Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Immune Checkpoint Inhibitors in Cancer Immunotherapy
2.1. PD-1/PD-L1
2.2. CTLA-4
2.3. CD137 and CD47
2.4. Limitations in Targeting GBM by Immune Checkpoint Inhibitors
2.4.1. Immune-Microenvironment in the CNS and Local Delivery of Immunotherapeutics
2.4.2. The Proposed Solution: The Effector Immune Cells Reaching the Tumor Area
2.4.3. Immunosuppressive Nature of GBM
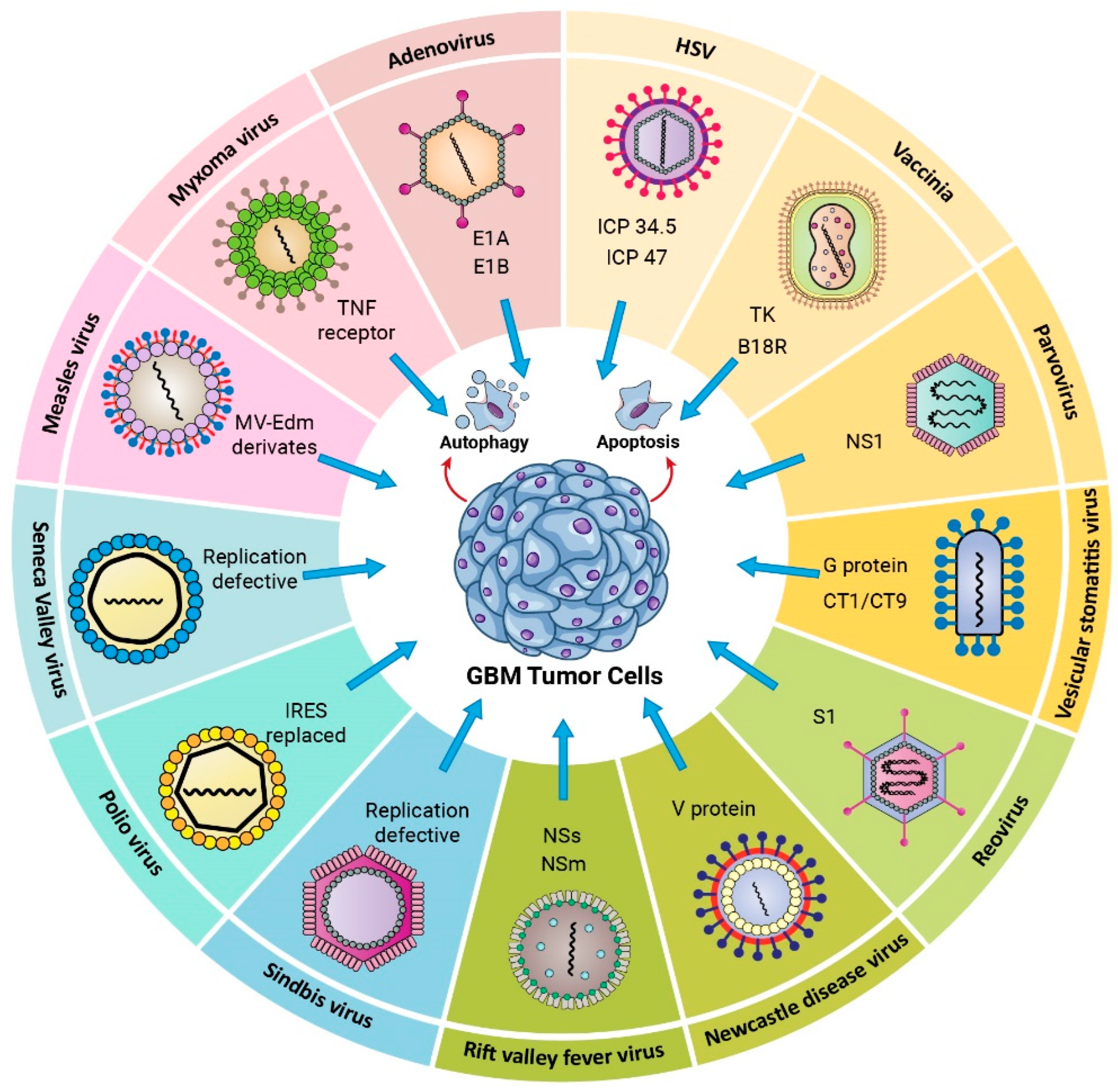
3. Oncolytic Viruses
3.1. Viruses Proposed as Glioma Oncolytic Agents
3.1.1. DNA Viruses
Herpes Simplex Virus Type I
Adenovirus
Vaccinia Virus (VV)
Myxoma
Parvovirus
3.1.2. RNA Viruses
Measles
Vesicular Stomatitis Virus (VSV)
Reovirus
Newcastle Disease Virus (NDV)
Seneca Valley Virus Isolate 001 (SVV-001)
Poliovirus
Sindbis
Rift Valley Fever Virus (RVFV)
3.2. Oncolytic Viruses Expressing Immunomodulatory Transgenes
3.2.1. Interleukins
3.2.2. TRAIL and Flt3L
3.2.3. Immune Checkpoint Inhibitors
3.2.4. Immune Stimulators
3.2.5. E-Cadherin

3.3. Limitations of Targeting GBM by Oncolytic Viruses and Possible Solutions
3.3.1. Innate Antiviral Response
Proposed Solution: Genetic Engineering
Proposed Solution: MicroRNA
3.3.2. Virus Delivery
The Proposed Solution: Intra-Tumoral Administration
The Proposed Solution: Promote CNS Tropism
3.3.3. Targeting Autophagy to Enhance Oncolytic Virus-Based Cancer Therapy
4. GBM–Immunotherapy–Bioengineering
4.1. Engineering and Biology: Two Pairs of Eyes Are Better Than One
4.2. Nanomedicine and Glioblastoma Immunotherapy
4.2.1. Different Types of Nanomaterials with Applications in GBM Treatment
Lipid-Based Nanoparticles
Exosomes
Polymeric Nanoparticles
Injectable Hydrogels
DNA-Based Nanocarriers
Other Types of Nanomaterials
4.2.2. Nanomaterials as Carrier for Virus Compounds
4.3. Three-Dimensional Bioprinted Platforms and GBM Immunotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzegar Behrooz, A.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/mTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastghaib, S.; Shojaei, S.; Mostafavi-Pour, Z.; Sharma, P.; Patterson, J.B.; Samali, A.; Mokarram, P.; Ghavami, S. Simvastatin induces unfolded protein response and enhances temozolomide-induced cell death in glioblastoma cells. Cells 2020, 9, 2339. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Nandhu, M.S.; Behera, P.; Chiocca, E.A.; Viapiano, M.S. Strategies in gene therapy for glioblastoma. Cancers 2013, 5, 1271–1305. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Lim, M.; Hafler, D.A.; Reardon, D.A.; Sampson, J.H. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat. Rev. Neurol. 2015, 11, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrível, M.; Gromicho, C.; Matos, A.M. Oncolytic viruses: What to expect from their use in cancer treatment. Microbiol. Immunol. 2020, 64, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef]
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Boussiotis, V.A.; Chatterjee, P.; Li, L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014, 20, 265. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [Green Version]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Twomey, J.D.; Zhang, B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Chang, E.; Pelosof, L.; Lemery, S.; Gong, Y.; Goldberg, K.B.; Farrell, A.T.; Keegan, P.; Veeraraghavan, J.; Wei, G.; Blumenthal, G.M. Systematic review of PD-1/PD-L1 inhibitors in oncology: From personalized medicine to public health. Oncol. 2021, 26, e1786–e1799. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 2008, 13, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Khattri, R.; Auger, J.A.; Griffin, M.D.; Sharpe, A.H.; Bluestone, J.A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 1999, 162, 5784–5791. [Google Scholar]
- Mitsuiki, N.; Schwab, C.; Grimbacher, B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol. Rev. 2019, 287, 33–49. [Google Scholar] [CrossRef]
- Jaberipour, M.; Habibagahi, M.; Hosseini, A.; Habibabad, S.R.; Talei, A.; Ghaderi, A. Increased CTLA-4 and FOXP3 transcripts in peripheral blood mononuclear cells of patients with breast cancer. Pathol. Oncol. Res. 2010, 16, 547–551. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Ouyang, W.; Lo, B.; Deenick, E.K.; Niemela, J.E.; Avery, D.T.; Schickel, J.-N.; Tran, D.Q.; Stoddard, J.; Zhang, Y. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014, 345, 1623–1627. [Google Scholar] [CrossRef] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Romano, E.; Kusio-Kobialka, M.; Foukas, P.G.; Baumgaertner, P.; Meyer, C.; Ballabeni, P.; Michielin, O.; Weide, B.; Romero, P.; Speiser, D.E. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc. Natl. Acad. Sci. USA 2015, 112, 6140–6145. [Google Scholar] [CrossRef] [Green Version]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Q.; Dong, M.; Guo, D.; Wu, X.; Wang, B. Glioblastoma immunotherapy targeting the innate immune checkpoint CD47-SIRPα axis. Front. Immunol. 2020, 11, 593219. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3. [Google Scholar]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Lossinsky, A.S.; Shivers, R. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol. Histopathol. 2004, 19, 535–564. [Google Scholar]
- Sabbagh, A.; Beccaria, K.; Ling, X.; Marisetty, A.; Ott, M.; Caruso, H.; Barton, E.; Kong, L.-Y.; Fang, D.; Latha, K. Opening of the blood–brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin. Cancer Res. 2021, 27, 4325–4337. [Google Scholar] [CrossRef]
