarek, M.; Chodyński, M.; Filip-Psurska, B.; Martowicz, A.; Krupa, M.; Krajewski, K.; Kutner, A.; Wietrzyk, J. Synthesis and Biological Activity of Diastereomeric and Geometric Analogs of Calcipotriol, PRI-2202 and PRI-2205, against Human HL-60 Leukemia and MCF-7 Breast Cancer Cells. Cancers 2013, 5, 1355–1378. [Google Scholar] [CrossRef] [Green Version]
- Chiang, K.-C.; Yeh, C.-N.; Yeh, T.-S.; Juang, H.-H.; Chen, L.-W.; Kuo, S.-F.; Chen, M.-H.; Chen, T.C.; Takano, M.; Kittaka, A.; et al. MART-10, a 1α,25(OH)2D3 Analog, Potently Represses Metastasis of ER+ Breast Cancer Cells with VEGF-A Overexpression. Anticancer Res. 2018, 38, 3879–3887. [Google Scholar] [CrossRef]
- Wilmanski, T.; Barnard, A.; Parikh, M.R.; Kirshner, J.; Buhman, K.; Burgess, J.; Teegarden, D. 1α,25-Dihydroxyvitamin D Inhibits the Metastatic Capability of MCF10CA1a and MDA-MB-231 Cells in an In Vitro Model of Breast to Bone Metastasis. Nutr. Cancer 2016, 68, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Horas, K.; Zheng, Y.; Fong-Yee, C.; Macfarlane, E.; Manibo, J.; Chen, Y.; Qiao, J.; Gao, M.; Haydar, N.; McDonald, M.M.; et al. Loss of the Vitamin D Receptor in Human Breast Cancer Cells Promotes Epithelial to Mesenchymal Cell Transition and Skeletal Colonization. J. Bone Miner. Res. 2019, 34, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.L.; Zinser, G.M.; Waltz, S.E. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of β-catenin activity. Oncotarget 2015, 6, 16304–16320. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, T.; Zheng, Y.; Fournier, P.G.; Murthy, S.; John, S.; Schillo, S.; Dunstan, C.R.; Mohammad, K.S.; Zhou, H.; Seibel, M.J.; et al. The vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm. Oncotarget 2017, 8, 26687–26701. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Du, Y.; Liu, F.; Feng, Y.; Cheng, S.; Guan, S.; Wang, Y.; Li, X.; Li, B.; Jin, F.; et al. Vitamin D aggravates breast cancer by inducing immunosuppression in the tumor bearing mouse. Immunotherapy 2018, 10, 555–566. [Google Scholar] [CrossRef]
- Ajibade, A.A.; Kirk, J.S.; Karasik, E.; Gillard, B.; Moser, M.T.; Johnson, C.S.; Trump, D.L.; Foster, B.A. Early Growth Inhibition Is Followed by Increased Metastatic Disease with Vitamin D (Calcitriol) Treatment in the TRAMP Model of Prostate Cancer. PLoS ONE 2014, 9, e89555. [Google Scholar] [CrossRef] [PubMed]
- Anisiewicz, A.; Pawlik, A.; Filip-Psurska, B.; Turlej, E.; Dzimira, S.; Milczarek, M.; Gdesz, K.; Papiernik, D.; Jarosz, J.; Klopotowska, D.; et al. Unfavorable effect of calcitriol and its low-calcemic analogs on metastasis of 4T1 mouse mammary gland cancer. Int. J. Oncol. 2017, 52, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Anisiewicz, A.; Kowalski, K.; Banach, J.; Łabędź, N.; Stachowicz-Suhs, M.; Piotrowska, A.; Milczarek, M.; Kłopotowska, D.; Dzięgiel, P.; Wietrzyk, J. Vitamin D Metabolite Profile in Cholecalciferol- or Calcitriol-Supplemented Healthy and Mammary Gland Tumor-Bearing Mice. Nutrients 2020, 12, 3416. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Swami, S.; Peng, L.; Wang, J.; Moreno, J.; Feldman, D. Tissue-Selective Regulation of Aromatase Expression by Calcitriol: Implications for Breast Cancer Therapy. Endocrinology 2010, 151, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip-Psurska, B.; Psurski, M.; Anisiewicz, A.; Libako, P.; Zbrojewicz, E.; Maciejewska, M.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Vitamin D Compounds PRI-2191 and PRI-2205 Enhance Anastrozole Activity in Human Breast Cancer Models. Int. J. Mol. Sci. 2021, 22, 2781. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Peng, L.; Albertelli, M.A.; Feldman, D. Inhibitory Effects of Calcitriol on the Growth of MCF-7 Breast Cancer Xenografts in Nude Mice: Selective Modulation of Aromatase Expression in vivo. Horm. Cancer 2011, 2, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Lundqvist, J.; Hansen, S.K.; Lykkesfeldt, A.E. Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter—a new regulatory pathway for aromatase. Biochim. Biophys. Acta 2013, 1833, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segovia-Mendoza, M.; García-Quiroz, J.; Díaz, L.; García-Becerra, R. Combinations of Calcitriol with Anticancer Treatments for Breast Cancer: An Update. Int. J. Mol. Sci. 2021, 22, 12741. [Google Scholar] [CrossRef]
- Anisiewicz, A.; Filip-Psurska, B.; Pawlik, A.; Nasulewicz-Goldeman, A.; Piasecki, T.; Kowalski, K.; Maciejewska, M.; Jarosz, J.; Banach, J.; Papiernik, D.; et al. Calcitriol Analogues Decrease Lung Metastasis but Impair Bone Metabolism in Aged Ovariectomized Mice Bearing 4T1 Mammary Gland Tumours. Aging Dis. 2019, 10, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Karkeni, E.; Morin, S.O.; Tayeh, B.B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferronato, M.J.; Obiol, D.J.; Fermento, M.E.; Gandini, N.A.; Alonso, E.N.; Salomón, D.G.; Vitale, C.; Mascaró, E.; Fall, Y.; Raimondi, A.R.; et al. The alkynylphosphonate analogue of calcitriol EM1 has potent anti-metastatic effects in breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 154, 285–293. [Google Scholar] [CrossRef]
- Verma, A.; Cohen, D.J.; Jacobs, T.W.; Boyan, B.D.; Schwartz, Z. The Relative Expression of ERα Isoforms ERα66 and ERα36 Controls the Cellular Response to 24R,25-Dihydroxyvitamin D3 in Breast Cancer. Mol. Cancer Res. 2021, 19, 99–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Zhang, Z.; Bai, N.; Liu, Z.; Xiong, M.; Wei, Y.; Xiang, R.; Tan, X. VDR Status Arbitrates the Prometastatic Effects of Tumor-Associated Macrophages. Mol. Cancer Res. 2014, 12, 1181–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wietrzyk, J.; Pełczyńska, M.; Madej, J.; Dzimira, S.; Kuśnierczyk, H.; Kutner, A.; Szelejewski, W.; Opolski, A. Toxicity and antineoplastic effect of (24R)-1,24-dihydroxyvitamin D3 (PRI-2191). Steroids 2004, 69, 629–635. [Google Scholar] [CrossRef]
- Wietrzyk, J.; Nevozhay, D.; Milczarek, M.; Filip-Psurska, B.; Kutner, A. Toxicity and antitumor activity of the vitamin D analogs PRI-1906 and PRI-1907 in combined treatment with cyclophosphamide in a mouse mammary cancer model. Cancer Chemother. Pharmacol. 2008, 62, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Rollakanti, K.R.; Anand, S.; Maytin, E.V. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015, 4, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, S.; Krishnan, A.V.; Williams, J.; Aggarwal, A.; Albertelli, M.; Horst, R.L.; Feldman, B.J.; Feldman, D. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr. -Relat. Cancer 2016, 23, 251–264. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: Importance of mammary CYP27B1 for treatment and prevention. J. Steroid Biochem. Mol. Biol. 2012, 136, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.; Feldman, D. Dietary Vitamin D3 and 1,25-Dihydroxyvitamin D3 (Calcitriol) Exhibit Equivalent Anticancer Activity in Mouse Xenograft Models of Breast and Prostate Cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-Dihydroxyvitamin D 3 Inhibits Angiogenesis In Vitro and In Vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlinden, L.; Verstuyf, A.; Van Camp, M.; Marcelis, S.; Sabbe, K.; Zhao, X.Y.; De Clercq, P.; Vandewalle, M.; Bouillon, R. Two novel 14-Epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 2673–2679. [Google Scholar]
