The Significance of External Quality Assessment Schemes for Molecular Testing in Clinical Laboratories
Abstract
:Simple Summary
Abstract
1. Introduction
Why Are Good EQA Schemes Important?
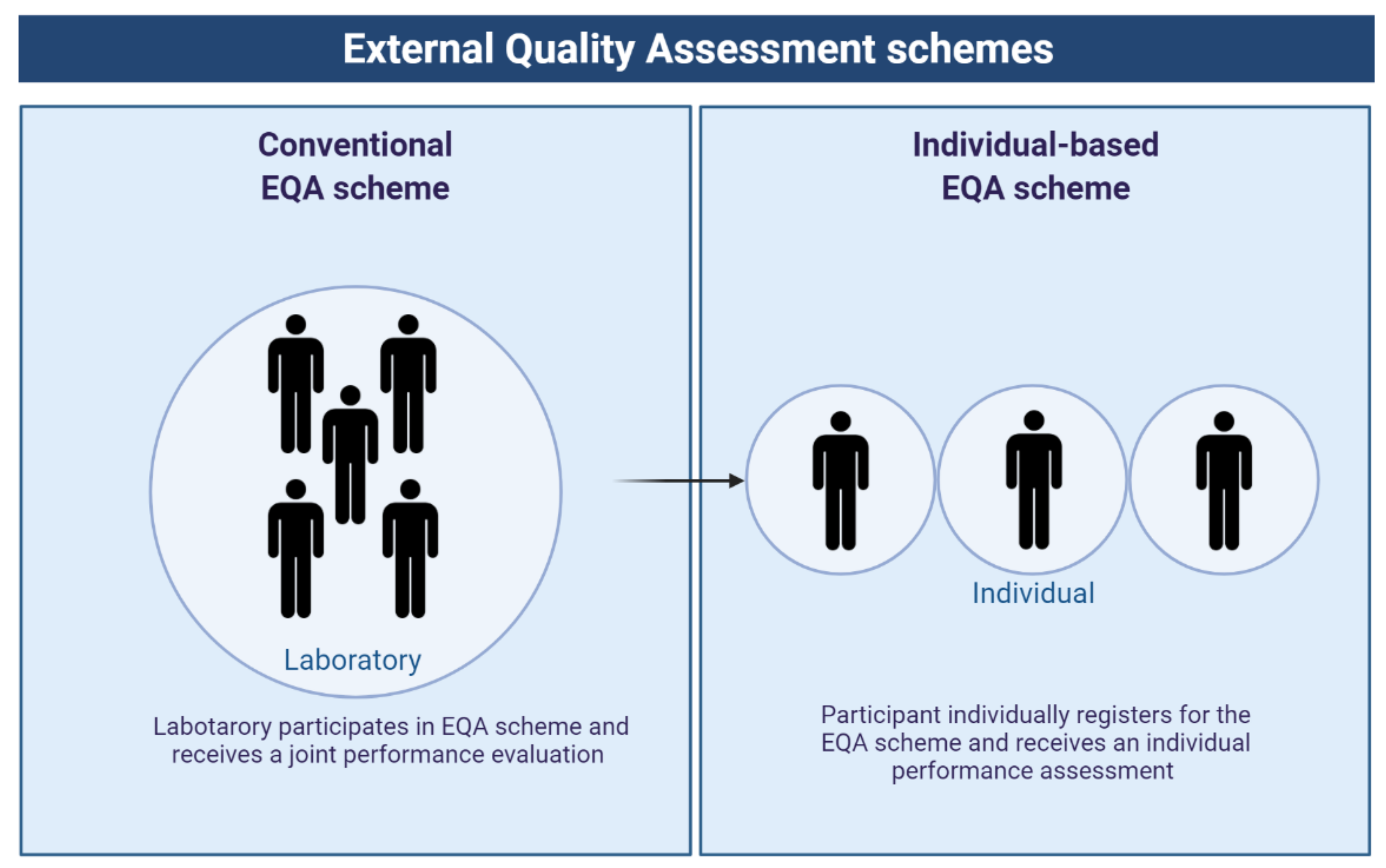
2. How to Organize Different Types of EQA Schemes?
2.1. Different Types of EQA Schemes Have Different Purposes and Aim to Improve Different Phases/Parts of the Process
2.2. EQA Providers Should Adhere to Several Guidelines to Guarantee High-Quality EQA Schemes
2.3. Risks That Might Influence the Quality of an EQA Scheme Should Be Addressed
2.4. Types of EQA Providers and Schemes
2.4.1. EQA Providers with ISO/IEC 17043 Accreditation
2.4.2. Goals of EQA Providers
2.4.3. Research by EQA Providers Can Highlight Points of Concern
3. Importance of EQA Schemes for Laboratories and for Clinical Practice
4. The Scientific Value of EQA Schemes and Importance for Industry: What Can EQA Schemes Learn Us?
4.1. Insights from EQA Schemes: Performance of Laboratories
4.2. Insights for Industry: Performance of Available Kits and Test Methods
5. Challenges and Future of EQA Schemes
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keppens, C.; Boone, E.; Gameiro, P.; Tack, V.; Moreau, E.; Hodges, E.; Evans, P.; Brüggemann, M.; Carter, I.; Lenze, D.; et al. Evaluation of a worldwide EQA scheme for complex clonality analysis of clinical lymphoproliferative cases demonstrates a learning effect. Virchows Arch. 2021, 479, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Tack, V.; Ligtenberg, M.J.L.; Tembuyser, L.; Normanno, N.; Vander Borght, S.; van Krieken, H.J.; Dequeker, E.M. External Quality Assessment Unravels Interlaboratory Differences in Quality of RAS Testing for Anti-EGFR Therapy in Colorectal Cancer. Oncologist 2015, 20, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Bellon, E.; Ligtenberg, M.J.; Tejpar, S.; Cox, K.; de Hertogh, G.; de Stricker, K.; Edsjö, A.; Gorgoulis, V.; Höfler, G.; Jung, A.; et al. External quality assessment for KRAS testing is needed: Setup of a European program and report of the first joined