- Idbaih, A.; Ducray, F.; Stupp, R.; Baize, N.; Chinot, O.L.; De Groot, J.F.; Guyotat, J.; Sonabend, A.M.; Menei, P.; Dufour, H. A phase I/IIa study to evaluate the safety and efficacy of blood-brain barrier (BBB) opening with the SonoCloud-9 implantable ultrasound device in recurrent glioblastoma patients receiving IV carboplatin. J. Clin. Oncol. 2021, 39, 2049. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, re342–re343. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuntova, P.; Chow, F.; Watchmaker, P.B.; Galvez, M.; Heimberger, A.B.; Newell, E.W.; Diaz, A.; DePinho, R.A.; Li, M.O.; Wherry, E.J. Unique challenges for glioblastoma immunotherapy—Discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank. Neuro-Oncol. 2021, 23, 356–375. [Google Scholar] [CrossRef]
- Müller, S.; Kohanbash, G.; Liu, S.J.; Alvarado, B.; Carrera, D.; Bhaduri, A.; Watchmaker, P.B.; Yagnik, G.; Di Lullo, E.; Malatesta, M. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017, 18, 234. [Google Scholar] [CrossRef]
- Mu, X.; Shi, W.; Xu, Y.; Xu, C.; Zhao, T.; Geng, B.; Yang, J.; Pan, J.; Hu, S.; Zhang, C. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle 2018, 17, 428–438. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Kipnis, J. Bypassing the blood-brain barrier. Science 2019, 366, 1448–1449. [Google Scholar] [CrossRef]
- Rutledge, W.C.; Kong, J.; Gao, J.; Gutman, D.A.; Cooper, L.A.D.; Appin, C.; Park, Y.; Scarpace, L.; Mikkelsen, T.; Cohen, M.L. Tumor-Infiltrating Lymphocytes in Glioblastoma Are Associated with Specific Genomic Alterations and Related to Transcriptional ClassTumor-Infiltrating Lymphocytes in Glioblastoma. Clin. Cancer Res. 2013, 19, 4951–4960. [Google Scholar] [CrossRef] [Green Version]
- Frederico, S.C.; Hancock, J.C.; Brettschneider, E.E.; Ratnam, N.M.; Gilbert, M.R.; Terabe, M. Making a cold tumor hot: The role of vaccines in the treatment of glioblastoma. Front. Oncol. 2021, 11, 672508. [Google Scholar] [CrossRef]
- Grabowski, M.M.; Sankey, E.W.; Ryan, K.J.; Chongsathidkiet, P.; Lorrey, S.J.; Wilkinson, D.S.; Fecci, P.E. Immune suppression in gliomas. J. Neuro-Oncol. 2021, 151, 3–12. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Liu, X.; Cheng, Q.; Luo, C.; Liu, Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int. 2020, 20, 7. [Google Scholar] [CrossRef]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef] [Green Version]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in GlioblastomaT-Cell Exhaustion Signatures in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef] [Green Version]
- Grossman, S.A.; Ye, X.; Lesser, G.; Sloan, A.; Carraway, H.; Desideri, S.; Piantadosi, S.; Consortium, N.C. Immunosuppression in Patients with High-Grade Gliomas Treated with Radiation and TemozolomideImmunosuppression in High-Grade Gliomas. Clin. Cancer Res. 2011, 17, 5473–5480. [Google Scholar] [CrossRef] [Green Version]
- Huff, W.X.; Bam, M.; Shireman, J.M.; Kwon, J.H.; Song, L.; Newman, S.; Cohen-Gadol, A.A.; Shapiro, S.; Jones, T.; Fulton, K. Aging-and Tumor-Mediated Increase in CD8+ CD28− T Cells Might Impose a Strong Barrier to Success of Immunotherapy in Glioblastoma. ImmunoHorizons 2021, 5, 395–409. [Google Scholar] [CrossRef]
- Didenko, V.V.; Ngo, H.N.; Minchew, C.; Baskin, D.S. Apoptosis of T lymphocytes invading glioblastomas multiforme: A possible tumor defense mechanism. J. Neurosurg. 2002, 96, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Andaloussi, A.E.; Lesniak, M.S. An increase in CD4+ CD25+ FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncol. 2006, 8, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Saas, P.; Walker, P.R.; Hahne, M.; Quiquerez, A.-L.; Schnuriger, V.; Perrin, G.; French, L.; Van Meir, E.G.; de Tribolet, N.; Tschopp, J. Fas ligand expression by astrocytoma in vivo: Maintaining immune privilege in the brain? J. Clin. Investig. 1997, 99, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-cell Dysfunction in Glioblastoma: Applying a New FrameworkT-cell Dysfunction in Glioblastoma. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Mazor, G.; Levin, L.; Picard, D.; Ahmadov, U.; Carén, H.; Borkhardt, A.; Reifenberger, G.; Leprivier, G.; Remke, M.; Rotblat, B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Orzan, F.; De Bacco, F.; Crisafulli, G.; Pellegatta, S.; Mussolin, B.; Siravegna, G.; D’Ambrosio, A.; Comoglio, P.M.; Finocchiaro, G.; Boccaccio, C. Genetic evolution of glioblastoma stem-like cells from primary to recurrent tumor. Stem Cells 2017, 35, 2218–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha-Thakurta, N.; Wierda, W.G. Cerebral edema secondary to chimeric antigen receptor T-cell immunotherapy. Neurology 2018, 91, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coniglio, S.J.; Eugenin, E.; Dobrenis, K.; Stanley, E.R.; West, B.L.; Symons, M.H.; Segall, J.E. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol. Med. 2012, 18, 519–527. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Almahariq, M.F.; Quinn, T.J.; Kesarwani, P.; Kant, S.; Miller, C.R.; Chinnaiyan, P. Inhibition of colony-stimulating factor-1 receptor enhances the efficacy of radiotherapy and reduces immune suppression in glioblastoma. In Vivo 2021, 35, 119–129. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef]
- Kortylewski, M.; Kujawski, M.; Wang, T.; Wei, S.; Zhang, S.; Pilon-Thomas, S.; Niu, G.; Kay, H.; Mulé, J.; Kerr, W.G. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat. Med. 2005, 11, 1314–1321. [Google Scholar] [CrossRef]
