Differences in Treatment Modalities and Prognosis of Elderly Patients with Ovarian Cancer: A Two-Center Propensity Score-Matched Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Surgery
2.3. Systemic Chemotherapy and Chemotherapy-Related Toxicity
2.4. Prognosis and Follow-Up Evaluation
2.5. Statistical Analysis
3. Results
3.1. Demographics of the Original Cohort
3.2. Development of the Cohort after PSM
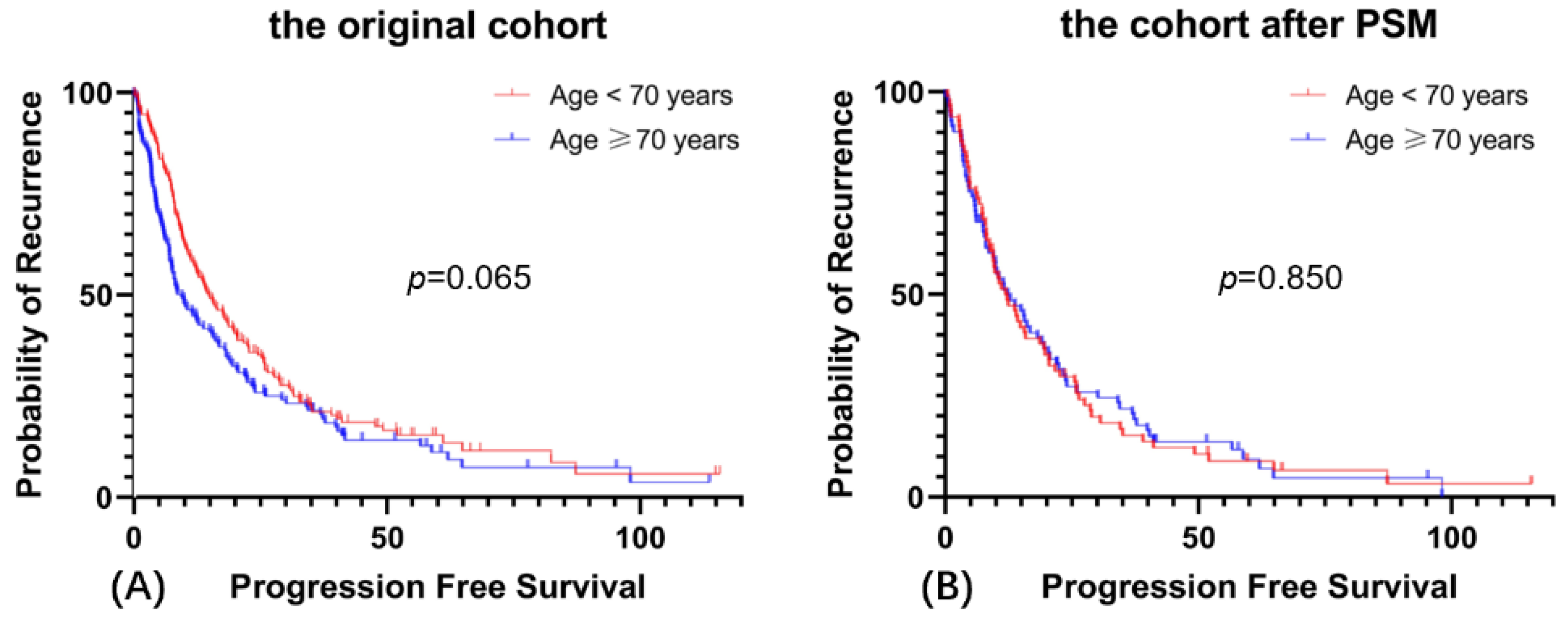
3.3. Prognosis
3.4. Severe Chemotherapy-Related Toxicity
3.5. Cause of Discontinuation of Adjuvant Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malvezzi, M.; Carioli, G.; Rodriguez, T.; Negri, E.; La Vecchia, C. Global trends and predictions in ovarian cancer mortality. Ann. Oncol. 2016, 27, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. Chin. Med. J. 2021, 134, 1959–1966. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.-M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef] [Green Version]
- Gondos, A.; Holleczek, B.; Arndt, V.; Stegmaier, C.; Ziegler, H.; Brenner, H. Trends in population-based cancer survival in Germany: To what extent does progress reach older patients? Ann. Oncol. 2007, 18, 1253–1259. [Google Scholar] [CrossRef]
- Colloca, G.; Corsonello, A.; Marzetti, E.; Balducci, L.; Landi, F.; Extermann, M.; Scambia, G.; Cesari, M.; Carreca, I.; Monfardini, S.; et al. Treating Cancer in Older and Oldest Old Patients. Curr. Pharm. Des. 2015, 21, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Loizzi, V.; Lin, Y.G.; Osann, K.; Brewster, W.R.; DiSaia, P.J. Stages III and IV invasive epithelial ovarian carcinoma in younger versus older women: What prognostic factors are important? Obstet. Gynecol. 2003, 102, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Youlden, D.; Baade, P.; Jackson, D.; Obermair, A. Elderly patients with stage III or IV ovarian cancer: Should they receive standard care? Int. J. Gynecol. Cancer 2008, 18, 896–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, N.C.; Enserro, D.; Brady, M.F.; Moxley, K.; Walker, J.L.; Cosgrove, C.; Bixel, K.; Tewari, K.S.; Thaker, P.; Hendrickson, A.E.W.; et al. Prognostic significance of ethnicity and age in advanced stage epithelial ovarian cancer: An NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2021, 164, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; Legner, V.J.; Symons, R.G.; McCormick, W.C.; Flum, D.R. Impact of Advancing Age on Abdominal Surgical Outcomes. Arch. Surg. 2009, 144, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, T.L.; Teiblum, S.; Paludan, M.; Poulsen, L.; Jørgensen, A.Y.S.; Bruun, K.H.; Hallas, J.; Herrstedt, J. Significance of age and comorbidity on treatment modality, treatment adherence, and prognosis in elderly ovarian cancer patients. Gynecol. Oncol. 2012, 127, 367–374. [Google Scholar] [CrossRef]
- Hilpert, F.; du Bois, A.; Greimel, E.; Hedderich, J.; Krause, G.; Venhoff, L.; Loibl, S.; Pfisterer, J. Feasibility, toxicity and quality of life of first-line chemotherapy with platinum/paclitaxel in elderly patients aged ≥70 years with advanced ovarian cancer—a study by the AGO OVAR Germany. Ann. Oncol. 2007, 18, 282–287. [Google Scholar] [CrossRef]