- Sundaram, S.; Sea, A.; Feldman, S.; Strawbridge, R.; Hoopes, P.J.; Demidenko, E.; Binderup, L.; Gewirtz, D.A. The Combination of a Potent Vitamin D3 Analog, EB 1089, with Ionizing Radiation Reduces Tumor Growth and induces Apoptosis of MCF-7 Breast Tumor Xenografts in Nude Mice. Clin. Cancer Res. 2003, 9, 2350–2356. [Google Scholar]
- García-Quiroz, J.; Rivas-Suárez, M.; García-Becerra, R.; Barrera, D.; Martínez-Reza, I.; Ordaz-Rosado, D.; Santos-Martinez, N.; Villanueva, O.; Santos-Cuevas, C.L.; Avila, E.; et al. Calcitriol reduces thrombospondin-1 and increases vascular endothelial growth factor in breast cancer cells: Implications for tumor angiogenesis. J. Steroid Biochem. Mol. Biol. 2014, 144, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wahler, J.; So, J.Y.; Kim, Y.C.; Liu, F.; Maehr, H.; Uskokovic, M.; Suh, N. Inhibition of the Transition of Ductal Carcinoma In Situ to Invasive Ductal Carcinoma by a Gemini Vitamin D Analog. Cancer Prev. Res. 2014, 7, 617–626. [Google Scholar] [CrossRef] [Green Version]
- So, J.Y.; Lee, H.J.; Smolarek, A.K.; Paul, S.; Wang, C.-X.; Maehr, H.; Uskokovic, M.; Zheng, X.; Conney, A.H.; Cai, L.; et al. A Novel Gemini Vitamin D Analog Represses the Expression of a Stem Cell Marker CD44 in Breast Cancer. Mol. Pharmacol. 2010, 79, 360–367. [Google Scholar] [CrossRef]
- Schaefer, R.J. Targeting of Calcitriol to Inflammatory Breast Cancer Tumors and Metastasis In Vitro and In Vivo. Biol. Syst. Open Access 2012, 1, 104. [Google Scholar] [CrossRef]
- Milliken, E.L.; Zhang, X.; Flask, C.; Duerk, J.L.; MacDonald, P.N.; Keri, R.A. EB1089, a vitamin D receptor agonist, reduces proliferation and decreases tumor growth rate in a mouse model of hormone-induced mammary cancer. Cancer Lett. 2005, 229, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Filho, V.C.N.; Katayama, M.L.H.; Lyra, E.C.; Maria, D.A.; Basso, R.A.; Nonogaki, S.; Guerra, J.M.; Maistro, S.; Góes, J.C.G.S.; Folgueira, M.A.A.K. Orthotopic tumorgrafts in nude mice as a model to evaluate calcitriol effects in breast cancer. Braz. J. Biol. 2017, 77, 856–867. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Paul, S.; Atalla, N.; Thomas, P.E.; Lin, X.; Yang, I.; Buckley, B.; Lu, G.; Zheng, X.; Lou, Y.-R.; et al. Gemini Vitamin D Analogues Inhibit Estrogen Receptor–Positive and Estrogen Receptor–Negative Mammary Tumorigenesis without Hypercalcemic Toxicity. Cancer Prev. Res. 2008, 1, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo, G.; Mehta, R.G. Chemoprevention of chemically-induced mammary and colon carcinogenesis by 1α-hydroxyvitamin D5. J. Steroid Biochem. Mol. Biol. 2005, 97, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.; Bailey, S.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Orekhov, A.N. T Helper Lymphocyte Subsets and Plasticity in Autoimmunity and Cancer: An Overview. BioMed. Res. Int. 2015, 2015, 327470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulubova, M.; Ananiev, J.; Ignatova, M.; Halacheva, K. Pro-Tumor and Anti-Tumor Functions of IL-17 and of TH17 Cells in Tumor Microenvironment. Acta Med. Bulg. 2016, 43, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Lopes, J.E.; Chong, M.; Ivanov, I.I.; Min, R.; Victora, G.; Shen, Y.; Du, J.; Rubtsov, Y.P.; Rudensky, A.Y.; et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008, 453, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Yosef, N.; Shalek, A.K.; Gaublomme, J.T.; Jin, H.; Lee, Y.; Awasthi, A.; Wu, C.; Karwacz, K.; Xiao, S.; Jorgolli, M.; et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 2013, 496, 461–468. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 Regulates Cytokine-mediated Generation of Inflammatory Helper T Cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.O.; Pappu, B.P.; Nurieva, R.; Akimzhanov, A.; Kang, H.S.; Chung, Y.; Ma, L.; Shah, B.; Panopoulos, A.D.; Schluns, K.S.; et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity 2008, 28, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Nurieva, R.; Yang, X.O.; Martinez, G.; Zhang, Y.; Panopoulos, A.; Ma, L.; Schluns, K.; Tian, Q.; Watowich, S.S.; Jetten, A.; et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007, 448, 480–483. [Google Scholar] [CrossRef]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmin, F.; Mignot, G.; Bruchard, M.; Chevriaux, A.; Végran, F.; Hichami, A.; Ladoire, S.; Derangère, V.; Vincent, J.; Masson, D.; et al. Stat3 and Gfi-1 Transcription Factors Control Th17 Cell Immunosuppressive Activity via the Regulation of Ectonucleotidase Expression. Immunity 2012, 36, 362–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowatzky, J.; Manches, O.; Khan, S.A.; Godefroy, E.; Bhardwaj, N. Modulation of human Th17 cell responses through complement receptor 3 (CD11 b/CD18) ligation on monocyte-derived dendritic cells. J. Autoimmun. 2018, 92, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroman, H.; van den Blink, B.; Kool, M. Mode of Dendritic Cell Activation: The Decisive Hand in Th2/Th17 Cell Differentiation. Implications in Asthma Severity? Immunobiology 2015, 220, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, C.; Klein, C.; von Bergen, M.; Simon, J.C.; Saalbach, A. Human fibroblasts support the expansion of IL-17–producing T cells via up-regulation of IL-23 production by dendritic cells. Blood 2010, 116, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Fernández, G.; Mariblanca, I.R.; Navarro, M.N. Decoding IL-23 Signaling Cascade for New Therapeutic Opportunities. Cells 2020, 9, 2044. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Jung, H.-J.; Kim, T.S. IL-33 changes CD25hi Tregs to Th17 cells through a dendritic cell-mediated pathway. Immunol. Lett. 2019, 218, 5–10. [Google Scholar] [CrossRef]
- Shan, M.; Yuan, X.; Song, L.-Z.; Roberts, L.; Zarinkamar, N.; Seryshev, A.; Zhang, Y.; Hilsenbeck, S.; Chang, S.-H.; Dong, C.; et al. Cigarette Smoke Induction of Osteopontin (SPP1) Mediates T H 17 Inflammation in Human and Experimental Emphysema. Sci. Transl. Med. 2012, 4, 117ra9. [Google Scholar] [CrossRef] [Green Version]
- Murugaiyan, G.; Mittal, A.; Weiner, H.L. Increased Osteopontin Expression in Dendritic Cells Amplifies IL-17 Production by CD4+ T Cells in Experimental Autoimmune Encephalomyelitis and in Multiple Sclerosis. J. Immunol. 2008, 181, 7480–7488. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wang, Z.; Deng, L.; Yuan, X.; Ma, Y.; Zhang, G.; Gantier, M.P.; Liu, J.-P.; Shen, L.; Xu, D. Osteopontin promotes inflammation in patients with acute coronary syndrome through its activity on IL-17 producing cells. Eur. J. Immunol. 2012, 42, 2803–2814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Cheng, W.; Xi, Y.; Cao, Z.; Xu, Y.; Wu, T.; Li, C.; Niu, X.; Chen, G. IFN-β regulates Th17 differentiation partly through the inhibition of osteopontin in experimental autoimmune encephalomyelitis. Mol. Immunol. 2017, 93, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Baaten, B.J.; Li, C.-R.; Bradley, L.M. Multifaceted regulation of T cells by CD44. Commun. Integr. Biol. 2010, 3, 508–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zhang, X.; Li, R.; Fang, L.; Niu, X.; Zheng, Y.; He, D.; Xu, R.; Zhang, J.Z. Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthritis Care Res. 2010, 62, 2900–2908. [Google Scholar] [CrossRef]