- Wei, J.; Wang, F.; Kong, L.-Y.; Xu, S.; Doucette, T.; Ferguson, S.D.; Yang, Y.; McEnery, K.; Jethwa, K.; Gjyshi, O. miR-124 Inhibits STAT3 Signaling to Enhance T Cell–Mediated Immune Clearance of GliomamiRNA-Mediated Glioma Immunosuppression. Cancer Res. 2013, 73, 3913–3926. [Google Scholar] [CrossRef] [Green Version]
- Ott, M.; Kassab, C.; Marisetty, A.; Hashimoto, Y.; Wei, J.; Zamler, D.; Leu, J.-S.; Tomaszowski, K.-H.; Sabbagh, A.; Fang, D. Radiation with STAT3 blockade triggers dendritic cell–T cell interactions in the glioma microenvironment and therapeutic efficacy. Clin. Cancer Res. 2020, 26, 4983–4994. [Google Scholar] [CrossRef]
- Yang, J.; Yao, Y.; Tong, L.; Zhu, Z.; Wang, L.; Yang, J. CD47 is highly expressed in gliomas and targeting CD47 is a promising therapeutic strategy. Eur. J. Inflamm. 2021, 19, 20587392211000899. [Google Scholar] [CrossRef]
- von Roemeling, C.A.; Wang, Y.; Qie, Y.; Yuan, H.; Zhao, H.; Liu, X.; Yang, Z.; Yang, M.; Deng, W.; Bruno, K.A. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun. 2020, 11, 1508. [Google Scholar] [CrossRef] [Green Version]
- Fecci, P.E.; Sweeney, A.E.; Grossi, P.M.; Nair, S.K.; Learn, C.A.; Mitchell, D.A.; Cui, X.; Cummings, T.J.; Bigner, D.D.; Gilboa, E. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin. Cancer Res. 2006, 12, 4294–4305. [Google Scholar] [CrossRef] [Green Version]
- El Andaloussi, A.; Han, Y.; Lesniak, M.S. Prolongation of survival following depletion of CD4+ CD25+ regulatory T cells in mice with experimental brain tumors. J. Neurosurg. 2006, 105, 430–437. [Google Scholar] [CrossRef]
- Sampson, J.H.; Schmittling, R.J.; Archer, G.E.; Congdon, K.L.; Nair, S.K.; Reap, E.A.; Desjardins, A.; Friedman, A.H.; Friedman, H.S.; Herndon, J.E. A pilot study of IL-2Rα blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE 2012, 7, e31046. [Google Scholar] [CrossRef]
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E.; McLendon, R.E.; Mitchell, D.A. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [Green Version]
- Batich, K.A.; Mitchell, D.A.; Healy, P.; Herndon, J.E.; Sampson, J.H. Once, Twice, Three Times a Finding: Reproducibility of Dendritic Cell Vaccine Trials Targeting Cytomegalovirus in GlioblastomaReproducibility of Dendritic Cell Vaccines in Glioblastoma. Clin. Cancer Res. 2020, 26, 5297–5303. [Google Scholar] [CrossRef]
- Yeo, E.C.; Brown, M.P.; Gargett, T.; Ebert, L.M. The role of cytokines and chemokines in shaping the immune microenvironment of glioblastoma: Implications for immunotherapy. Cells 2021, 10, 607. [Google Scholar] [CrossRef]
- Stenken, J.A.; Poschenrieder, A.J. Bioanalytical chemistry of cytokines–a review. Anal. Chim. Acta 2015, 853, 95–115. [Google Scholar] [CrossRef]
- Urbantat, R.M.; Vajkoczy, P.; Brandenburg, S. Advances in chemokine signaling pathways as therapeutic targets in glioblastoma. Cancers 2021, 13, 2983. [Google Scholar] [CrossRef]
- Chen, W.; Xia, T.; Wang, D.; Huang, B.; Zhao, P.; Wang, J.; Qu, X.; Li, X. Human astrocytes secrete IL-6 to promote glioma migration and invasion through upregulation of cytomembrane MMP14. Oncotarget 2016, 7, 62425. [Google Scholar] [CrossRef] [Green Version]
- Wollmann, G.; Ozduman, K.; Van Den Pol, A.N. Oncolytic virus therapy of glioblastoma multiforme–concepts and candidates. Cancer J. 2012, 18, 69. [Google Scholar] [CrossRef]
- Li, J.; Meng, Q.; Zhou, X.; Zhao, H.; Wang, K.; Niu, H.; Wang, Y. Gospel of malignant Glioma: Oncolytic virus therapy. Gene 2022, 818, 146217. [Google Scholar] [CrossRef]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Krummenacher, C.; Nicola, A.V.; Whitbeck, J.C.; Lou, H.; Hou, W.; Lambris, J.D.; Geraghty, R.J.; Spear, P.G.; Cohen, G.H.; Eisenberg, R.J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 1998, 72, 7064–7074. [Google Scholar] [CrossRef] [Green Version]
- Friedman, G.K.; Langford, C.P.; Coleman, J.M.; Cassady, K.A.; Parker, J.N.; Markert, J.M.; Yancey Gillespie, G. Engineered herpes simplex viruses efficiently infect and kill CD133+ human glioma xenograft cells that express CD111. J. Neuro-Oncol. 2009, 95, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, K.; Iwai, M.; Ito, H.; Tanaka, M.; Seto, Y.; Todo, T. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition via dynamic intratumoral immune modulation. Mol. Ther.-Oncolytics 2021, 22, 129–142. [Google Scholar] [CrossRef]
- Sharma, A.; Li, X.; Bangari, D.S.; Mittal, S.K. Adenovirus receptors and their implications in gene delivery. Virus Res. 2009, 143, 184–194. [Google Scholar] [CrossRef]
- Kiyokawa, J.; Wakimoto, H. Preclinical and clinical development of oncolytic adenovirus for the treatment of malignant glioma. Oncolytic Virotherapy 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghchi, C.A.; Zhang, Z.; Alusi, G.; Lemoine, N.R.; Wang, Y. Vaccinia virus, a promising new therapeutic agent for pancreatic cancer. Immunotherapy 2015, 7, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Ma, Y.; Barrett, J.W.; Gao, X.; Loh, J.; Barton, E.; Virgin, H.W.; McFadden, G. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat. Immunol. 2004, 5, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Yang, W.; Alain, T.; Shi, Z.-Q.; Muzik, H.; Barrett, J.W.; McFadden, G.; Bell, J.; Hamilton, M.G.; Senger, D.L. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005, 65, 9982–9990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogbomo, H.; Zemp, F.J.; Lun, X.; Zhang, J.; Stack, D.; Rahman, M.M.; McFadden, G.; Mody, C.H.; Forsyth, P.A. Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS ONE 2013, 8, e66825. [Google Scholar]