regional quality assessment rounds. Oncologist 2011, 16, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Overview of External Quality Assessment (EQA). Available online: https://cdn.who.int/media/docs/default-source/essential-medicines/norms-and-standards/10-b-eqa-contents.pdf?sfvrsn=181d9a32_4&download=true (accessed on 7 June 2022).
- Johnson, P.; Cabuang, L. Proficiency testing and ring trials. Rev. Sci. Tech. 2021, 40, 189–203. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Ames, D.; Lopez, B.; Still, R.; Simpson, W.; Twomey, P. External quality assessment: Best practice. J. Clin. Pathol. 2014, 67, 651–655. [Google Scholar] [CrossRef] [PubMed]
- NordiQC. Immunohistochemical Quality Control. Available online: https://www.nordiqc.org/ (accessed on 7 June 2022).
- Biomedical Quality Assurance, KU Leuven. Available online: https://eqascheme.org/ (accessed on 7 June 2022).
- The College of American Pathologists. Available online: https://www.cap.org/laboratory-improvement/proficiency-testing (accessed on 7 June 2022).
- The European Molecular Genetics Quality Network. Available online: https://www.emqn.org/ (accessed on 7 June 2022).
- ISO/IEC17043:2010. Conformity Assessment—General Requirements for Proficiency Testing. The International Organization for Standardization: Geneva, Switzerland, 2022.
- Dufraing, K.; Fenizia, F.; Torlakovic, E.; Wolstenholme, N.; Deans, Z.C.; Rouleau, E.; Vyberg, M.; Parry, S.; Schuuring, E.; Dequeker, E.M.C. Biomarker testing in oncology—Requirements for organizing external quality assessment programs to improve the performance of laboratory testing: Revision of an expert opinion paper on behalf of IQNPath ABSL. Virchows Arch. 2021, 478, 553–565. [Google Scholar] [CrossRef] [PubMed]
- ISO15189:2012. Medical Laboratories—Requirements for Quality and Competence. The International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO/IEC17025:2017. General Requirements for the Competence of Testing and Calibration Laboratories. The International Organization for Standardization: Geneva, Switzerland, 2022.