- Chen, R.-Y.; Fan, Y.-M.; Zhang, Q.; Liu, S.; Li, Q.; Ke, G.-L.; Li, C.; You, Z. Estradiol Inhibits Th17 Cell Differentiation through Inhibition of RORγT Transcription by Recruiting the ERα/REA Complex to Estrogen Response Elements of the RORγT Promoter. J. Immunol. 2015, 194, 4019–4028. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.M.; Srivastava, K.; Mansoori, M.N.; Trivedi, R.; Chattopadhyay, N.; Singh, D. Estrogen Deficiency Induces the Differentiation of IL-17 Secreting Th17 Cells: A New Candidate in the Pathogenesis of Osteoporosis. PLoS ONE 2012, 7, e44552. [Google Scholar] [CrossRef]
- Andersson, A.; Stubelius, A.; Karlsson, M.N.; Engdahl, C.; Erlandsson, M.; Grahnemo, L.; Lagerquist, M.K.; Islander, U. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res. Ther. 2015, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Fuseini, H.; Cephus, J.-Y.; Wu, P.; Davis, J.B.; Contreras, D.C.; Gandhi, V.D.; Rathmell, J.C.; Newcomb, D.C. ERα Signaling Increased IL-17A Production in Th17 Cells by Upregulating IL-23R Expression, Mitochondrial Respiration, and Proliferation. Front. Immunol. 2019, 10, 2740. [Google Scholar] [CrossRef] [Green Version]
- Fuseini, H.; Cephus, J.; Davis, J.B.; Peebles, S.; Newcomb, D.C. Estrogen receptor α signaling promotes IL-17A production in Th17 cells through the Let7/IL-23R signaling pathway. J. Allergy Clin. Immunol. 2019, 143, AB213. [Google Scholar] [CrossRef]
- Newcomb, D.C.; Cephus, J.Y.; Boswell, M.G.; Fahrenholz, J.M.; Langley, E.W.; Feldman, A.S.; Zhou, W.; Dulek, D.E.; Goleniewska, K.; Woodward, K.B.; et al. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J. Allergy Clin. Immunol. 2015, 136, 1025–1034.e11. [Google Scholar] [CrossRef] [Green Version]
- Kurschus, F.C.; Croxford, A.L.; Heinen, A.P.; Wörtge, S.; Ielo, D.; Waisman, A. Genetic proof for the transient nature of the Th17 phenotype. Eur. J. Immunol. 2010, 40, 3336–3346. [Google Scholar] [CrossRef]
- Gagliani, N.; Amezcua Vesely, M.C.; Iseppon, A.; Brockmann, L.; Xu, H.; Palm, N.W.; De Zoete, M.R.; Licona-Limón, P.; Paiva, R.S.; Ching, T.; et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015, 523, 221–225. [Google Scholar] [CrossRef]
- Downs-Canner, S.; Berkey, S.; Delgoffe, G.M.; Edwards, R.P.; Curiel, T.; Odunsi, K.; Bartlett, D.L.; Obermajer, N. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat. Commun. 2017, 8, 14649. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef] [Green Version]
- Kotake, S.; Yago, T.; Kobashigawa, T.; Nanke, Y. The Plasticity of Th17 Cells in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Med. 2017, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Lindahl, H.; Olsson, T. Interleukin-22 Influences the Th1/Th17 Axis. Front. Immunol. 2021, 12, 618110. [Google Scholar] [CrossRef]
- Hirota, K.; Turner, J.-E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of TH17 cells in Peyer’s patches is responsible for the induction of T cell–dependent IgA responses. Nat. Immunol. 2013, 14, 372–379. [Google Scholar] [CrossRef]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Muranski, P.; Restifo, N.P. Essentials of Th17 cell commitment and plasticity. Blood 2013, 121, 2402–2414. [Google Scholar] [CrossRef]
- Agalioti, T.; Villablanca, E.J.; Huber, S.; Gagliani, N. TH17 cell Plasticity: The Role of Dendritic Cells and Molecular Mechanisms. J. Autoimmun. 2018, 87, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology 2020, 163, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Kurebayashi, Y.; Koyasu, S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann. N. Y. Acad. Sci. 2013, 1280, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Bruno, L.; Hertweck, A.; Finlay, D.; Leleu, M.; Spivakov, M.; Knight, Z.A.; Cobb, B.S.; Cantrell, D.; O’Connor, E.; et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 2008, 105, 7797–7802. [Google Scholar] [CrossRef] [Green Version]
- Céline, M.; Dubéa, A.L.; Brewstera, T.Z.B. T Helper Cell Polarization in Healthy People: Implications for Cardiovascular Disease. Bone 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Hu, J.; Tang, L.; Zhao, S.; Shan, B. Expression of Th17 Cells in Breast Cancer Tissue and Its Association with Clinical Parameters. Cell Biophys. 2011, 62, 153–159. [Google Scholar] [CrossRef]
- Kryczek, I.; Banerjee, M.; Cheng, P.; Vatan, L.; Szeliga, W.; Wei, S.; Huang, E.; Finlayson, E.; Simeone, D.; Welling, T.H.; et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009, 114, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
- Wilke, C.M.; Kryczek, I.; Wei, S.; Zhao, E.; Wu, K.; Wang, G.; Zou, W. Th17 cells in cancer: Help or hindrance? Carcinogenesis 2011, 32, 643–649. [Google Scholar] [CrossRef] [Green Version]
- DeNardo, D.G.; Johansson, M.; Coussens, L.M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2007, 27, 11–18. [Google Scholar] [CrossRef]
- Su, X.; Ye, J.; Hsueh, E.C.; Zhang, Y.; Hoft, D.F.; Peng, G. Tumor Microenvironments Direct the Recruitment and Expansion of Human Th17 Cells. J. Immunol. 2009, 184, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Hu, G. Prognostic role of intratumoral IL-17A expression by immunohistochemistry in solid tumors: A meta-analysis. Oncotarget 2017, 8, 66382–66391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allaoui, R.; Hagerling, C.; Desmond, E.; Warfvinge, C.-F.; Jirström, K.; Leandersson, K. Infiltration of Γδ T cells, IL-17+ T cells and FoxP3+ T cells in human breast cancer. Cancer Biomark. 2018, 20, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Yang, L.; Shen, R.; Kong, B.; Chen, W.; Liang, J.; Tang, G.; Zhang, B. Th17 cells regulate the production of CXCL1 in breast cancer. Int. Immunopharmacol. 2018, 56, 320–329. [Google Scholar] [CrossRef]
- Avalos-Navarro, G.; Muñoz-Valle, J.F.; Daneri-Navarro, A.; Quintero-Ramos, A.; Franco-Topete, R.A.; Morán-Mendoza, A.D.J.; Oceguera-Villanueva, A.; Bautista-Herrera, L.A.; Topete-Camacho, A.; Del Toro-Arreola, A. Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes: Relationship with Th17 cytokine profile. Clin. Exp. Med. 2019, 19, 385–391. [Google Scholar] [CrossRef]
- Eiró, N.; González, L.; González, L.O.; Fernandez-Garcia, B.; Lamelas, M.L.; Marín, L.; González-Reyes, S.; del Casar, J.M.; Vizoso, F.J. Relationship between the Inflammatory Molecular Profile of Breast Carcinomas and Distant Metastasis Development. PLoS ONE 2012, 7, e49047. [Google Scholar] [CrossRef] [Green Version]
- Welsh, J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol. Cell. Endocrinol. 2017, 453, 88–95. [Google Scholar] [CrossRef]
- Chen, W.-C.; Lai, Y.-H.; Chen, H.-Y.; Guo, H.-R.; Su, I.-J.; Chen, H.H.W. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology 2013, 63, 225–233. [Google Scholar] [CrossRef]
- Kaur, R.P.; Vasudeva, K.; Singla, H.; Benipal, R.P.S.; Khetarpal, P.; Munshi, A. Analysis of pro- and anti-inflammatory cytokine gene variants and serum cytokine levels as prognostic markers in breast cancer. J. Cell. Physiol. 2018, 233, 9716–9723. [Google Scholar] [CrossRef]
- Kaewkangsadan, V.; Verma, C.; Eremin, J.M.; Cowley, G.; Ilyas, M.; Eremin, O. Crucial Contributions by T Lymphocytes (Effector, Regulatory, and Checkpoint Inhibitor) and Cytokines (TH1, TH2, and TH17) to a Pathological Complete Response Induced by Neoadjuvant Chemotherapy in Women with Breast Cancer. J. Immunol. Res. 2016, 2016, 4757405. [Google Scholar] [CrossRef] [Green Version]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.-M.; Curé, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Yang, X.; Pan, H.; Han, W. Estrogen Receptor Downregulates Expression of PD-1/PD-L1 and Infiltration of CD8+ T Cells by Inhibiting IL-17 Signaling Transduction in Breast Cancer. Front. Oncol. 2020, 10, 582863. [Google Scholar] [CrossRef]