- Liontos, M.; Papatheodoridi, A.; Andrikopoulou, A.; Thomakos, N.; Haidopoulos, D.; Rodolakis, A.; Zagouri, F.; Bamias, A.; Dimopoulos, M.-A. Management of the Elderly Patients with High-Grade Serous Ovarian Cancer in the REAL-WORLD Setting. Curr. Oncol. 2021, 28, 1143–1152. [Google Scholar] [CrossRef]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Salutari, V.; Selle, F.; Frezzini, S.; et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol. 2021, 22, 267–276. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Mallen, A.; Todd, S.; Robertson, S.E.; Kim, J.; Sehovic, M.; Wenham, R.M.; Extermann, M.; Chon, H.S. Impact of age, comorbidity, and treatment characteristics on survival in older women with advanced high grade epithelial ovarian cancer. Gynecol. Oncol. 2021, 161, 693–699. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. Available online: https://pubmed.ncbi.nlm.nih.gov/7722560/ (accessed on 2 March 2022). [CrossRef]
- Aletti, G.D.; Eisenhauer, E.L.; Santillan, A.; Axtell, A.; Aletti, G.; Holschneider, C.; Chi, D.S.; Bristow, R.E.; Cliby, W.A. Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment. Gynecol. Oncol. 2011, 120, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Mohile, S.; Gajra, A.; Klepin, H.; Muss, H.; Chapman, A.; Feng, T.; Smith, D.; Sun, C.-L.; De Glas, N.; et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults with Cancer. J. Clin. Oncol. 2016, 34, 2366–2371. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-H.; Lim, M.C.; Lee, D.; Won, Y.-J.; Ha, H.I.; Chang, H.K.; Cho, H. Cause-specific mortality rate of ovarian cancer in the presence of competing risks of death: A nationwide population-based cohort study. J. Gynecol. Oncol. 2022, 33. [Google Scholar] [CrossRef] [PubMed]
- Joueidi, Y.; Dion, L.; Bendifallah, S.; Mimoun, C.; Bricou, A.; Timoh, K.N.; Collinet, P.; Touboul, C.; Ouldamer, L.; Azaïs, H.; et al. Management and Survival of Elderly and Very Elderly Patients with Ovarian Cancer: An Age-Stratified Study of 1123 Women from the FRANCOGYN Group. J. Clin. Med. 2020, 9, 1451. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2016, CD007565. [Google Scholar] [CrossRef]
- Winter, W.E., 3rd; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P.; et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta-Analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Piedimonte, S.; Bernardini, M.Q.; May, T.; Cybulska, P.; Ferguson, S.E.; Laframboise, S.; Bouchard-Fortier, G.; Avery, L.; Hogen, L. Treatment outcomes and predictive factors in patients ≥70 years old with advanced ovarian cancer. J. Surg. Oncol. 2021, 125, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Koual, M.; Le Frère-Belda, M.-A.; Zerbib, J.; Fournier, L.; Nguyen-Xuan, H.-T.; Delanoy, N.; Bentivegna, E.; Bats, A.-S.; Azaïs, H. Risks and benefits of systematic lymphadenectomy during interval debulking surgery for advanced high grade serous ovarian cancer. Eur. J. Surg. Oncol. (EJSO) 2021, 48, 275–282. [Google Scholar] [CrossRef]