- Keppens, C.; Dequeker, E.M.; Pauwels, P.; Ryska, A.; Hart, N.; von der Thüsen, J.H. PD-L1 immunohistochemistry in non-small-cell lung cancer: Unraveling differences in staining concordance and interpretation. Virchows Arch. 2021, 478, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Keppens, C.; von der Thüsen, J.; Pauwels, P.; Ryska, A.; Hart, N.; Schuuring, E.; Miller, K.; Thunnissen, E.; Zwaenepoel, K.; Dequeker, E.M.C. Staining Performance of ALK and ROS1 Immunohistochemistry and Influence on Interpretation in Non-Small-Cell Lung Cancer. J. Mol. Diagn. 2020, 22, 1438–1452. [Google Scholar] [CrossRef]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; van de Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 2020, 6, 17. [Google Scholar] [CrossRef]
- Dufraing, K.; van Krieken, J.H.; De Hertogh, G.; Hoefler, G.; Oniscu, A.; Kuhlmann, T.P.; Weichert, W.; Marchiò, C.; Ristimäki, A.; Ryška, A.; et al. Neoplastic cell percentage estimation in tissue samples for molecular oncology: Recommendations from a modified Delphi study. Histopathology 2019, 75, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Jurmeister, P.; Vollbrecht, C.; Jöhrens, K.; Aust, D.; Behnke, A.; Stenzinger, A.; Penzel, R.; Endris, V.; Schirmacher, P.; Fisseler-Eckhoff, A.; et al. Status quo of ALK testing in lung cancer: Results of an EQA scheme based on in-situ hybridization, immunohistochemistry, and RNA/DNA sequencing. Virchows Arch. 2021, 479, 247–255. [Google Scholar] [CrossRef]
- Horn, L.; Whisenant, J.G.; Wakelee, H.; Reckamp, K.L.; Qiao, H.; Leal, T.A.; Du, L.; Hernandez, J.; Huang, V.; Blumenschein, G.R.; et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J. Thorac. Oncol. 2019, 14, 1901–1911. [Google Scholar] [CrossRef]
- Buchta, C.; Görzer, I.; Chiba, P.; Camp, J.V.; Holzmann, H.; Puchhammer-Stöckl, E.; Mayerhofer, M.; Müller, M.M.; Aberle, S.W. Variability of cycle threshold values in an external quality assessment scheme for detection of the SARS-CoV-2 virus genome by RT-PCR. Clin. Chem. Lab. Med. 2020, 59, 987–994. [Google Scholar] [CrossRef]
- Ast, V.; Costina, V.; Eichner, R.; Bode, A.; Aida, S.; Gerhards, C.; Thiaucourt, M.; Dobler, G.; Geilenkeuser, W.J.; Wölfel, R.; et al. Assessing the Quality of Serological Testing in the COVID-19 Pandemic: Results of a European External Quality Assessment (EQA) Scheme for Anti-SARS-CoV-2 Antibody Detection. J. Clin. Microbiol. 2021, 59, e0055921. [Google Scholar] [CrossRef]
- Haselmann, V.; Özçürümez, M.K.; Klawonn, F.; Ast, V.; Gerhards, C.; Eichner, R.; Costina, V.; Dobler, G.; Geilenkeuser, W.J.; Wölfel, R.; et al. Results of the first pilot external quality assessment (EQA) scheme for anti-SARS-CoV2-antibody testing. Clin. Chem. Lab. Med. 2020, 58, 2121–2130. [Google Scholar] [CrossRef]
- Weng, B.; Li, X. An external quality assessment scheme for prenatal detection of rare chromosomal abnormalities. Clin. Chim. Acta 2012, 413, 1721–1724. [Google Scholar] [CrossRef]
- Kristensen, G.B.; Aakre, K.M.; Kristoffersen, A.H.; Sandberg, S. How to conduct External Quality Assessment Schemes for the pre-analytical phase? Biochem. Med. 2014, 24, 114–122. [Google Scholar] [CrossRef]
- Malentacchi, F.; Pazzagli, M.; Simi, L.; Orlando, C.; Wyrich, R.; Hartmann, C.C.; Verderio, P.; Pizzamiglio, S.; Ciniselli, C.M.; Tichopad, A.; et al. SPIDIA-DNA: An External Quality Assessment for the pre-analytical phase of blood samples used for DNA-based analyses. Clin. Chim. Acta 2013, 424, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Malentacchi, F.; Pizzamiglio, S.; Ibrahim-Gawel, H.; Pazzagli, M.; Verderio, P.; Ciniselli, C.M.; Wyrich, R.; Gelmini, S. Second SPIDIA-DNA External Quality Assessment (EQA): Influence of pre-analytical phase of blood samples on genomic DNA quality. Clin. Chim. Acta 2016, 454, 10–14. [Google Scholar] [CrossRef]