- Horlock, C.; Stott, B.; Dyson, P.J.; Morishita, M.; Coombes, R.C.; Savage, P.; Stebbing, J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br. J. Cancer 2009, 100, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cai, D.; Ma, B.; Wu, G.; Wu, J. Skewing the Balance of Regulatory T-Cells and T-Helper 17 Cells in Breast Cancer Patients. J. Int. Med. Res. 2011, 39, 691–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faucheux, L.; Grandclaudon, M.; Perrot-Dockès, M.; Sirven, P.; Berger, F.; Hamy, A.; Fourchotte, V.; Vincent-Salomon, A.; Mechta-Grigoriou, F.; Reyal, F.; et al. A multivariate Th17 metagene for prognostic stratification in T cell non-inflamed triple negative breast cancer. OncoImmunology 2019, 8, e1624130. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, S.; Cochaud, S.; Merrouche, Y.; Garbar, C.; Antonicelli, F.; Laprevotte, E.; Alberici, G.; Bonnefoy, N.; Eliaou, J.-F.; Bastid, J.; et al. IL-17A and its homologs IL-25/IL-17E recruit the c-RAF/S6 kinase pathway and the generation of pro-oncogenic LMW-E in breast cancer cells. Sci. Rep. 2015, 5, 11874. [Google Scholar] [CrossRef] [Green Version]
- Merrouche, Y.; Fabre, J.; Cure, H.; Garbar, C.; Fuselier, C.; Bastid, J.; Antonicelli, F.; Al-Daccak, R.; Bensussan, A.; Giustiniani, J. IL-17E synergizes with EGF and confers in vitro resistance to EGFR-targeted therapies in TNBC cells. Oncotarget 2016, 7, 53350–53361. [Google Scholar] [CrossRef] [Green Version]
- Laprevotte, E.; Cochaud, S.; du Manoir, S.; Lapierre, M.; Dejou, C.; Philippe, M.; Giustiniani, J.; Frewer, K.A.; Sanders, A.J.; Jiang, W.G.; et al. The IL-17B-IL-17 receptor B pathway promotes resistance to paclitaxel in breast tumors through activation of the ERK1/2 pathway. Oncotarget 2017, 8, 113360–113372. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-K.; Yang, C.-Y.; Jeng, Y.-M.; Chen, C.-L.; Wu, H.-H.; Chang, Y.-C.; Ma, C.; Kuo, W.-H.; Chang, K.-J.; Shew, J.-Y.; et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene 2013, 33, 2968–2977. [Google Scholar] [CrossRef] [Green Version]
- Bastid, J.; Dejou, C.; Docquier, A.; Bonnefoy, N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020, 11, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyomi, A.; Makita, M.; Ozeki, T.; Li, N.; Satomura, A.; Tanaka, S.; Onda, K.; Sugiyama, K.; Iwase, T.; Hirano, T. Characterization and Clinical Implication of Th1/Th2/Th17 Cytokines Produced from Three-Dimensionally Cultured Tumor Tissues Resected from Breast Cancer Patients. Transl. Oncol. 2015, 8, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibaudin, M.; Chaix, M.; Boidot, R.; Végran, F.; Derangère, V.; Limagne, E.; Berger, H.; Ladoire, S.; Apetoh, L.; Ghiringhelli, F. Human ectonucleotidase-expressing CD25highTh17 cells accumulate in breast cancer tumors and exert immunosuppressive functions. OncoImmunology 2015, 5, e1055444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benevides, L.; Cardoso, C.R.B.; Tiezzi, D.G.; Marana, H.R.C.; Andrade, J.M.; Silva, J.S. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur. J. Immunol. 2013, 43, 1518–1528. [Google Scholar] [CrossRef]
- Mathias, C.; Muzzi, J.C.D.; Antunes, B.B.; Gradia, D.F.; Castro, M.A.A.; de Oliveira, J.C. Unraveling Immune-Related lncRNAs in Breast Cancer Molecular Subtypes. Front. Oncol. 2021, 11, 1936. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Yu, L.; Lin, S.; Zhang, C.; Xu, H.; Leng, Z.; Huang, W.; Lei, J.; Li, T.; et al. Comprehensive landscape of epigenetic-dysregulated lncRNAs reveals a profound role of enhancers in carcinogenesis in BC subtypes. Mol. Ther.-Nucleic Acids 2020, 23, 667–681. [Google Scholar] [CrossRef]
- Dai, Z.-M.; Zhang, T.-S.; Lin, S.; Zhang, W.-G.; Liu, J.; Cao, X.-M.; Li, H.-B.; Wang, M.; Liu, X.-H.; Liu, K.; et al. Role of IL-17A rs2275913 and IL-17F rs763780 polymorphisms in risk of cancer development: An updated meta-analysis. Sci. Rep. 2016, 6, 20439. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jiang, Y.; Zhang, Y.; Wang, Y.; Huang, S.; Wang, Z.; Tian, B.; Yang, Y.; Jiang, W.; Pang, D. Association Analysis of IL-17A and IL-17F Polymorphisms in Chinese Han Women with Breast Cancer. PLoS ONE 2012, 7, e34400. [Google Scholar] [CrossRef] [Green Version]
- Naeimi, S.; Erfani, N.; Ardekani, A.M.; Ghaderi, A. Variation of IL-17A and IL-17F Genes in Patients with Breast Cancer in a Population from Southern Iran. Adv. Environ. Biol. 2014, 8, 892–897. [Google Scholar]
- Dupre, S.A.; Hunter, K.W. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp. Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef]
- Du, J.-W.; Xu, K.-Y.; Fang, L.-Y.; Qi, X.-L. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol. Med. Rep. 2012, 6, 1099–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, X.; Gu, L.; Ning, H.; Zhang, Y.; Hsueh, E.C.; Fu, M.; Hu, X.; Wei, L.; Hoft, D.F.; Liu, J. Increased Th17 Cells in the Tumor Microenvironment Is Mediated by IL-23 via Tumor-Secreted Prostaglandin E2. J. Immunol. 2013, 190, 5894–5902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, A.C.; Bonomo, A. Dendritic cells development into osteoclast-type APCs by 4T1 breast tumor T cells milieu boost bone consumption. Bone 2020, 143, 115755. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Leal, A.C.; Gonçalves-Silva, T.; Mercadante, A.C.T.; Kestelman, F.; Chaves, S.; Azevedo, R.B.; Monteiro, J.P.; Bonomo, A. T Cells Induce Pre-Metastatic Osteolytic Disease and Help Bone Metastases Establishment in a Mouse Model of Metastatic Breast Cancer. PLoS ONE 2013, 8, e68171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewell, A.N.; Swamydas, M.; Castillo, C.I.; Wyan, H.; Allen, L.D.; McDermott, K.A.; Eddy, J.M.; Dréau, D. The Endothelin Axis Stimulates the Expression of Pro-Inflammatory Cytokines and Pro-Migratory Molecules in Breast Cancer. Cancer Investig. 2010, 28, 932–943. [Google Scholar] [CrossRef]
- Nam, J.-S.; Terabe, M.; Kang, M.-J.; Chae, H.; Voong, N.; Yang, Y.-A.; Laurence, A.; Michalowska, A.; Mamura, M.; Lonning, S.; et al. Transforming Growth Factor β Subverts the Immune System into Directly Promoting Tumor Growth through Interleukin-17. Cancer Res. 2008, 68, 3915–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das Roy, L.; Ghosh, S.; Pathangey, L.B.; Tinder, T.L.; Gruber, H.E.; Mukherjee, P. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer 2011, 11, 365. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Khanal, P.; Lim, S.-C.; Yun, H.J.; Ahn, S.-G.; Ki, S.H.; Choi, H.S. Interleukin-17 induces AP-1 activity and cellular transformation via upregulation of tumor progression locus 2 activity. Carcinogenesis 2012, 34, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Mulcahy, L.A.; Mohammed, R.A.A.; Lee, A.H.S.; Franks, H.A.; Kilpatrick, L.; Yilmazer, A.; Paish, E.C.; Ellis, I.O.; Patel, P.M.; et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008, 10, R95. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Awaji, M.; Saxena, S.; Varney, M.L.; Sharma, B.; Singh, R.K. IL-17–CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am. J. Pathol. 2019, 190, 222–233. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.C.; Fiala, G.J.; Agudelo, L.Z.; Dyck, L.; Kane, H.; et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 2021, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Benatar, T.; Cao, M.Y.; Lee, Y.; Lightfoot, J.; Feng, N.; Gu, X.; Lee, V.; Jin, H.; Wang, M.; Wright, J.A.; et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol. Immunother. 2009, 59, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Jeng, Y.-M.; Zhou, L.; Huang, L.; Kuhn, I.; Bissell, M.J.; Lee, W.-H. IL-25 Causes Apoptosis of IL-25R–Expressing Breast Cancer Cells without Toxicity to Nonmalignant Cells. Sci. Transl. Med. 2011, 3, 78ra31. [Google Scholar] [CrossRef] [Green Version]