- Narasimhulu, D.M.; Kumar, A.; Weaver, A.L.; McGree, M.E.; Langstraat, C.L.; Cliby, W.A. Using an evidence-based triage algorithm to reduce 90-day mortality after primary debulking surgery for advanced epithelial ovarian cancer. Gynecol. Oncol. 2019, 155, 58–62. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Fukuda, T.; Uemura, R.; Matsubara, H.; Wada, T.; Kawanishi, M.; Tasaka, R.; Kasai, M.; Hashiguchi, Y.; Ichimura, T.; et al. Age-related differences in prognosis and prognostic factors among patients with epithelial ovarian cancer. Mol. Clin. Oncol. 2018, 9, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.S.; He, W.; Harrison, R.; Zhao, H.; Sun, C.C.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. Disparities in treatment and survival among elderly ovarian cancer patients. Gynecol. Oncol. 2018, 151, 269–274. [Google Scholar] [CrossRef]
- Hay, C.M.; Donovan, H.S.; Campbell, G.B.; Taylor, S.E.; Wang, L.; Courtney-Brooks, M. Chemotherapy in older adult gynecologic oncology patients: Can a phenotypic frailty score predict tolerance? Gynecol. Oncol. 2019, 152, 304–309. [Google Scholar] [CrossRef]
- Amadio, G.; Marchetti, C.; Villani, E.R.; Fusco, D.; Stollagli, F.; Bottoni, C.; Di Stefano, M.; Colloca, G.; Scambia, G.; Fagotti, A. ToleRability of BevacizUmab in elderly Ovarian cancer patients (TURBO study): A case-control study of a real-life experience. J. Gynecol. Oncol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, Y.; Kim, T.-J.; Lee, J.-W.; Kim, B.-G.; Bae, D.-S.; Choi, C.H. Optimal cutoff age for predicting prognosis associated with serous epithelial ovarian cancer: What is the best age cutoff? J. Gynecol. Oncol. 2019, 30. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Ehling, G.; Kauffmann, H.-M.; Unger, C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum. Exp. Toxicol. 2007, 26, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Ortega, R.M.M.; Villanueva-Rodríguez, G.; Serna-Thomé, M.G.; Flores-Estrada, D.; Diaz-Romero, C.; Rodríguez, C.M.; Martínez, L.; Sánchez-Lara, K. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer 2010, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Characteristics | N (%) | Groups, n (%) | p Value | ||
|---|---|---|---|---|---|
| Age < 70 (n = 186) | Age ≥ 70 (n = 138) | ||||
| Age (years) | Median ± SD | 68.5 ± 5.3 | 64.8 ± 3.1 | 73.4 ± 3.3 | <0.001 |
| BMI (kg/m2) | Median ± SD | 24.4 ± 3.3 | 24.5 ± 3.4 | 24.3 ± 3.0 | 0.909 |
| BRCA testing | No | 248 (76.5) | 135 (72.6) | 113 (81.9) | 0.051 |
| Yes | 76 (23.5) | 51 (27.4) | 25 (18.1) | ||
| BRCA 1/2 mutation | negative | 60 (78.9) | 40 (78.4) | 20 (80.0) | 0.875 |
| positive | 16 (21.1) | 11 (21.6) | 5 (20.0) | ||
| Performance status | 0 | 253 (79.3) | 151 (83.4) | 102 (73.9) | 0.062 |
| 1 | 65 (20.4) | 29 (16.0) | 36 (26.1) | ||
| 2 | 1 (0.3) | 1 (0.6) | 0 (0.0) | ||
| Hemoglobin (g/L) | ≥110 | 202 (62.3) | 122 (65.6) | 80 (58.0) | 0.331 |