- Thunnissen, E.; Bubendorf, L.; Dietel, M.; Elmberger, G.; Kerr, K.; Lopez-Rios, F.; Moch, H.; Olszewski, W.; Pauwels, P.; Penault-Llorca, F.; et al. EML4-ALK testing in non-small cell carcinomas of the lung: A review with recommendations. Virchows Arch. 2012, 461, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Parry, S.; Wilkinson, D.; Bilbe, N.; Allen, D.; Forrest, S.; Maxwell, P.; O’Grady, A.; Starczynski, J.; Tanier, P.; et al. ALK Immunohistochemistry in NSCLC: Discordant Staining Can Impact Patient Treatment Regimen. J. Thorac. Oncol. 2016, 11, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Barberis, M.; Papotti, M.; Rossi, G.; Franco, R.; Malatesta, S.; Buttitta, F.; Ardizzoni, A.; Crinò, L.; Gridelli, C.; et al. ALK rearrangement testing by FISH analysis in non-small-cell lung cancer patients: Results of the first italian external quality assurance scheme. J. Thorac. Oncol. 2014, 9, 1470–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tembuyser, L.; Dequeker, E.M. Endorsing good quality assurance practices in molecular pathology: Risks and recommendations for diagnostic laboratories and external quality assessment providers. Virchows Arch. 2016, 468, 31–41. [Google Scholar] [CrossRef] [PubMed]
- CF Network. Available online: http://cf.eqascheme.org/ (accessed on 7 June 2022).
- UK NEQAS. Available online: https://ukneqas.org.uk/ (accessed on 7 June 2022).
- Dequeker, E.; Stuhrmann, M.; Morris, M.A.; Casals, T.; Castellani, C.; Claustres, M.; Cuppens, H.; des Georges, M.; Ferec, C.; Macek, M.; et al. Best practice guidelines for molecular genetic diagnosis of cystic fibrosis and CFTR-related disorders—Updated European recommendations. Eur. J. Hum. Genet. 2009, 17, 51–65. [Google Scholar] [CrossRef]
- Langerak, A.W.; Groenen, P.J.; Brüggemann, M.; Beldjord, K.; Bellan, C.; Bonello, L.; Boone, E.; Carter, G.I.; Catherwood, M.; Davi, F.; et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012, 26, 2159–2171. [Google Scholar] [CrossRef]
- Regulation (EC) No 765/2008 of the European Parliament and of the Council of 9 July 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R0765&from=NL (accessed on 7 June 2022).
- Sciensano. Available online: https://www.sciensano.be/en (accessed on 7 June 2022).
- Laudus, N.; Audrézet, M.P.; Girodon, E.; Morris, M.A.; Radojkovic, D.; Raynal, C.; Seia, M.; Štambergová, A.; Torkler, H.; Yamamoto, R.; et al. Laboratory reporting on the clinical spectrum of CFTR p.Arg117His: Still room for improvement. J. Cyst. Fibros. 2020, 19, 969–974. [Google Scholar] [CrossRef]
- Armand, M.; Derrieux, C.; Beldjord, K.; Wabeke, T.; Lenze, D.; Boone, E.; Bruggemann, M.; Evans, P.; Gameiro, P.; Hummel, M.; et al. A New and Simple TRG Multiplex PCR Assay for Assessment of T-cell Clonality: A Comparative Study from the EuroClonality Consortium. HemaSphere 2019, 3, e255. [Google Scholar] [CrossRef]
- Kirchner, M.; Glade, J.; Lehmann, U.; Merkelbach-Bruse, S.; Hummel, M.; Lehmann, A.; Trautmann, M.; Kumbrink, J.; Jung, A.; Dietmaier, W.; et al. NTRK testing: First results of the QuiP-EQA scheme and a comprehensive map of NTRK fusion variants and their diagnostic coverage by targeted RNA-based NGS assays. Genes Chromosomes Cancer 2020, 59, 445–453. [Google Scholar] [CrossRef]
- Gen&tiss. Available online: http://www.genetiss.org/ (accessed on 7 June 2022).