- Reuter, D.; Schneider-Schaulies, J. Measles virus infection of the CNS: Human disease, animal models, and approaches to therapy. Med. Microbiol. Immunol. 2010, 199, 261–271. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.-W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef] [Green Version]
- Nikolic, J.; Belot, L.; Raux, H.; Legrand, P.; Gaudin, Y.; Albertini, A.A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 2018, 9, 1029. [Google Scholar] [CrossRef] [Green Version]
- Barber, G.N. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004, 17, 516–527. [Google Scholar] [CrossRef]
- Balachandran, S.; Barber, G.N. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 2000, 50, 135–138. [Google Scholar] [CrossRef]
- Strong, J.E.; Coffey, M.C.; Tang, D.; Sabinin, P.; Lee, P.W.K. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998, 17, 3351–3362. [Google Scholar] [CrossRef] [Green Version]
- Strong, J.E.; Tang, D.; Lee, P.W. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology 1993, 197, 405–411. [Google Scholar] [CrossRef]
- Schirrmacher, V. Signaling through RIG-I and type I interferon receptor: Immune activation by Newcastle disease virus in man versus immune evasion by Ebola virus. Int. J. Mol. Med. 2015, 36, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Morton, C.L.; Houghton, P.J.; Kolb, E.A.; Gorlick, R.; Reynolds, C.P.; Kang, M.H.; Maris, J.M.; Keir, S.T.; Wu, J.; Smith, M.A. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatric Blood Cancer 2010, 55, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.S.; Burroughs, K.D.; Hales, L.M.; Ganesh, S.; Jones, B.H.; Idamakanti, N.; Hay, C.; Li, S.S.; Skele, K.L.; Vasko, A.-J. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J. Natl. Cancer Inst. 2007, 99, 1623–1633. [Google Scholar] [CrossRef]
- Mehndiratta, M.M.; Mehndiratta, P.; Pande, R. Poliomyelitis: Historical facts, epidemiology, and current challenges in eradication. Neurohospitalist 2014, 4, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Gromeier, M.; Alexander, L.; Wimmer, E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. USA 1996, 93, 2370–2375. [Google Scholar] [CrossRef] [Green Version]
- Tseng, J.-C.; Levin, B.; Hirano, T.; Yee, H.; Pampeno, C.; Meruelo, D. In vivo antitumor activity of Sindbis viral vectors. J. Natl. Cancer Inst. 2002, 94, 1790–1802. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-S.; Kuhn, R.J.; Strauss, E.G.; Ou, S.; Strauss, J.H. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 1992, 66, 4992–5001. [Google Scholar] [CrossRef] [Green Version]
- Harmon, B.; Schudel, B.R.; Maar, D.; Kozina, C.; Ikegami, T.; Tseng, C.-T.K.; Negrete, O.A. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J. Virol. 2012, 86, 12954–12970. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized interleukin-12 for cancer immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef]
- Cheema, T.A.; Wakimoto, H.; Fecci, P.E.; Ning, J.; Kuroda, T.; Jeyaretna, D.S.; Martuza, R.L.; Rabkin, S.D. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl. Acad. Sci. USA 2013, 110, 12006–12011. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Fulci, G.; Wakimoto, H.; Cheema, T.A.; Buhrman, J.S.; Jeyaretna, D.S.; Rachamimov, A.O.S.; Rabkin, S.D.; Martuza, R.L. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia 2013, 15, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Passer, B.J.; Cheema, T.; Wu, S.; Wu, C.; Rabkin, S.D.; Martuza, R.L. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013, 20, 17–24. [Google Scholar] [CrossRef]
- Friedman, G.K.; Bernstock, J.D.; Chen, D.; Nan, L.; Moore, B.P.; Kelly, V.M.; Youngblood, S.L.; Langford, C.P.; Han, X.; Ring, E.K. Enhanced sensitivity of patient-derived pediatric high-grade brain tumor xenografts to oncolytic HSV-1 virotherapy correlates with nectin-1 expression. Sci. Rep. 2018, 8, 13930. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Rohena-Rivera, K.; Sanchez-Vazquez, M.M.; Aponte-Colon, D.A.; Forestier-Roman, I.S.; Quintero-Aguilo, M.E.; Martinez-Ferrer, M. IL-15 regulates migration, invasion, angiogenesis and genes associated with lipid metabolism and inflammation in prostate cancer. PLoS ONE 2017, 12, e0172786. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Li, S.; Jia, T.; Du, X.; Xu, Y.; Zhao, Y.; Li, L.; Liang, K.; Liang, W.; Sun, H. Combined therapy with CTL cells and oncolytic adenovirus expressing IL-15-induced enhanced antitumor activity. Tumor Biol. 2015, 36, 4535–4543. [Google Scholar] [CrossRef]
- Barnard, Z.; Wakimoto, H.; Zaupa, C.; Patel, A.P.; Klehm, J.; Martuza, R.L.; Rabkin, S.D.; Curry Jr, W.T. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery 2012, 71, E749–E756. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Jeong, M.; Mushiake, H.; Kim, B.; Kim, W.; Ko, J.; Kim, M.; Kim, M.; Kim, T.; Robbins, P. Cancer gene therapy using a novel secretable trimeric TRAIL. Gene Ther. 2006, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.H.F.; Hu, R.; Lin, H.; Davis, T.; Iliev, D.; Frye, C.; Swedlund, B.; Hansen, K.L.; Vinson, V.L.; Gumpper, K.L. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997, 57, 5221–5225. [Google Scholar] [PubMed]
- Tamura, K.; Wakimoto, H.; Agarwal, A.S.; Rabkin, S.D.; Bhere, D.; Martuza, R.L.; Kuroda, T.; Kasmieh, R.; Shah, K. Multimechanistic tumor targeted oncolytic virus overcomes resistance in brain tumors. Mol. Ther. 2013, 21, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahan, N.; Lee, J.M.; Shah, K.; Wakimoto, H. Therapeutic targeting of chemoresistant and recurrent glioblastoma stem cells with a proapoptotic variant of oncolytic herpes simplex virus. Int. J. Cancer 2017, 141, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Martikainen, M.; Essand, M. Virus-based immunotherapy of glioblastoma. Cancers 2019, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 2017, 32, 253–267.e255. [Google Scholar] [CrossRef] [Green Version]