- Fabre, J.A.S.; Giustiniani, J.; Garbar, C.; Merrouche, Y.; Antonicelli, F.; Bensussan, A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018, 19, 3880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qianmei, Y.; Zehong, S.; Guang, W.; Hui, L.; Lian, G. Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy. Immunol. Res. 2021, 69, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.; Paredes, J.; Costa, J.L.; Ylstra, B.; Schmitt, F. Vitamin D and the mammary gland: A review on its role in normal development and breast cancer. Breast Cancer Res. 2012, 14, 211. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, A.; Feldman, D.; Feldman, B.J. Identification of tumor-autonomous and indirect effects of vitamin D action that inhibit breast cancer growth and tumor progression. J. Steroid Biochem. Mol. Biol. 2018, 177, 155–158. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.; Zhou, Y.; Xiao, Z.; Zhang, L.; Zhang, H.; Li, X.; Li, W.; et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B 2018, 9, 203–219. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Tan, Y.; Tang, Y.; Ye, J.; Yuan, B.; Yu, W. Cancer-Associated Fibroblasts Suppress Cancer Development: The Other Side of the Coin. Front. Cell Dev. Biol. 2021, 9, 146. [Google Scholar] [CrossRef]
- Campos, L.T.; Brentani, H.; Roela, R.A.; Katayama, M.L.H.; Lima, L.; Rolim, C.F.; Milani, C.; Folgueira, M.A.A.K.; Brentani, M.M. Differences in transcriptional effects of 1α,25 dihydroxyvitamin D3 on fibroblasts associated to breast carcinomas and from paired normal breast tissues. J. Steroid Biochem. Mol. Biol. 2013, 133, 12–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Barral, A.; Bustamante-Madrid, P.; Ferrer-Mayorga, G.; Barbáchano, A.; Larriba, M.J.; Muñoz, A. Vitamin D Effects on Cell Differentiation and Stemness in Cancer. Cancers 2020, 12, 2413. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1α,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, R.J.; Johnson, C.S.; Modzelewski, R.A.; Trump, D.L. Antiproliferative Effects of 1α,25-Dihydroxyvitamin D3 and Vitamin D Analogs on Tumor-Derived Endothelial Cells. Endocrinology 2002, 143, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Simpson, E.R.; Brown, K.A. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. J. Steroid Biochem. Mol. Biol. 2015, 153, 35–44. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef] [Green Version]
- Adorini, L.; Daniel, K.C.; Penna, G. Vitamin D receptor agonists, cancer and the immune system: An intricate relationship. Curr. Top. Med. Chem. 2006, 6, 1297–1301. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Min, D.; Lv, X.-B.; Wang, X.; Zhang, B.; Meng, W.; Yu, F.; Hu, H. Downregulation of miR-302c and miR-520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell-mediated cytotoxicity. Br. J. Cancer 2013, 109, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Pawlik, A.; Anisiewicz, A.; Filip-Psurska, B.; Nowak, M.; Turlej, E.; Trynda, J.; Banach, J.; Gretkierewicz, P.; Wietrzyk, J. Calcitriol and Its Analogs Establish the Immunosuppressive Microenvironment That Drives Metastasis in 4T1 Mouse Mammary Gland Cancer. Int. J. Mol. Sci. 2018, 19, 2116. [Google Scholar] [CrossRef] [Green Version]
- Anisiewicz, A.; Pawlik, A.; Filip-Psurska, B.; Wietrzyk, J. Differential Impact of Calcitriol and Its Analogs on Tumor Stroma in Young and Aged Ovariectomized Mice Bearing 4T1 Mammary Gland Cancer. Int. J. Mol. Sci. 2020, 21, 6359. [Google Scholar] [CrossRef] [PubMed]
- Anisiewicz, A.; Łabędź, N.; Krauze, I.; Wietrzyk, J. Calcitriol in the Presence of Conditioned Media from Metastatic Breast Cancer Cells Enhances Ex Vivo Polarization of M2 Alternative Murine Bone Marrow-Derived Macrophages. Cancers 2020, 12, 3485. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- Rőszer, T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, J.; Sun, Y.; Wang, J.; Liu, X.; Wang, F.; Zhang, H.; Wang, W.; Ma, X.; Gao, X.; et al. Prognostic significance of tumor-associated macrophages in breast cancer: A meta-analysis of the literature. Oncotarget 2017, 8, 30576–30586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, C.E.; Hubler, S.L.; Moore, J.R.; Barta, L.E.; Praska, C.E.; Nashold, F.E. Vitamin D Actions on CD4+ T Cells in Autoimmune Disease. Front. Immunol. 2015, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; He, X.-Y.; Xu, M. Correlation of Vitamin D3 with the Expression of RORγt and Foxp3 mRNAs in the Peripheral Blood of Myasthenia Gravis Patients. Neuroimmunomodulation 2020, 27, 97–103. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Hobrath, J.V.; Oak, A.S.; Tang, E.K.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as “biased” agonists on VDR and inverse agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 2016, 173, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Yi, A.-K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Thangamani, S.; Kim, M.; Son, Y.; Huang, X.; Kim, H.; Lee, J.H.; Cho, J.; Ulrich, B.; Broxmeyer, H.E.; Kim, C.H. Cutting edge: Progesterone directly upregulates vitamin d receptor gene expression for efficient regulation of T cells by calcitriol. J. Immunol. 2014, 194, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Smolders, J.; Thewissen, M.; Peelen, E.; Menheere, P.; Tervaert, J.W.C.; Damoiseaux, J.; Hupperts, R. Vitamin D Status Is Positively Correlated with Regulatory T Cell Function in Patients with Multiple Sclerosis. PLoS ONE 2009, 4, e6635. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Peelen, E.; Thewissen, M.; Cohen Tervaert, J.W.; Menheere, P.; Hupperts, R.; Damoiseaux, J. Safety and T Cell Modulating Effects of High Dose Vitamin D3 Supplementation in Multiple Sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.; Pantalena, L.-C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-Dihydroxyvitamin D 3 Ameliorates Th17 Autoimmunity via Transcriptional Modulation of Interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Chung, Y.; Dong, C. Vitamin D Suppresses Th17 Cytokine Production by Inducing C/EBP Homologous Protein (CHOP) Expression. J. Biol. Chem. 2010, 285, 38751–38755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, K.; Sharif, Y.; Watad, A.; Yavne, Y.; Lichtbroun, B.; Bragazzi, N.L.; Amital, H.; Shoenfeld, Y. Vitamin D, autoimmunity and recurrent pregnancy loss: More than an association. Am. J. Reprod. Immunol. 2018, 80, e12991. [Google Scholar] [CrossRef]
- Ji, J.; Zhai, H.; Zhou, H.; Song, S.; Mor, G.; Liao, A. The role and mechanism of vitamin D-mediated regulation of Treg/Th17 balance in recurrent pregnancy loss. Am. J. Reprod. Immunol. 2019, 81, e13112. [Google Scholar] [CrossRef]