- Miller, W.G.; Jones, G.R.; Horowitz, G.L.; Weykamp, C. Proficiency testing/external quality assessment: Current challenges and future directions. Clin. Chem. 2011, 57, 1670–1680. [Google Scholar] [CrossRef] [Green Version]
- Berwouts, S.; Girodon, E.; Schwarz, M.; Stuhrmann, M.; Morris, M.A.; Dequeker, E. Improvement of interpretation in cystic fibrosis clinical laboratory reports: Longitudinal analysis of external quality assessment data. Eur. J. Hum. Genet. 2012, 20, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Keppens, C.; Tack, V.; Hart, N.; Tembuyser, L.; Ryska, A.; Pauwels, P.; Zwaenepoel, K.; Schuuring, E.; Cabillic, F.; Tornillo, L.; et al. A stitch in time saves nine: External quality assessment rounds demonstrate improved quality of biomarker analysis in lung cancer. Oncotarget 2018, 9, 20524–20538. [Google Scholar] [CrossRef] [Green Version]
- Tack, V.; Schuuring, E.; Keppens, C.; Hart, N.; Pauwels, P.; van Krieken, H.; Dequeker, E.M.C. Accreditation, setting and experience as indicators to assure quality in oncology biomarker testing laboratories. Br. J. Cancer 2018, 119, 605–614. [Google Scholar] [CrossRef]
- Keppens, C.; Dufraing, K.; van Krieken, H.J.; Siebers, A.G.; Kafatos, G.; Lowe, K.; Demonty, G.; Dequeker, E.M.C. European follow-up of incorrect biomarker results for colorectal cancer demonstrates the importance of quality improvement projects. Virchows Arch. 2019, 475, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.C.; Hill, R.H.; Black, R.S.; Winkelman, J.; Tholen, D. CDC 2008. Review of Proficiency Testing Services for Clinical Laboratories in the United States—Final Report of a Technical Working; Battelle Memorial Institute: Columbus, OH, USA, 2008. [Google Scholar]
- Dufraing, K.; De Hertogh, G.; Tack, V.; Keppens, C.; Dequeker, E.M.C.; van Krieken, J.H. External Quality Assessment Identifies Training Needs to Determine the Neoplastic Cell Content for Biomarker Testing. J. Mol. Diagn. 2018, 20, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Smits, A.J.; Kummer, J.A.; de Bruin, P.C.; Bol, M.; van den Tweel, J.G.; Seldenrijk, K.A.; Willems, S.M.; Offerhaus, G.J.; de Weger, R.A.; van Diest, P.J.; et al. The estimation of tumor cell percentage for molecular testing by pathologists is not accurate. Mod. Pathol. 2014, 27, 168–174. [Google Scholar] [CrossRef]
- Mikubo, M.; Seto, K.; Kitamura, A.; Nakaguro, M.; Hattori, Y.; Maeda, N.; Miyazaki, T.; Watanabe, K.; Murakami, H.; Tsukamoto, T.; et al. Calculating the Tumor Nuclei Content for Comprehensive Cancer Panel Testing. J. Thorac. Oncol. 2020, 15, 130–137. [Google Scholar] [CrossRef]
- Denkert, C.; Wienert, S.; Poterie, A.; Loibl, S.; Budczies, J.; Badve, S.; Bago-Horvath, Z.; Bane, A.; Bedri, S.; Brock, J.; et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod. Pathol. 2016, 29, 1155–1164. [Google Scholar] [CrossRef]
- Buchta, C.; Coucke, W.; Mayr, W.R.; Müller, M.M.; Oeser, R.; Schweiger, C.R.; Körmöczi, G.F. Evidence for the positive impact of ISO 9001 and ISO 15189 quality systems on laboratory performance—Evaluation of immunohaematology external quality assessment results during 19 years in Austria. Clin. Chem. Lab. Med. 2018, 56, 2039–2046. [Google Scholar] [CrossRef]