- Lukas, R.V.; Rodon, J.; Becker, K.; Wong, E.T.; Shih, K.; Touat, M.; Fassò, M.; Osborne, S.; Molinero, L.; O’Hear, C. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neuro-Oncol. 2018, 140, 317–328. [Google Scholar] [CrossRef]
- Passaro, C.; Alayo, Q.; DeLaura, I.; McNulty, J.; Grauwet, K.; Ito, H.; Bhaskaran, V.; Mineo, M.; Lawler, S.E.; Shah, K. Arming an Oncolytic Herpes Simplex Virus Type 1 with a Single-chain Fragment Variable Antibody against PD-1 for Experimental Glioblastoma TherapyA scFvPD1-encoding Oncolytic HSV. Clin. Cancer Res. 2019, 25, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Puigdelloses, M.; Garcia-Moure, M.; Labiano, S.; Laspidea, V.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; De la Nava, D.; Ausejo, I. CD137 and PD-L1 targeting with immunovirotherapy induces a potent and durable antitumor immune response in glioblastoma models. J. Immunother. Cancer 2021, 9, e002644. [Google Scholar] [CrossRef]
- Rivera-Molina, Y.; Jiang, H.; Fueyo, J.; Nguyen, T.; Shin, D.H.; Youssef, G.; Fan, X.; Gumin, J.; Alonso, M.M.; Phadnis, S. GITRL-armed Delta-24-RGD oncolytic adenovirus prolongs survival and induces anti-glioma immune memory. Neuro-Oncol. Adv. 2019, 1, vdz009. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Rivera-Molina, Y.; Gomez-Manzano, C.; Clise-Dwyer, K.; Bover, L.; Vence, L.M.; Yuan, Y.; Lang, F.F.; Toniatti, C.; Hossain, M.B. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer VaccinationCancer Vaccination Induced by Oncolytic Adenovirus. Cancer Res. 2017, 77, 3894–3907. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Ma, R.; Russell, L.; Yoo, J.Y.; Han, J.; Cui, H.; Yi, P.; Zhang, J.; Nakashima, H.; Dai, H. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat. Biotechnol. 2019, 37, 45–54. [Google Scholar] [CrossRef]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, M.; Yu, D.; Dyczynski, M.; Baskaran, S.; Zhang, L.; Lulla, A.; Lulla, V.; Saul, S.; Nelander, S.; Dimberg, A. Safe and Effective Treatment of Experimental Neuroblastoma and Glioblastoma Using Systemically Delivered Triple MicroRNA-Detargeted Oncolytic Semliki Forest VirusSFVmiRT for Treatment of Glioblastoma and Neuroblastoma. Clin. Cancer Res. 2017, 23, 1519–1530. [Google Scholar] [CrossRef] [Green Version]
- Martikainen, M.; Ramachandran, M.; Lugano, R.; Ma, J.; Martikainen, M.-M.; Dimberg, A.; Yu, D.; Merits, A.; Essand, M. IFN-I-tolerant oncolytic Semliki Forest virus in combination with anti-PD1 enhances T cell response against mouse glioma. Mol. Ther.-Oncolytics 2021, 21, 37–46. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R.; Nace, R.A.; Russell, S.J.; Schulze, A.J. MicroRNA-detargeting proves more effective than leader gene deletion for improving safety of oncolytic Mengovirus in a nude mouse model. Mol. Ther.-Oncolytics 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Ferguson, M.S.; Lemoine, N.R.; Wang, Y. Systemic delivery of oncolytic viruses: Hopes and hurdles. Adv. Virol. 2012, 2012, 805629. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R.; Schulze, A.J. Oncolytic viruses for malignant glioma: On the verge of success? Viruses 2021, 13, 1294. [Google Scholar] [CrossRef]
- Rius-Rocabert, S.; García-Romero, N.; García, A.; Ayuso-Sacido, A.; Nistal-Villan, E. Oncolytic virotherapy in glioma tumors. Int. J. Mol. Sci. 2020, 21, 7604. [Google Scholar] [CrossRef]
- Ilett, E.J.; Bárcena, M.; Errington-Mais, F.; Griffin, S.; Harrington, K.J.; Pandha, H.S.; Coffey, M.; Selby, P.J.; Limpens, R.W.; Mommaas, M. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 2011, 17, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Mader, E.K.; Maeyama, Y.; Lin, Y.; Butler, G.W.; Russell, H.M.; Galanis, E.; Russell, S.J.; Dietz, A.B.; Peng, K.-W. Mesenchymal Stem Cell Carriers Protect Oncolytic Measles Viruses from Antibody Neutralization in an Orthotopic Ovarian Cancer Therapy ModelVirotherapy in Immune Mice Using MSC Cell Carriers. Clin. Cancer Res. 2009, 15, 7246–7255. [Google Scholar] [CrossRef] [Green Version]
- Denton, N.L.; Chen, C.-Y.; Scott, T.R.; Cripe, T.P. Tumor-associated macrophages in oncolytic virotherapy: Friend or foe? Biomedicines 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xiang, W.; Yi, D.-Y.; Xue, B.-Z.; Wen, W.-W.; Abdelmaksoud, A.; Xiong, N.-X.; Jiang, X.-B.; Zhao, H.-Y.; Fu, P. Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas. Stem Cell Res. Ther. 2018, 9, 228. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, N.; Lei, D.; Ali, S. Emerging role of oncolytic viruses and stem cells in gene therapy: Should they be integrated? Drug Discov. Today 2022, 27, 2244–2251. [Google Scholar] [CrossRef]
- Schneider-Schaulies, J. Cellular receptors for viruses: Links to tropism and pathogenesis. J. Gen. Virol. 2000, 81, 1413–1429. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Fujita, M.; Miyazaki, Y.; Adachi, A. Viral tropism. Recept.-Indep./-Assoc. Viral Trop. 2012, 4, 281. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, S.; Suresh, M.; Klionsky, D.J.; Labouta, H.I.; Ghavami, S. Autophagy and SARS-CoV-2 infection: A possible smart targeting of the autophagy pathway. Virulence 2020, 11, 805–810. [Google Scholar] [CrossRef]