- Abdollahi, E.; Saghafi, N.; Rezaee, S.A.R.; Rastin, M.; Jarahi, L.; Clifton, V.; Rafatpanah, H. Evaluation of 1,25(OH)2D3 Effects on FOXP3, ROR-γt, GITR, and CTLA-4 Gene Expression in the PBMCs of Vitamin D-Deficient Women with Unexplained Recurrent Pregnancy Loss (URPL). Iran. Biomed. J. 2020, 24, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Li, Y.; Zhang, J.; Zhou, C.; Wu, C.; Zhu, Q.; Shen, T. Maternal vitamin D supplementation inhibits bisphenol A-induced proliferation of Th17 cells in adult offspring. Food Chem. Toxicol. 2020, 144, 111604. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, J.; Qu, H.; Li, X.; Tian, B.; Ge, S.; Chen, F. Calcitriol suppresses lipopolysaccharide-induced alveolar bone damage in rats by regulating T helper cell subset polarization. J. Periodontal Res. 2019, 54, 612–623. [Google Scholar] [CrossRef]
- Bi, C.; Li, X.; Qu, H.; Sun, L.; An, Y.; Hong, Y.; Tian, B.; Chen, F. Calcitriol inhibits osteoclastogenesis in an inflammatory environment by changing the proportion and function of T helper cell subsets (Th2/Th17). Cell Prolif. 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Huang, H.; Zhang, F.; Lu, Y.; Hu, H. High salt diet may promote progression of breast tumor through eliciting immune response. Int. Immunopharmacol. 2020, 87, 106816. [Google Scholar] [CrossRef]
- Pawlik, A.; Anisiewicz, A.; Filip-Psurska, B.; Klopotowska, D.; Maciejewska, M.; Mazur, A.; Wietrzyk, J. Divergent Effect of Tacalcitol (PRI-2191) on Th17 Cells in 4T1 Tumor Bearing Young and Old Ovariectomized Mice. Aging Dis. 2020, 11, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Yang, J.M. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1–8. [Google Scholar] [CrossRef]
- Nashold, F.E.; Spach, K.M.; Spanier, J.A.; Hayes, C.E. Estrogen Controls Vitamin D3-Mediated Resistance to Experimental Autoimmune Encephalomyelitis by Controlling Vitamin D3 Metabolism and Receptor Expression. J. Immunol. 2009, 183, 3672–3681. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.L.; Prince, C.W. 1α,25-Dihydroxyvitamin D3 Stimulates Synthesis and Secretion of Nonphosphory-lated Osteopontin (Secreted Phosphoprotein 1) in Mouse JB6 Epidermal Cells. Cancer Res. 1991, 51, 2144–2150. [Google Scholar]
- Lau, W.L.; Leaf, E.M.; Hu, M.C.; Takeno, M.M.; Kuro, M.-O.; Moe, O.W.; Giachelli, C.M. Vitamin D receptor agonists increase klotho and osteopontin while decreasing aortic calcification in mice with chronic kidney disease fed a high phosphate diet. Kidney Int. 2012, 82, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.-M.; Shin, E.-A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 20. [Google Scholar] [CrossRef] [Green Version]
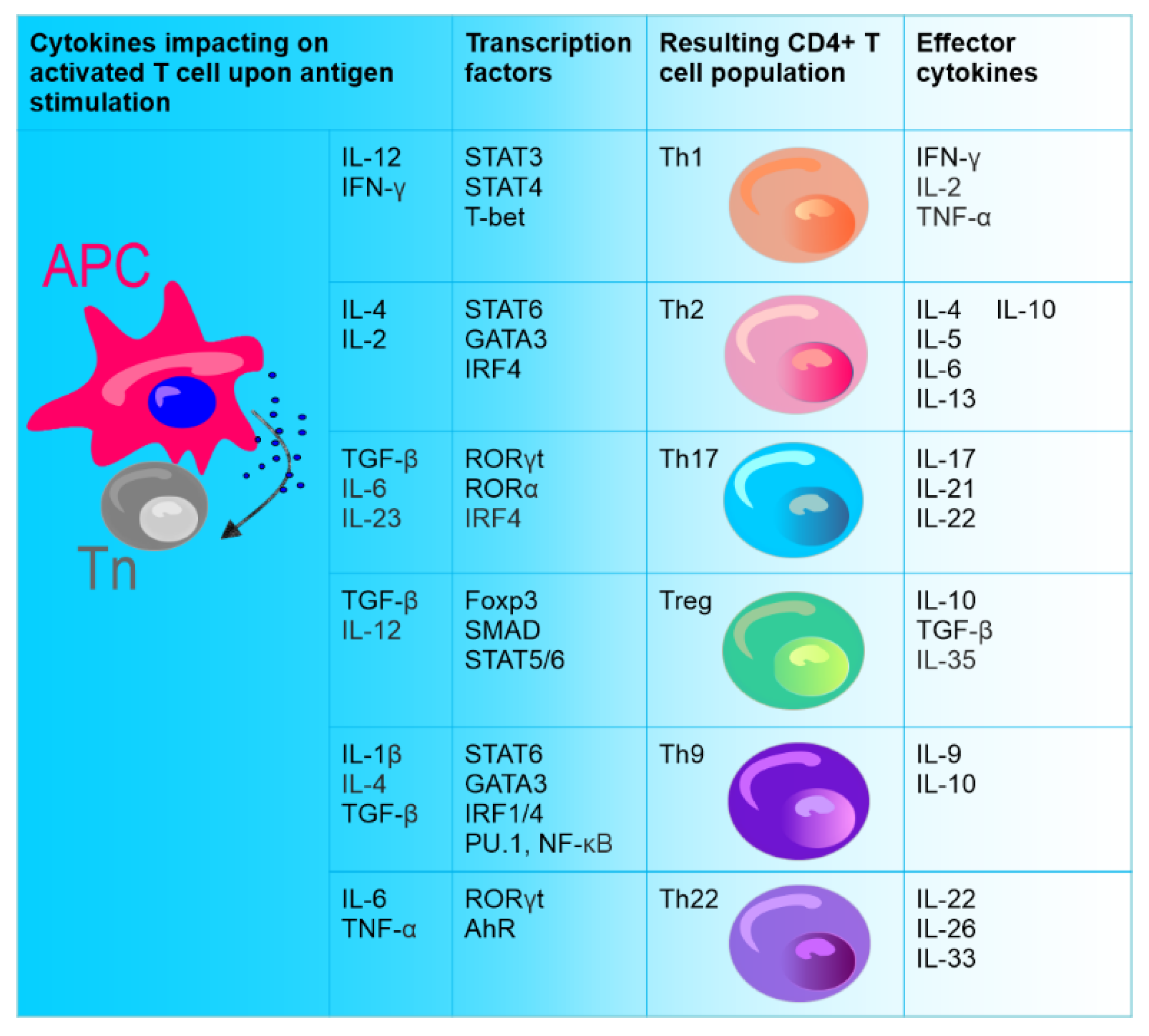
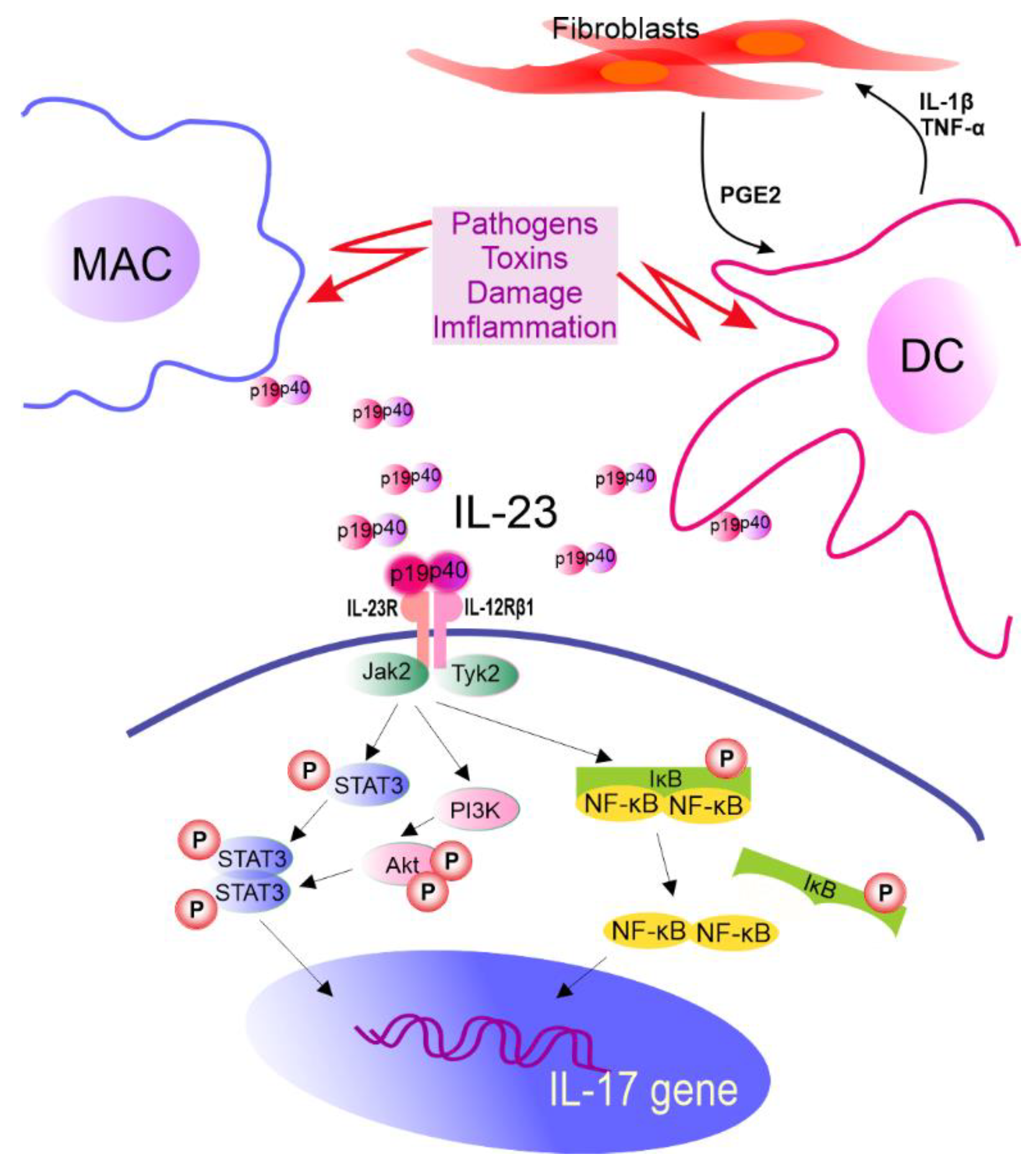

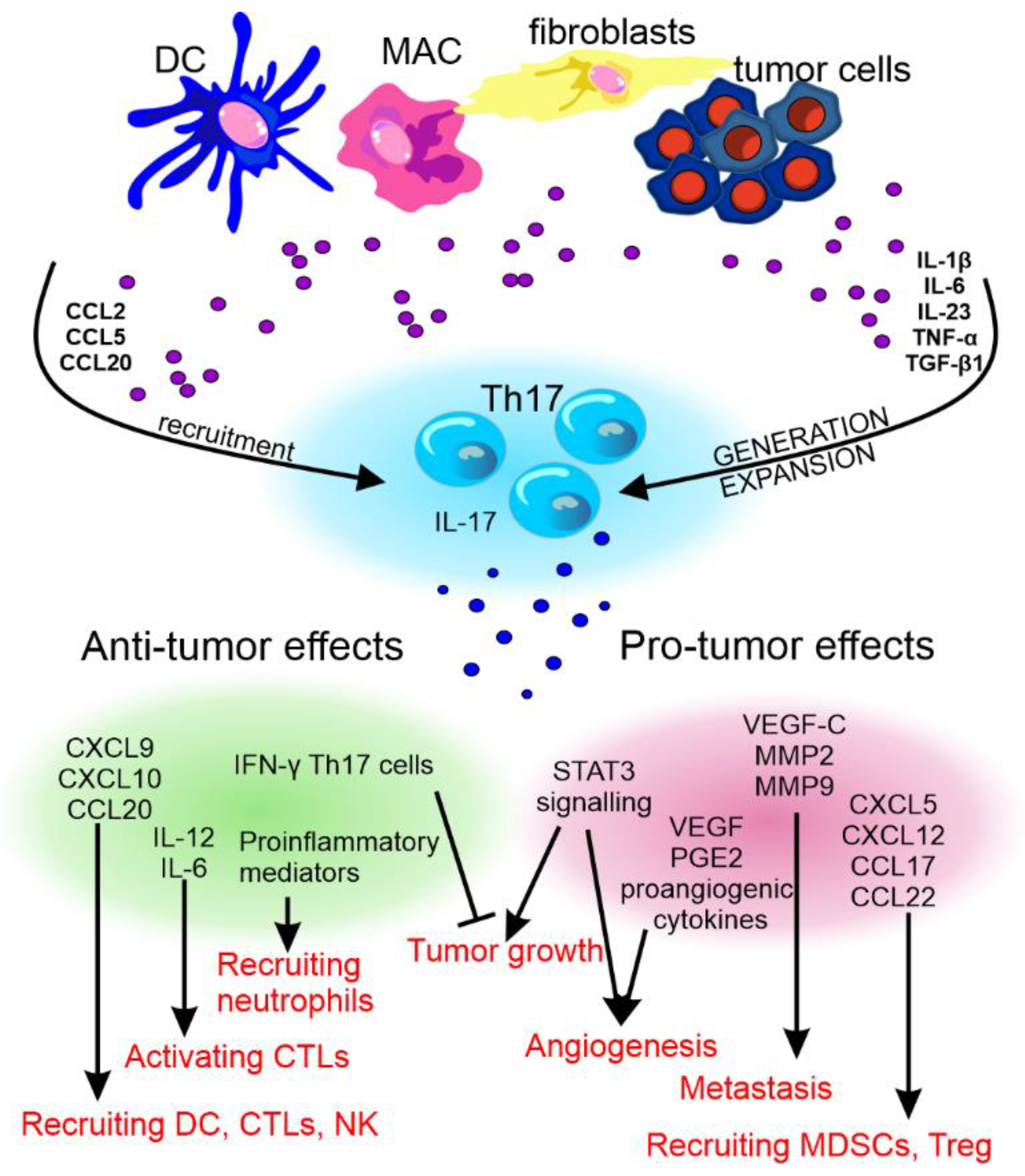


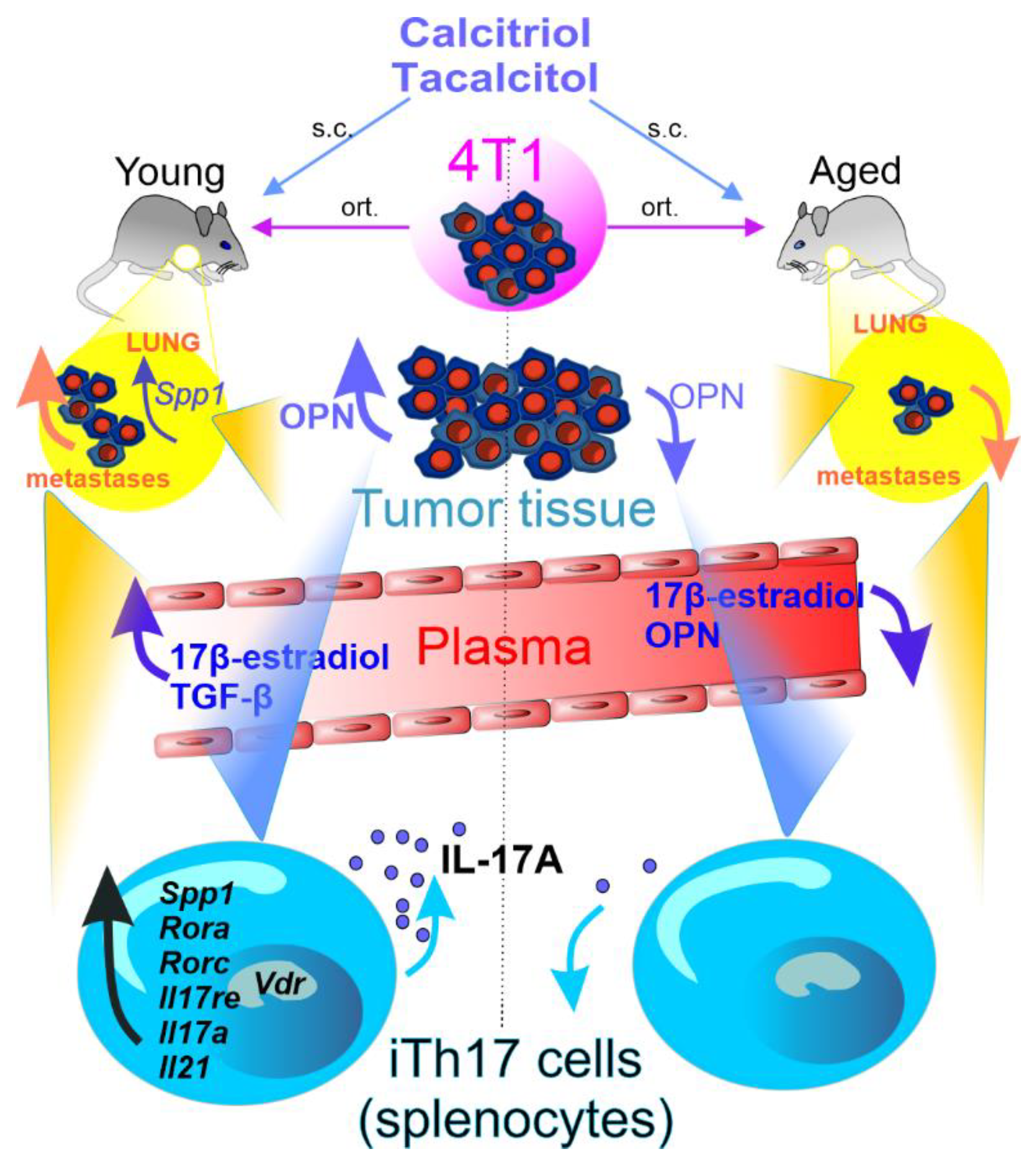
| Factor Studied | Type of Studies | Observed Effects | References |
|---|---|---|---|
| Sunlight exposition | Case–control | Sunlight measures are not associated with breast cancer risk but may depend on timing of exposure and genetic background | [34] |
| Prospective | UV radiation has no association with breast cancer risk | [35] | |
| Systematic review and meta-analysis | Exposure to sun for longer than an hour a day during the summer could decrease the risk of breast cancer | [36] | |
| Cancer database analysis | Breast cancer is diagnosed more often in spring and fall; breast cancer seasonality is latitude-dependent | [37] | |
| Retrospective analysis | Sunlight exposure may be associated with more prevalent TNBC | [38] | |
| Population-based | Spending more daylight hours outdoors in a year was associated with lower risk of ER+, ER−, and TNBC | [39] | |
| Population-based case–control | Factors suggestive of increased cutaneous production of vitamin D are associated with reduced breast cancer risk | [40] | |
| Death certificate based case–control | Breast cancer was negatively associated with residential and occupational sunlight | [41] | |
| Vitamin D dietary intake | Population-based | In the pooled analysis, dietary vitamin D and calcium were not associated with risk of breast cancer subtypes. Vitamin D: possible inverse associations between intake of ≤800 IU/d (compared with nonuse) and risk of several subtypes, with strongest effects observed for TNBC; no association was found for >800 IU/d | [39] |
| Prospective | Higher intakes of calcium and vitamin D may be associated with lower risk of premenopausal breast cancer, but not among postmenopausal women | [42] | |
| Nutrition cohort | Women with the highest intake of dietary calcium (>1250 mg/d) were at a lower risk of breast cancer than ≤500 mg/d; but neither use of supplemental calcium nor vitamin D intake was associated with breast cancer risk | [43] | |
| Double-blind, placebo-controlled clinical trial and cohort study | Only some evidence for a reduction in breast cancer risk and total invasive cancer risk among calcium and vitamin D users | [44] | |
| Case–control | High calcium, phosphorus, and vitamin D nutrient intake pattern was associated with a significant decrease in breast cancer risk | [45] | |
| Case–control | Supplementation with vitamin D, fatty acids EPA, and DHA was inversely associated with breast cancer | [46] | |
| Case–control | The risk of breast cancer was lower by 67% if the serum level of 25(OH)D was ≥24.6 ng/mL and lower by 68% if the serum level of calcium was ≥9.6 mg/dL; higher (than-normal) calcium serum level, considered separately, and a slightly lower-than- normal vitamin D serum level may protect against breast cancer among postmenopausal women, independent of dietary patterns and supplements | [47] | |
| 25(OH)D plasma level | Observational | Low 25(OH)D serum levels, alone and in combination with BsmI VDR genotype, may increase the risk of breast cancer in a UK Caucasian population | [48] |
| Cross-sectional analytical study | 25(OH)D deficiency is widespread among breast cancer patients | [49] | |
| Nested case–control | No overall association was found between plasma 25(OH)D and breast cancer risk; women with high, compared with low, plasma 25(OH)D levels in the summer have a reduced breast cancer risk, and plasma 25(OH)D may be inversely associated with risk of tumors expressing high levels of VDR | [50] | |
| Case–control | Low serum 25(OH)D levels, high tissue VDR levels, and high ERα gene expression were associated with increased risk of breast cancer | [51] | |
| Unmatched case–control | Severe vitamin D deficiency (<25 nmol/L) was significantly higher in chemotherapy-naïve (41.1%) than in TAM-treated (11.2%) patients; vitamin D deficiency was not significantly associated with tumor characteristics or VDR genotype | [52] | |
| Nested case–control | No association was found between plasma level of free 25(OH)D and overall risk of breast cancer; no association was found for plasma vitamin D binding protein (DBP) as well; neither the total nor the calculated free 25(OH)D and breast cancer association substantially varied by plasma DBP levels | [53] | |
| Cross-sectional | Significant correlation was observed between low vitamin D levels and advanced stage of breast cancer, particularly in postmenopausal patients | [22] | |
| Prospective | Maintaining an optimal 25(OH)D status at diagnosis and during the 1-year follow-up period is important for improving breast cancer patient survival | [21] | |