- Deans, Z.C.; Bilbe, N.; O’Sullivan, B.; Lazarou, L.P.; de Castro, D.G.; Parry, S.; Dodson, A.; Taniere, P.; Clark, C.; Butler, R. Improvement in the quality of molecular analysis of EGFR in non-small-cell lung cancer detected by three rounds of external quality assessment. J. Clin. Pathol. 2013, 66, 319–325. [Google Scholar] [CrossRef]
- Keppens, C.; Dequeker, E.M.C.; Rouleau, E.; Hart, N.; Bubendorf, L.; Dufraing, K.; Garrec, C.; Guéguen, P.; Lamy, A.; Marchetti, A.; et al. Sensitive detection methods are key to identify secondary EGFR c.2369C>T p.(Thr790Met) in non-small cell lung cancer tissue samples. BMC Cancer 2020, 20, 366. [Google Scholar] [CrossRef]
- Nielsen, S. External quality assessment for immunohistochemistry: Experiences from NordiQC. Biotech. Histochem. 2015, 90, 331–340. [Google Scholar] [CrossRef]
- Vyberg, M.; Nielsen, S. Proficiency testing in immunohistochemistry—Experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 2016, 468, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Lantuejoul, S.; Sound-Tsao, M.; Cooper, W.A.; Girard, N.; Hirsch, F.R.; Roden, A.C.; Lopez-Rios, F.; Jain, D.; Chou, T.Y.; Motoi, N.; et al. PD-L1 Testing for Lung Cancer in 2019: Perspective From the IASLC Pathology Committee. J. Thorac. Oncol. 2020, 15, 499–519. [Google Scholar] [CrossRef]
- Torlakovic, E.; Lim, H.J.; Adam, J.; Barnes, P.; Bigras, G.; Chan, A.W.H.; Cheung, C.C.; Chung, J.H.; Couture, C.; Fiset, P.O.; et al. “Interchangeability” of PD-L1 immunohistochemistry assays: A meta-analysis of diagnostic accuracy. Mod. Pathol. 2020, 33, 4–17. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Van Krieken, J.H.; Normanno, N.; Blackhall, F.; Boone, E.; Botti, G.; Carneiro, F.; Celik, I.; Ciardiello, F.; Cree, I.A.; Deans, Z.C.; et al. Guideline on the requirements of external quality assessment programs in molecular pathology. Virchows Arch. 2013, 462, 27–37. [Google Scholar] [CrossRef]
- The European Society of Pathology. Available online: https://www.esp-pathology.org/ (accessed on 7 June 2022).
- Tack, V.; Deans, Z.C.; Wolstenholme, N.; Patton, S.; Dequeker, E.M. What’s in a Name? A Coordinated Approach toward the Correct Use of a Uniform Nomenclature to Improve Patient Reports and Databases. Hum. Mutat. 2016, 37, 570–575. [Google Scholar] [CrossRef] [Green Version]
- Cree, I.; Deans, Z.; Ligtenberg, M.; Normanno, N.; Edsjö, A.; Rouleau, E.; Solé, F.; Thunnissen, E.; Timens, W.; Schuuring, E.; et al. Guidance for laboratories performing molecular pathology for cancer patients. J. Clin. Pathol. 2014, 67, 923–931. [Google Scholar] [CrossRef] [Green Version]
- The College of American Pathologists. Molecular Pathology Checklist. Available online: https://elss.cap.org/elss/ShowProperty?nodePath=/UCMCON/Contribution%20Folders/DctmContent/education/OnlineCourseContent/2017/LAP-TLTM/checklists/cl-mol.pdf (accessed on 7 June 2022).