- Habibzadeh, P.; Dastsooz, H.; Eshraghi, M.; Łos, M.J.; Klionsky, D.J.; Ghavami, S. Autophagy: The Potential Link between SARS-CoV-2 and Cancer. Cancers 2021, 13, 5721. [Google Scholar] [CrossRef]
- Adelipour, M.; Saleth, L.R.; Ghavami, S.; Alagarsamy, K.N.; Dhingra, S.; Allameh, A. The role of autophagy in the metabolism and differentiation of stem cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166412. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Yarjanli, Z.; Pakniya, F.; Bidram, E.; Los, M.J.; Eshraghi, M.; Klionsky, D.J.; Ghavami, S.; Zarrabi, A. Targeting autophagy, oxidative stress, and ER stress for neurodegenerative diseases treatment. J. Control. Release 2022, 345, 147–175. [Google Scholar] [CrossRef]
- Meng, S.; Xu, J.; Wu, Y.; Ding, C. Targeting autophagy to enhance oncolytic virus-based cancer therapy. Expert Opin. Biol. Ther. 2013, 13, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.-T.; Tao, X.-H.; Fan, Y.-B.; Wang, S.-B. Crosstalk between oncolytic viruses and autophagy in cancer therapy. Biomed. Pharmacother. 2021, 134, 110932. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rocha, H.; Gomez-Gutierrez, J.G.; Garcia-Garcia, A.; Rao, X.-M.; Chen, L.; McMasters, K.M.; Zhou, H.S. Adenoviruses induce autophagy to promote virus replication and oncolysis. Virology 2011, 416, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, G.; Passaro, C.; Libertini, S.; Abagnale, A.; Barbato, S.; Maione, A.S.; Hallden, G.; Beguinot, F.; Formisano, P.; Portella, G. Inhibition of autophagy enhances the effects of E1A-defective oncolytic adenovirus dl 922–947 against glioma cells in vitro and in vivo. Hum. Gene Ther. 2012, 23, 623–634. [Google Scholar] [CrossRef]
- Liikanen, I.; Ahtiainen, L.; Hirvinen, M.L.; Bramante, S.; Cerullo, V.; Nokisalmi, P.; Hemminki, O.; Diaconu, I.; Pesonen, S.; Koski, A. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol. Ther. 2013, 21, 1212–1223. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Hu, C.; Xing, F.; Gao, M.; Liang, J.; Xiao, X.; Cai, J.; Tan, Y.; Hu, J.; Zhu, W. Deficiency of the IRE1α-autophagy axis enhances the antitumor effects of the oncolytic virus M1. J. Virol. 2018, 92, e01317–e01331. [Google Scholar] [CrossRef] [Green Version]
- Calcott, B.; Levy, A.; Siegal, M.L.; Soyer, O.S.; Wagner, A. Engineering and biology: Counsel for a continued relationship. Biol. Theory 2015, 10, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Lyon, J.G.; Mokarram, N.; Saxena, T.; Carroll, S.L.; Bellamkonda, R.V. Engineering challenges for brain tumor immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 19–32. [Google Scholar] [CrossRef]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Ferreira, N.N.; de Oliveira Junior, E.; Granja, S.; Boni, F.I.; Ferreira, L.M.; Cury, B.S.; Santos, L.C.; Reis, R.M.; Lima, E.M.; Baltazar, F. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int. J. Pharm. 2021, 603, 120714. [Google Scholar] [CrossRef]
- Rezaei, V.; Rabiee, A.; Khademi, F. Glioblastoma multiforme: A glance at advanced therapies based on nanotechnology. J. Chemother. 2020, 32, 107–117. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Bae, S.; Li, Y.; Kim, C.; Choi, B.; Nam, H.Y.; Kim, S.H.; Yun, C.-O.; Lee, K.Y. Nose-to-brain delivery of cancer-targeting paclitaxel-loaded nanoparticles potentiates antitumor effects in malignant glioblastoma. Mol. Pharm. 2020, 17, 1193–1204. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Z.; Liu, R.; Wu, Y.; Zhang, X. Combined-therapeutic strategies synergistically potentiate glioblastoma multiforme treatment via nanotechnology. Theranostics 2020, 10, 3223. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Rdv. Drug. Deliv. Rev. 2011, 64, 640–665. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef]
- Liu, Q.; Duo, Y.; Fu, J.; Qiu, M.; Sun, Z.; Adah, D.; Kang, J.; Xie, Z.; Fan, T.; Bao, S. Nano-immunotherapy: Unique mechanisms of nanomaterials in synergizing cancer immunotherapy. Nano Today 2021, 36, 101023. [Google Scholar] [CrossRef]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.; Zhu, D.; Yu, X.; Ouyang, X.; Xie, Z.; Zhao, Y. Recent advances in nanomaterials-based chemo-photothermal combination therapy for improving cancer treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef]
- Sang, W.; Zhang, Z.; Dai, Y.; Chen, X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 2019, 48, 3771–3810. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef]
- Han, X.; Shen, S.; Fan, Q.; Chen, G.; Archibong, E.; Dotti, G.; Liu, Z.; Gu, Z.; Wang, C. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci. Adv. 2019, 5, eaaw6870. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA nanotechnology-mediated cancer immunotherapy. Theranostics 2020, 10, 281. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, R.; Jiang, W.; Bo, Y.; Zhang, T.; Yu, J.; Cheng, M.; Wu, Y.; Cheng, J.; Ma, W. Recent progress in nanomaterials for nucleic acid delivery in cancer immunotherapy. Biomater. Sci. 2019, 7, 2640–2651. [Google Scholar] [CrossRef]
- Abdou, P.; Wang, Z.; Chen, Q.; Chan, A.; Zhou, D.R.; Gunadhi, V.; Gu, Z. Advances in engineering local drug delivery systems for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1632. [Google Scholar] [CrossRef]
- Singh, J.; Garg, T.; Rath, G.; Goyal, A.K. Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis–a critical review. Drug Deliv. 2016, 23, 1676–1698. [Google Scholar] [CrossRef]