| Prospective | Serum levels of 25(OH)D were significantly higher in patients with early-stage breast cancer than in those with locally advanced or metastatic disease | [23] | |
| Case–control | Women with low levels of 25(OH)D, as compared to women in the middle tertile, had a high risk of breast cancer with an unfavorable prognosis | [26] | |
| Case–cohort | In women with elevated risk of breast cancer, high serum 25(OH)D levels and regular vitamin D supplement use were associated with lower rates of postmenopausal breast cancer over 5-year follow-up | [54] | |
| Cross-sectional | Low and decreased level of vitamin D might correlate with progression and metastasis of breast cancer | [27] | |
| Retrospective cohort study | 25(OH)D serum level < 16 ng/mL was associated with poor survival in breast cancer patients, regardless of age, lymph node status, stage, or breast cancer subtype | [55] | |
| Single-center prospective cohort study | Low 25(OH)D status is associated with better breast cancer survival; high 25(OH)D levels (>110 nmol/L) are associated with poorer breast cancer survival | [33] | |
| Meta-analysis | Lower 25(OH)D concentrations were not significantly associated with increased incidence of most cancers assessed; increased risk of breast cancer and decreased risk of lymphoma were noted with higher 25(OH)D concentrations | [32] | |
| Systematic review and meta-analysis | Association of low levels of vitamin D with increased risk of recurrence and death was noted in early-stage breast cancer patients | [56] | |
| Meta-analysis | A protective relationship was observed between circulating vitamin D (measured as 25(OH)D) and breast cancer development in premenopausal women | [31] | |
| Genome-wide association studies | No evidence was found supporting the association between 25(OH)D and risk of breast cancer | [57] | |
| Retrospective | Vitamin D deficiency was associated with inability to achieve complete pathological response following neoadjuvant chemotherapy | [58] | |
| Pooled cohort | Higher 25(OH)D concentrations, ≥60 ng/mL, were associated with a dose–response decrease in breast cancer risk. | [59] | |
| 1,25(OH)2D3 plasma level | Prospective | breast cancer patients have lower 1,25(OH)2D3 (40 ± 21) levels than healthy women (53 ± 23 pg/mL) | [60] |
| VDR tumor tissue expression | Observational | VDR expression inversely related to aggressive tumor characteristics (large tumor size, hormonal receptor negativity, and triple-negative subtype); VDR expression did not influence any patient survival outcomes | [28] |
| Retrospective | VDR expression was negatively associated with tumor size and lymph node involvement. High VDR expression speaks for better patient outcome | [61] | |
| Prospective cohort study | High VDR expression in invasive breast tumors was associated with favorable prognostic factors and lower risk of breast cancer death | [29] | |
| Case–control | VDR was upregulated in breast cancer tissues especially in hormone-negative breast cancer | [51] | |
| State-of-the-science review | The role of VDRs in cancer etiology is still equivocal | [62] | |
| VDR CTCs expression | Observational | VDR+ CTCs were detected in 46% of CTC+ patients | [30] |
| CYP27B1/CYP24A1 tumor tissue expression | Observational | mRNA expression of CYP27B1 was downregulated in tumor tissues, compared with normal tissues; the mRNA expression of CYP24A1 was significantly upregulated in the tumor tissues | [63] |
| Observational | Expression of CYP24A1 mRNA was reduced by about 58% in breast cancer tissues | [64] | |
| Observational | CYP27B1 expression was lower in invasive carcinomas (44.6%) than in benign lesions (55.8%). In contrast, CYP24A1 expression was inreased in carcinomas (56.0% in in situ and 53.7% in invasive carcinomas) compared to benign lesions (19.0%) | [18] | |
| Prospective | No correlation was observed between 24-hydroxylase, 1α-hydroxylase, and VDR expression in tumor tissue | [60] | |
| Vitamin D-binding protein | Meta-analysis | Borderline decrease in cancer risk was noted for subjects with high levels of DBP | [65] |
| Vitamin D-related gene polymorphism | Case–control | Two CYP24A1 polymorphisms (rs34043203, rs2762934) were associated with increased breast cancer risk; one with reduced breast cancer risk (rs1570669); minor alleles for the VDR Bsm1 polymorphism (rs1544410) but not Fok1 (rs2228570) were inversely associated with breast cancer risk | [34] |
| Population-based, case–control | None of the analyzed polymorphisms (FokI and TaqI) were associated with overall risk for postmenopausal breast cancer; TaqI polymorphism–a significantly increased risk for ER+ tumors but not for ER–tumors; haplotype FtCA was associated with a significantly greater breast cancer risk as compared with the most frequent haplotype FTCG | [66] | |
| Large population-based case–control | Increased risk for breast cancer was found in postmenopausal Caucasian women with the BsmI bb genotype | [67] | |
| Case–control | The VDR Tru9I “uu” genotype may increase the risk of premenopausal breast cancer | [68] | |
| Case–control | VDR FokI ff (rs2228570) polymorphism was significantly associated with an increased risk of breast cancer | [69] | |
| Unmatched case–control | VDR common variant alleles rs7975232 (ApaI), rs2228570 (FokI), and rs731236 (TaqI) were analyzed; rs2228570 GG genotype was associated with increased risk of breast cancer | [52] | |
| Case–control | GG genotype of Cdx2-VDR gene polymorphism may increase the risk of developing breast cancer in young female patients in South Pakistan | [70] | |
| Observational | VDR gene polymorphisms (BsmI and ApaI) may contribute to breast cancer risk among postmenopausal women | [71] | |
| Case–control | Significantly increased risk of breast cancer was associated with BsmI bb or even Bb genotype. Significant association between FokI genotypes and breast cancer risk was not observed | [72] | |
| Comparative | Early-onset patients revealed an association between rs10735810 and increased breast cancer risk; rs1544410, rs731236, and rs4516035 showed no association with disease | [73] | |
| Case–control | BsmI polymorphism in VDR gene may be associated with an increased breast cancer risk in Pakistani women negative for BRCA1/2 germline mutations | [74] | |
| Multicenter, prospective | Odds ratio for the rs2228570 (FokI) ff versus FF genotype in the overall population was statistically significantly elevated; no association; BB genotype was associated with a significantly lower risk of advanced breast cancer | [75] | |
| Case–control correlative | Vitamin D levels were not significantly associated with development of AI-induced arthralgia (AIA); patients with FokI VDR genotype were less likely to develop AIA | [76] | |
| Systematic review and network meta-analysis | Recessive polymorphism model with the rs2228570 (FokI) polymorphism is the best predictor of breast cancer in Caucasian patients; homozygote model with the CDX2 polymorphism is the best predictor of breast cancer in African-American patients | [77] | |
| State-of-the-science review | VDR polymorphisms may affect the risk and mortality of breast cancer, according tomenopausal status, vitamin D level, and breast cancer risk and mortality | [62] | |
| Systematic review of the literature | VDR polymorphisms Fok1, Bsm1, Taq1, Apa1, and Cdx2 were analyzed, but no; conflicting data were obtained | [78] |