- Van Krieken, J.H.; Jung, A.; Kirchner, T.; Carneiro, F.; Seruca, R.; Bosman, F.T.; Quirke, P.; Fléjou, J.F.; Plato, H.T.; de Hertogh, G.; et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: Proposal for an European quality assurance program. Virchows Arch. 2008, 453, 417–431. [Google Scholar] [CrossRef] [Green Version]
- Bubendorf, L.; Büttner, R.; Al-Dayel, F.; Dietel, M.; Elmberger, G.; Kerr, K.; López-Ríos, F.; Marchetti, A.; Öz, B.; Pauwels, P.; et al. Testing for ROS1 in non-small cell lung cancer: A review with recommendations. Virchows Arch. 2016, 469, 489–503. [Google Scholar] [CrossRef]
- Gulley, M.L.; Braziel, R.M.; Halling, K.C.; Hsi, E.D.; Kant, J.A.; Nikiforova, M.N.; Nowak, J.A.; Ogino, S.; Oliveira, A.; Polesky, H.F.; et al. Clinical laboratory reports in molecular pathology. Arch. Pathol. Lab. Med. 2007, 131, 852–863. [Google Scholar] [CrossRef] [PubMed]
- HUGO Gene Nomenclature Committee. Available online: https://www.genenames.org/ (accessed on 7 June 2022).
- The Human Genome Variation Society. Available online: https://varnomen.hgvs.org/ (accessed on 7 June 2022).
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Moncur, J.T.; Bartley, A.N.; Bridge, J.A.; Kamel-Reid, S.; Lazar, A.J.; Lindeman, N.I.; Long, T.A.; Merker, J.D.; Rai, A.J.; Rimm, D.L.; et al. Performance Comparison of Different Analytic Methods in Proficiency Testing for Mutations in the BRAF, EGFR, and KRAS Genes: A Study of the College of American Pathologists Molecular Oncology Committee. Arch. Pathol. Lab. Med. 2019, 143, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.S.; Bartley, A.N.; Bridge, J.A.; Kamel-Reid, S.; Lazar, A.J.; Lindeman, N.I.; Long, T.A.; Merker, J.D.; Rai, A.J.; Rimm, D.L.; et al. Comparison of Laboratory-Developed Tests and FDA-Approved Assays for BRAF, EGFR, and KRAS Testing. JAMA Oncol. 2018, 4, 838–841. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Salgado, R.; Lively, T.; Sweep, F.; Schuh, A. A risk-management approach for effective integration of biomarkers in clinical trials: Perspectives of an NCI, NCRI, and EORTC working group. Lancet Oncol. 2014, 15, e184–e193. [Google Scholar] [CrossRef]
- Brunnström, H.; Johansson, A.; Westbom-Fremer, S.; Backman, M.; Djureinovic, D.; Patthey, A.; Isaksson-Mettävainio, M.; Gulyas, M.; Micke, P. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: Inter-pathologist variability is higher than assay variability. Mod. Pathol. 2017, 30, 1411–1421. [Google Scholar] [CrossRef] [Green Version]
- NHS England. Pathology Quality Assurance Review; NHS England: Leeds, UK, 2014.
- International TILS Working Group. Available online: https://www.tilsinbreastcancer.org/ (accessed on 7 June 2022).
- Burthem, J.; Brereton, M.; Ardern, J.; Hickman, L.; Seal, L.; Serrant, A.; Hutchinson, C.V.; Wells, E.; McTaggart, P.; De la Salle, B.; et al. The use of digital ‘virtual slides’ in the quality assessment of haematological morphology: Results of a pilot exercise involving UK NEQAS(H) participants. Br. J. Haematol. 2005, 130, 293–296. [Google Scholar] [CrossRef]
- Yu, K.H.; Zhang, C.; Berry, G.J.; Altman, R.B.; Ré, C.; Rubin, D.L.; Snyder, M. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun. 2016, 7, 12474. [Google Scholar] [CrossRef] [Green Version]
- Akbar, S.; Peikari, M.; Salama, S.; Panah, A.Y.; Nofech-Mozes, S.; Martel, A.L. Automated and Manual Quantification of Tumour Cellularity in Digital Slides for Tumour Burden Assessment. Sci. Rep. 2019, 9, 14099. [Google Scholar] [CrossRef] [Green Version]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology—New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, D.Y. Artificial intelligence and computational pathology. Lab. Investig. 2021, 101, 412–422. [Google Scholar] [CrossRef]