- Li, S.; Feng, X.; Wang, J.; He, L.; Wang, C.; Ding, J.; Chen, X. Polymer nanoparticles as adjuvants in cancer immunotherapy. Nano Res. 2018, 11, 5769–5786. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawakami, K.; Nomura, M.; Sato, Y.; Hyodo, M.; Hatakeyama, H.; Hayakawa, Y.; Harashima, H. Combined nano cancer immunotherapy based on immune status in a tumor microenvironment. J. Control. Release 2022, 345, 200–213. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Feng, Y.; Zhang, S.; Lin, L.; Guo, Z.; Sun, P.; Xu, C.; Tian, H.; Chen, X. An immune cocktail therapy to realize multiple boosting of the cancer-immunity cycle by combination of drug/gene delivery nanoparticles. Sci. Adv. 2020, 6, eabc7828. [Google Scholar] [CrossRef]
- Chen, J.; Lin, L.; Yan, N.; Hu, Y.; Fang, H.; Guo, Z.; Sun, P.; Tian, H.; Chen, X. Macrophages loaded CpG and GNR-PEI for combination of tumor photothermal therapy and immunotherapy. Sci. China Mater. 2018, 61, 1484–1494. [Google Scholar] [CrossRef] [Green Version]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- Šamec, N.; Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanomedicine and immunotherapy: A step further towards precision medicine for glioblastoma. Molecules 2020, 25, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Harford, J.B.; Moghe, M.; Rait, A.; Chang, E.H. Combination with SGT-53 overcomes tumor resistance to a checkpoint inhibitor. Oncoimmunology 2018, 7, e1484982. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, L.; Chen, J.; Fan, Y.; Wang, C.; Du, Y.; Guo, C.; Chen, F.; Li, W. Liposomal honokiol inhibits glioblastoma growth through regulating macrophage polarization. Ann. Transl. Med. 2021, 9, 1644. [Google Scholar] [CrossRef]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted delivery of chlorogenic acid by mannosylated liposomes to effectively promote the polarization of TAMs for the treatment of glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal TriCurin, a synergistic combination of curcumin, epicatechin gallate and resveratrol, repolarizes tumor-associated microglia/macrophages, and eliminates glioblastoma (GBM) and GBM stem cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Pinton, L.; Magri, S.; Masetto, E.; Vettore, M.; Schibuola, I.; Ingangi, V.; Marigo, I.; Matha, K.; Benoit, J.-P.; Della Puppa, A. Targeting of immunosuppressive myeloid cells from glioblastoma patients by modulation of size and surface charge of lipid nanocapsules. J. Nanobiotechnol. 2020, 18, 31. [Google Scholar] [CrossRef]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef]
- Lollo, G.; Vincent, M.; Ullio-Gamboa, G.; Lemaire, L.; Franconi, F.; Couez, D.; Benoit, J.-P. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int. J. Pharm. 2015, 495, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Jong, A.Y.; Wu, C.-H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef] [Green Version]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Petersen, T.R.; Sika-Paotonu, D.; Knight, D.A.; Dickgreber, N.; Farrand, K.J.; Ronchese, F.; Hermans, I.F. Potent anti-tumor responses to immunization with dendritic cells loaded with tumor tissue and an NKT cell ligand. Immunol. Cell Biol. 2010, 88, 596–604. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef]
- Adamus, T.; Hung, C.-Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther.-Nucleic Acids 2022, 27, 611–620. [Google Scholar] [CrossRef]
- Zhu, L.; Gangadaran, P.; Kalimuthu, S.; Oh, J.M.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Novel alternatives to extracellular vesicle-based immunotherapy–exosome mimetics derived from natural killer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, S166–S179. [Google Scholar] [CrossRef] [Green Version]
- Ung, N.; Yang, I. Nanotechnology to augment immunotherapy for the treatment of glioblastoma multiforme. J. Neuro-Oncol. 2015, 123, 473–481. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Spriggs, T.; Appiani La Rosa, S.; Kannan, S.; Kannan, R.M. Dendrimer-mediated targeted delivery of rapamycin to tumor-associated macrophages improves systemic treatment of glioblastoma. Biomacromolecules 2020, 21, 5148–5161. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef]
- Wei, J.; Wu, D.; Zhao, S.; Shao, Y.; Xia, Y.; Ni, D.; Qiu, X.; Zhang, J.; Chen, J.; Meng, F. Immunotherapy of Malignant Glioma by Noninvasive Administration of TLR9 Agonist CpG Nano-Immunoadjuvant. Adv. Sci. 2022, 9, 2103689. [Google Scholar] [CrossRef]
- Wang, H.; Chao, Y.; Zhao, H.; Zhou, X.; Zhang, F.; Zhang, Z.; Li, Z.; Pan, J.; Wang, J.; Chen, Q. Smart Nanomedicine to Enable Crossing Blood–Brain Barrier Delivery of Checkpoint Blockade Antibody for Immunotherapy of Glioma. ACS Nano 2022, 16, 664–674. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, X.; Zhang, Q.; Zheng, T.; Mohammadniaei, M.; Wang, W.; Shen, J.; Sun, Y. Biodegradable Polymeric Nanoparticles Containing an Immune Checkpoint Inhibitor (aPDL1) to Locally Induce Immune Responses in the Central Nervous System. Adv. Funct. Mater. 2021, 31, 2102274. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Chen, X. Injectable Hydrogels as Local Depots at Tumor Sites for Antitumor Immunotherapy and Immune-Based Combination Therapy. Macromol. Biosci. 2021, 21, 2100039. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Li, A.; Jing, W.; Sun, P.; Huang, X.; Liu, Y.; Zhang, S.; Du, W.; Zhang, R. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 2021, 16, 538–548. [Google Scholar] [CrossRef]