| Animal Model | Vitamin D Metabolite/Analog Used | Schedule of Treatment | Monotherapy/Combined Treatment/Other | Effect Observed | References |
|---|---|---|---|---|---|
| 4T1 mouse mammary gland cells | Vitamin D3 (VD) | Day 17 after tumor inoculation, 5 μg/kg of VD/d, 7 days treatment | - | Increase in tumor growth | [142] |
| 4T1, 67NR, E0771 mouse mammary gland cancers | Calcitriol (Cal) 1 µg/kg three times a week p.o. or 5000 IU of vitamin D3/kg of diet | Cal: 7 days after implantation till day 21; Diet: 6 weeks before transplantation and continued | - | 4T1: increased metastasis; 67NR: no effect; E0771: decreased tumor growth | [145] |
| 4T1 mouse mammary gland cells | Calcitriol, PRI-2191, and PRI-2205 0.5, 1, and 10 µg/kg, respectively, three times a week s.c. | 7 days after implantation till day 33 | Postmenopausal OVX model | Transiently decreased metastasis | [151] |
| 4T1 mouse mammary gland cells | Calcitriol, PRI-2191, and PRI-2205 0.5, 1, and 10 µg/kg, respectively, three times a week s.c. | 7 days after implantation till day 33 | - | Increased metastasis | [144] |
| E0771 mouse mammary gland cancer | Cholecalciferol gavage 40 IU/d/mouse | 7 days after cell injection for 2 weeks | Normal mice Obese mice | Antitumor, antimetastatic effect Stimulation of tumor growth and metastasis | [152] |
| LM3 mouse breast adenocarcinoma | EM1 20 and 50 µg/kg | Started when tumors were 50–70 mm3, 7 injections for 2 weeks | - | Tumor growth—no effect; decrease in lung metastasis | [153] |
| ER–breast cancer/ER+ breast cancer | 24R,25(OH)2D3 | HCC38, 2 weeks after inoculation, 24R,25(OH)2D3 100 ng/d, 3 times a week, 10-week treatment MCF-7: 25 ng or 100 ng per injection of 24R,25(OH)2D3 from week 5 | - | Protumorigenic when breast cancer was of Erα-66– and Erα-36 ± status: the response of breast cancer cells to 24R-24,25(OH)2D3. Enhanced or decreased epithelial-to-mesenchymal transition | [154] |
| 4T1 mouse mammary gland cells | Calcitriol 0.3 μg/kg b.w. once every other day i.p. | From the day before tumor cells were injected | - | Antimetastatic | [155] |
| 16/c mouse mammary adenocarcinoma | Calcitriol or PRI-2191 10 μg/kg for 5 consecutive days s.c. | From day 5 after tumor inoculation | Cisplatin: 3 mg/kg i.p. at day 6 after tumor cell inoculation; clodronate: days 5 and 8, i.p., 1.5 mg/mouse/d | 54% tumor growth inhibition by combined treatment with PRI-2191; 41% inhibition by treatment with PRI-2191 alone; no effect of calcitriol | [156] |
| 16/c mouse mammary adenocarcinoma | PRI-1906 or PRI-2191 1 μg/kg/d for 9 consecutive days s.c. | Started from day 1 or 5 after tumor cell inoculation | Cyclophosphamide (CY) i.p. 100 mg/kg on day 4 after tumor cell inoculation | Potentiation of CY antitumor effect by both analogs. PRI-1906 alone—no effect; PRI-2191—25% of inhibition | [157] |
| MDA-MB-231-luc | Calcitriol 1 µg/kg | 3 days prior to photodynamic therapy (PT) | 5-Aminolevulinate-based PT | Increased PT effect * | [158] |
| MMTV-Wnt1 mouse mammary gland cells | Calcitriol 25 ng/mouse or 5300 IU of vitamin D3/kg of diet | 10 weeks after standard (STD) and high-fat (HFD) diet | STD and HFD | Decreased tumor volume | [159] |
| MMTV-Wnt1 mouse mammary gland cells | Calcitriol 50 ng/mouse three times a week or 5000 IU of vitamin D3/kg of diet | 8 weeks prior to tumor inoculation and continued | - | Decreased tumor volume | [134] |
| MCF-7 human breast cancer | Calcitriol 50 ng/mouse three times a week i.p. or 5000 IU of vitamin D3/kg of diet | After 6 weeks of tumor growth continued for 4 weeks | - | Decreased tumor growth | [160] |
| MCF-7 human breast cancer | Calcitriol 0.025, 0.05, or 0.1 μg/mouse, three times a week i.p. or 5000 IU of vitamin D3/kg of diet | After 6 weeks of tumor growth continued for 4 weeks | Pre- and postmenopausal (OVX) models | Decreased tumor growth (~60%) | [161] |
| MCF-7 human breast cancer | PRI-2191 and PRI-2205 1.0 μg/kg/d or 10.0 μg/kg/d, respectively, three times a week s.c. | From day 39 after tumor cell inoculation up to day 67 | - | Decreased tumor growth by PRI-2205 | [136] |
| MCF-7 (VEGF-transfected) with MDA-435S human breast cancer cells | Calcitriol 12.5 pmol/d s.c. (micro-osmotic pumps)/28 days; next weekly s.c. dose of 12.5 pmol/d 7 times | 6 days before cell implantation and during 8 weeks | - | Decreased tumor angiogenesis | [162] |
| MCF-7 human breast cancer | Calcitriol and analogs: TX 522 and TX 527 5, 80, and 25 μg/kg, respectively, every other day i.p. | Started 4 days after tumor transplantation | - | Cal: no effect; both analogs: decrease in tumor volume and mitotic figures | [163] |
| MCF-7 human breast cancer | EB1089 45 pmol/d (osmotic pumps) | Started with 150–200 mm3 tumors for 8 days | Irradiation after end of EB1089 (2 × 5 Gy) | Delayed tumor growth and decreased tumor volume (50%) in combined treatment | [164] |
| MCF-7 human breast cancer | PRI-2191 1.0 µg/kg b.w./d, PRI-2205 10.0 µg/kg b.w./d | Started 5 days after tumor cell inoculation | Anastrozole: 5 days/week, 200 µg/mouse in each injection | Significantly decreased MCF-7 tumor volume after single or combined treatment | [147] |
| T47D or TDC human breast cancer | Calcitriol 0.03 μg/kg, 2 times a week i.p. | Started when tumors were palpable during 4 weeks | - | Decreased tumor growth, no effect on angiogenesis | [165] |
| MCF10DCIS.com human DCIS model | BXL0124 0.1 μg/kg, 6 times a week i.p. or 0.03 or 0.1 μg/kg, 6 times a week p.o. or i.p. | Next day after tumor inoculation for 4 weeks or for 5 weeks | - | Inhibition of DCIS to IDC progression; 43% reduction in tumor volume | [166,167] |
| SUM149 human inflammatory breast cancer cell line | Quantum dots with calcitriol 40 nM i.v. | Started with 80 mm3 tumors | Quantum dots with calcitriol conjugated with anti MUC-1 Ab | Enhanced concentration of quantum dots in tumor and lungs | [168] |
| Transgenic model of hormone-induced mammary cancer (LH-overexpression) | EB1089 0.027 μg per animal s.c. | From 3 to 5 weeks of age | - | Decreased growth to regression | [169] |
| Freshly collected breast cancer samples xenografted into animals | Calcitriol 0.06 μg intratumoral | 6 weeks after transplantation, weekly (6–11) | - | No effect | [170] |
| N-methyl-N-nitrosourea (NMU)-induced mammary tumor (rats) and MCF10DCIS.com | Gemini 0072 and Gemini 0097 0.1, 0.3, or 0.03 μg/kg, 5 days a week i.p. | 1 week after NMU; day 4 after MCF10DCIS.com cell implantation | 60% inhibition of NMU-induced mammary tumor and suppression of MCF10DCIS.com | [171] | |
| NMU- and DMBA (7,12-dimethylbenz(a)anthracene)-induced mammary carcinogenesis | 1α-Hydroxy-24-ethylcholecalciferol 25, 40, 50 μg/kg of diet | 2 weeks before carcinogen | - | Chemopreventive effects | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip-Psurska, B.; Zachary, H.; Strzykalska, A.; Wietrzyk, J. Vitamin D, Th17 Lymphocytes, and Breast Cancer. Cancers 2022, 14, 3649. https://doi.org/10.3390/cancers14153649
Filip-Psurska B, Zachary H, Strzykalska A, Wietrzyk J. Vitamin D, Th17 Lymphocytes, and Breast Cancer. Cancers. 2022; 14(15):3649. https://doi.org/10.3390/cancers14153649
Chicago/Turabian StyleFilip-Psurska, Beata, Honorata Zachary, Aleksandra Strzykalska, and Joanna Wietrzyk. 2022. "Vitamin D, Th17 Lymphocytes, and Breast Cancer" Cancers 14, no. 15: 3649. https://doi.org/10.3390/cancers14153649