- Colling, R.; Pitman, H.; Oien, K.; Rajpoot, N.; Macklin, P.; Snead, D.; Sackville, T.; Verrill, C. Artificial intelligence in digital pathology: A roadmap to routine use in clinical practice. J. Pathol. 2019, 249, 143–150. [Google Scholar] [CrossRef]
- Badrick, T.; Stavelin, A. Harmonising EQA schemes the next frontier: Challenging the status quo. Clin. Chem. Lab. Med. 2020, 58, 1795–1797. [Google Scholar] [CrossRef]
| EQA Provider | National versus International | Country of Headquarters | Use | Goals of EQA Types Organized by this Provider | Type of Sample Used | Genes Tested | ISO 17043 Accredited | Methods Assessed | References |
|---|---|---|---|---|---|---|---|---|---|
| ESP | International: worldwide | Belgium | Expertise & Research |
| Patient samples (FFPE) |
| Unclear | IHC, FISH, DRT, DNA analysis (PCR and NGS) | https://www.esp-pathology.org/ |
| EMQN | International: worldwide | United Kingdom | Expertise |
| Many types of samples | Many genes in many types of pathology | Some EQA schemes | Many methods assessed | https://www.emqn.org/eqa-scheme-catalogue/ |
| CF Network | International: worldwide | Belgium | Expertise & research |
| Patient sample (DNA) | CFTR | Yes | DNA analysis (PCR and NGS) | http://cf.eqascheme.org/ |
| EuroClonality | International: worldwide | The Netherlands | Expertise |
| Patient sample (DNA) | IG and TR clonal expansion | Yes | DNA analysis (PCR) | https://euroclonality.org/eqa-scheme |
| UK NEQAS | International: worldwide | United Kingdom | Expertise |
| Many types of samples | Many genes in many types of pathology | Some EQA schemes | Many methods assessed | https://ukneqas.org.uk/programmes/ |
| RCPAQAP | International: worldwide | Australia | Expertise & research |
| Many types of samples | Many genes in many types of pathology | Some EQA schemes | Many methods assessed | https://rcpaqap.com.au/products/ |
| Sciensano | National: Belgium | Belgium | Policy |
| Digital and artificial samples (DNA) | Many genes in many types of pathology | Yes | Many methods assessed | https://www.sciensano.be/en/about-sciensano/sciensanos-organogram/quality-laboratories/external-quality-assessment#want-to-know-more- and https://www.wiv-isp.be/QML/index_nl.htm |
| Gen&Tiss | National: France | France | Policy & research |
| Patient and artificial samples (DNA, ctDNA) | EGFR, KRAS, BRAF, NRAS, PIK3CA, MSI, ERBB2, KIT in different types of cancers | Yes | DNA analysis (PCR and NGS) and ctDNA analysis | http://www.genetiss.org/ |
| CAP | National: United States of America | United States of America | Expertise |
| Many types of samples | Many genes in many types of pathology | Yes | Many methods assessed | https://www.cap.org/laboratory-improvement/proficiency-testing |
| CIQC (split in CPQA-AQCP and CBQA-PCAB) | National: Canada | Canada | Expertise, policy & research |
| Origin unclear (DNA, FFPE) | BRCA, EVER, P16, NTRK, PD-L1, HER2 and more | No | IHC, FISH, DNA analysis (PCR and NGS) | https://www.cpqa.ca/ and www.cbqa.ca |
| NCCL | National: China | China | Policy |
| Unclear | Unclear | Yes | Many methods assessed | https://www.nccl.org.cn/planEn |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudus, N.; Nijs, L.; Nauwelaers, I.; Dequeker, E.M.C. The Significance of External Quality Assessment Schemes for Molecular Testing in Clinical Laboratories. Cancers 2022, 14, 3686. https://doi.org/10.3390/cancers14153686
Laudus N, Nijs L, Nauwelaers I, Dequeker EMC. The Significance of External Quality Assessment Schemes for Molecular Testing in Clinical Laboratories. Cancers. 2022; 14(15):3686. https://doi.org/10.3390/cancers14153686
Chicago/Turabian StyleLaudus, Nele, Lynn Nijs, Inne Nauwelaers, and Elisabeth M. C. Dequeker. 2022. "The Significance of External Quality Assessment Schemes for Molecular Testing in Clinical Laboratories" Cancers 14, no. 15: 3686. https://doi.org/10.3390/cancers14153686