| <110 | 112 (34.6) | 58 (31.2) | 54 (39.1) | ||
| unknown | 10 (3.1) | 6 (3.2) | 4 (2.9) | ||
| Albumin (g/L) | >40 | 144 (47.7) | 90 (51.4) | 54 (42.5) | 0.126 |
| ≤40 | 158 (52.3) | 85 (48.6) | 73 (57.5) | ||
| unknown | 22 (6.8) | 11 (5.9) | 11 (8.0) | ||
| Renal function | >90 | 84 (26.5) | 65 (35.9) | 19 (14.0) | <0.001 |
| (mL/min*1.73 m2) | 60–90 | 186 (58.7) | 105 (58.0) | 81 (59.6) | |
| 30–60 | 45 (14.2) | 11 (6.1) | 34 (25.0) | ||
| 15–30 | 2 (0.6) | 0 (0.0) | 2 (1.5) | ||
| <15 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Neoadjuvant chemotherapy | No | 180 (55.6) | 128 (68.8) | 52 (37.7) | <0.001 |
| Yes | 144 (44.4) | 58 (31.2) | 86 (62.3) | ||
| ASA | 1 | 20 (6.2) | 14 (7.5) | 6 (4.3) | 0.059 |
| 2 | 238 (73.5) | 142 (76.3) | 96 (69.6) | ||
| 3 | 66 (20.4) | 30 (16.1) | 36 (26.1) | ||
| Ascites | <200 mL | 202 (62.3) | 107 (57.5) | 95 (68.8) | 0.038 |
| ≥200 mL | 122 (37.7) | 79 (42.5) | 43 (31.2) | ||
| Tumor dissemination | Low | 108 (33.3) | 62 (33.3) | 46 (33.3) | 0.999 |
| Intermediate | 143 (44.1) | 82 (44.1) | 61 (44.2) | ||
| High | 73 (22.5) | 42 (22.6) | 31 (22.5) | ||
| Surgical complexity score | 1 | 224 (75.3) | 130 (69.9) | 114 (82.6) | 0.009 |
| 2 | 80 (24.7) | 56 (30.1) | 24 (17.4) | ||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Systemic lymph node dissection | No | 105 (60.2) | 96 (51.6) | 99 (71.7) | <0.001 |
| Yes | 129 (39.8) | 90 (48.4) | 39 (28.3) | ||
| FIGO stage | I | 13 (4.0) | 10 (5.4) | 3 (2.2) | 0.228 |
| II | 32 (9.9) | 21 (11.3) | 11 (8.0) | ||
| III | 229 (70.7) | 124 (66.7) | 105 (76.1) | ||
| IV | 50 (15.4) | 31 (16.7) | 19 (13.8) | ||
| Histology | HGSC | 297 (91.7) | 164 (88.2) | 133 (96.4) | 0.030 |
| EC | 11 (3.4) | 9 (4.8) | 2 (1.4) | ||
| Clear cell | 16 (4.9) | 13 (7.0) | 3 (2.2) | ||
| R0 cytoreduction | No | 185 (57.1) | 102 (54.8) | 83 (60.1) | 0.340 |
| Yes | 139 (42.9) | 84 (45.2) | 55 (39.9) | ||
| CARG | low risk | 209 (64.5) | 157 (84.4) | 52 (37.7) | <0.001 |
| medium risk | 113 (34.9) | 29 (15.6) | 84 (60.9) | ||
| high risk | 2 (0.6) | 0 (0.0) | 2 (1.4) | ||
| Cycles of chemotherapy | Median ± SD | 5.26 ± 1.84 | 5.72 ± 1.67 | 4.64 ± 1.85 | 0.014 |
| Completion of adjuvant chemotherapy | No | 63 (19.4) | 20 (10.8) | 43 (31.2) | <0.001 |
| Yes | 261 (80.6) | 166 (89.2) | 95 (68.8) | ||
| Chemotherapy | none | 2 (0.6) | 0 (0.0) | 2 (1.4) | 0.008 |
| regimen | monotherapy | 5 (1.5) | 0 (0.0) | 5 (3.6) | |
| double therapy | 317 (97.9) | 186 (1.0) | 131 (95.0) | ||
| Hematologic toxicity | I–II | 195 (60.9) | 121 (65.8) | 74 (54.4) | 0.040 |
| III–IV | 125 (39.1) | 63 (34.2) | 62 (45.6) | ||
| Clinical trial | No | 317 (97.8) | 182 (97.8) | 135 (97.8) | 0.989 |
| Yes | 7 (2.2) | 4 (2.2) | 3 (2.2) | ||
| Charlson comorbidity index | mild | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.004 |
| moderate | 35 (10.8) | 28 (15.1) | 7 (5.1) | ||
| sever | 289 (89.2) | 158 (84.9) | 131 (94.9) | ||
| Characteristics | The Original Cohort | The Cohort after PSM | |||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate Analysis | Univariate | Multivariate Analysis | ||||
| p | OR (95% CI) | p | p | OR (95% CI) | p | ||
| Age | <70 | 0.040 | 1 | 0.083 | 0.010 | 1 | 0.008 |
| ≥70 | 1.602 (0.940–2.729) | 2.639 (1.291–5.395) | |||||
| Renal function | <60 | 0.957 | 1 | 0.608 | 0.686 | 1 | 0.635 |
| >60 | 0.824 (0.393–1.727) | 1.330 (0.409–4.323) | |||||
| Albumin (g/L) | ≥40 | <0.001 | 1 | <0.001 | 0.010 | 1 | 0.018 |
| <40 | 2.860 (1.746–4.684) | 2.441 (1.168–5.100) | |||||
| Surgical complexity score | 1 | 0.085 | 1 | 0.093 | 0.313 | 1 | 0.561 |
| 2–3 | 1.614 (0.923–2.821) | 1.272 (0.565–2.863) | |||||
| Completion of adjuvant chemotherapy | No | 0.607 | 1 | 0.886 | 0.149 | 1 | 0.208 |
| Yes | 1.048 (0.553–1.984) | 0.554 (0.221–1.389) | |||||
| Chemotherapy regimen | monotherapy | 0.061 | 1 | 0.088 | N/A | N/A | N/A |
| double therapy | 7.355 (0.745–72.598) | ||||||
| Characteristics | The Original Cohort | The Cohort after PSM | |||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||
| p value | OR (95% CI) | p value | p value | OR (95% CI) | p value | ||
| Age | <70 | <0.001 | 1 | 0.182 | 0.539 | 1 | 0.497 |
| ≥70 | 1.634 (0.795–3.356) | 1.441 (0.556–3.733) | |||||
| Charlson comorbidity index | 3–4 | 0.098 | 1 | 0.991 | 0.380 | 1 | 0.952 |
| ≥5 | 1.008 (0.254–4.003) | 0.947 (0.165–5.443) | |||||
| Cancer Aging Research Group scores | Low risk | 0.001 | 1 | 0.666 | 0.460 | 1 | 0.575 |
| Medium risk | 0.858 (0.428–1.719) | 0.756 (0.285–2.006) | |||||
| ASA | 1–2 | 0.005 | 1 | 0.061 | 0.283 | 1 | 0.404 |
| 3 | 1.957 (0.968–3.953) | 0.640 (0.224–1.827) | |||||
| Renal function | >60 | <0.0001 | 1 | 0.010 | 0.001 | 1 | 0.002 |
| <60 | 2.717 (1.266–5.814) | 5.128 (1.789–14.71) | |||||
| Neoadjuvant chemotherapy | No | <0.0001 | 3.367 (1.626–6.944) | 0.001 | 0.041 | 2.326 (0.859–6.289) | 0.097 |
| Yes | |||||||
| Surgical complexity score | 1 | 0.141 | 0.445 | ||||
| 2–3 | |||||||
| Ascites | <200 mL | 0.027 | 1 | 0.089 | 0.174 | ||
| ≥200 mL | 1.893 (0.908–3.947) | ||||||
| Hematologic toxicity | 0–2 | 0.606 | 0.150 | ||||
| 3–4 | |||||||
| Chemotherapy | monotherapy | 0.247 | N/A | ||||
| regimen | double therapy | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zuo, J.; Li, N.; Zheng, R.; Yuan, G.; Shen, G.; Wu, L. Differences in Treatment Modalities and Prognosis of Elderly Patients with Ovarian Cancer: A Two-Center Propensity Score-Matched Study. Cancers 2022, 14, 3655. https://doi.org/10.3390/cancers14153655
Zhao Y, Zuo J, Li N, Zheng R, Yuan G, Shen G, Wu L. Differences in Treatment Modalities and Prognosis of Elderly Patients with Ovarian Cancer: A Two-Center Propensity Score-Matched Study. Cancers. 2022; 14(15):3655. https://doi.org/10.3390/cancers14153655
Chicago/Turabian StyleZhao, Yuxi, Jing Zuo, Ning Li, Rongshou Zheng, Guangwen Yuan, Guihua Shen, and Lingying Wu. 2022. "Differences in Treatment Modalities and Prognosis of Elderly Patients with Ovarian Cancer: A Two-Center Propensity Score-Matched Study" Cancers 14, no. 15: 3655. https://doi.org/10.3390/cancers14153655