- Tsao, C.-T.; Kievit, F.M.; Ravanpay, A.; Erickson, A.E.; Jensen, M.C.; Ellenbogen, R.G.; Zhang, M. Thermoreversible poly (ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules 2014, 15, 2656–2662. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ye, L.; He, W.; Teng, C.; Sun, S.; Lu, H.; Li, S.; Lv, L.; Cao, X.; Yin, H. In situ targeting nanoparticles-hydrogel hybrid system for combined chemo-immunotherapy of glioma. J. Control. Release 2022, 345, 786–797. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2018, 119, 6459–6506. [Google Scholar] [CrossRef]
- Huang, J.; Ma, W.; Sun, H.; Wang, H.; He, X.; Cheng, H.; Huang, M.; Lei, Y.; Wang, K. Self-assembled DNA nanostructures-based nanocarriers enabled functional nucleic acids delivery. ACS Appl. Bio Mater. 2020, 3, 2779–2795. [Google Scholar] [CrossRef]
- Fu, W.; You, C.; Ma, L.; Li, H.; Ju, Y.; Guo, X.; Shi, S.; Zhang, T.; Zhou, R.; Lin, Y. Enhanced efficacy of temozolomide loaded by a tetrahedral framework DNA nanoparticle in the therapy for glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 39525–39533. [Google Scholar] [CrossRef] [PubMed]
- Abadi, B.; Yazdanpanah, N.; Nokhodchi, A.; Rezaei, N. Smart biomaterials to enhance the efficiency of immunotherapy in glioblastoma: State of the art and future perspectives. Adv. Drug Deliv. Rev. 2021, 179, 114035. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, S.; Min, Q.; Song, J.; Tian, Y. Reconstructed adoptive-macrophages with DNA-tetrahedron-CpG/siRNA for synergistic solid tumor immunotherapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128184. [Google Scholar] [CrossRef]
- Lee, G.P.; Willis, A.; Pernal, S.; Phakatkar, A.; Shokuhfar, T.; Blot, V.; Engelhard, H.H. Targeted sonodynamic destruction of glioblastoma cells using antibody–titanium dioxide nanoparticle conjugates. Nanomedicine 2021, 16, 523–534. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Liu, H.; Ren, F.; Qiu, W.; Sun, Q.; Yan, F.; Zheng, H.; Li, Z.; Gao, M. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2−x Se nanoparticles. Nanoscale 2019, 11, 7600–7608. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Qiu, W.; Han, Y.; Liu, H.; Li, Z. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact. Mater. 2022, 16, 418–432. [Google Scholar] [CrossRef]
- Bielecki, P.A.; Lorkowski, M.E.; Becicka, W.M.; Atukorale, P.U.; Moon, T.J.; Zhang, Y.; Wiese, M.; Covarrubias, G.; Ravichandran, S.; Karathanasis, E. Immunostimulatory silica nanoparticle boosts innate immunity in brain tumors. Nanoscale Horiz. 2021, 6, 156–167. [Google Scholar] [CrossRef]
- Kim, G.B.; Aragon-Sanabria, V.; Randolph, L.; Jiang, H.; Reynolds, J.A.; Webb, B.S.; Madhankumar, A.; Lian, X.; Connor, J.R.; Yang, J. High-affinity mutant Interleukin-13 targeted CAR T cells enhance delivery of clickable biodegradable fluorescent nanoparticles to glioblastoma. Bioact. Mater. 2020, 5, 624–635. [Google Scholar] [CrossRef]
- Chen, M.-H.; Liu, T.-Y.; Chen, Y.-C.; Chen, M.-H. Combining augmented radiotherapy and immunotherapy through a nano-gold and bacterial outer-membrane vesicle complex for the treatment of glioblastoma. Nanomaterials 2021, 11, 1661. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Fang, J.; Xue, W.; Zhang, J.; Tong, H.; Liu, H.; Guo, Y.; Yang, Y.; Zhang, W. Non-invasive monitoring of the kinetic infiltration and therapeutic efficacy of nanoparticle-labeled chimeric antigen receptor T cells in glioblastoma via 7.0-Tesla magnetic resonance imaging. Cytotherapy 2021, 23, 211–222. [Google Scholar] [CrossRef]
- Rivera-Rodriguez, A.; Hoang-Minh, L.B.; Chiu-Lam, A.; Sarna, N.; Marrero-Morales, L.; Mitchell, D.A.; Rinaldi-Ramos, C.M. Tracking adoptive T cell immunotherapy using magnetic particle imaging. Nanotheranostics 2021, 5, 431. [Google Scholar] [CrossRef]
- Yang, R.; Sarkar, S.; Korchinski, D.J.; Wu, Y.; Yong, V.W.; Dunn, J.F. MRI monitoring of monocytes to detect immune stimulating treatment response in brain tumor. Neuro-Oncol. 2017, 19, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Zhu, L.; Wang, C.; Yang, Y.; He, Z. BSA–MnO 2–SAL multifunctional nanoparticle-mediated M 1 macrophages polarization for glioblastoma therapy. RSC Adv. 2021, 11, 35331–35341. [Google Scholar] [CrossRef]
- Kinoh, H.; Quader, S.; Shibasaki, H.; Liu, X.; Maity, A.; Yamasoba, T.; Cabral, H.; Kataoka, K. Translational nanomedicine boosts anti-PD1 therapy to eradicate orthotopic PTEN-negative glioblastoma. ACS Nano 2020, 14, 10127–10140. [Google Scholar] [CrossRef]
- Choi, J.; Pant, A.; Medikonda, R.; Kim, Y.-H.; Routkevitch, D.; Saleh, L.; Tong, L.; Chan, H.Y.; Nedrow, J.; Jackson, C. Sustained localized delivery of immunotherapy to lymph nodes reverses immunosuppression and increases long-term survival in murine glioblastoma. OncoImmunology 2021, 10, 1940673. [Google Scholar] [CrossRef]
- Kadiyala, P.; Li, D.; Nuñez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef]
- Kuang, J.; Song, W.; Yin, J.; Zeng, X.; Han, S.; Zhao, Y.P.; Tao, J.; Liu, C.J.; He, X.H.; Zhang, X.Z. iRGD modified chemo-immunotherapeutic nanoparticles for enhanced immunotherapy against glioblastoma. Adv. Funct. Mater. 2018, 28, 1800025. [Google Scholar] [CrossRef]
- Li, T.-F.; Xu, Y.-H.; Li, K.; Wang, C.; Liu, X.; Yue, Y.; Chen, Z.; Yuan, S.-J.; Wen, Y.; Zhang, Q. Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019, 86, 381–394. [Google Scholar] [CrossRef]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J. Traceable nanoparticles with dual targeting and ROS response for RNAi-based immunochemotherapy of intracranial glioblastoma treatment. Adv. Mater. 2018, 30, 1705054. [Google Scholar] [CrossRef]
- Chou, S.-T.; Patil, R.; Galstyan, A.; Gangalum, P.R.; Cavenee, W.K.; Furnari, F.B.; Ljubimov, V.A.; Chesnokova, A.; Kramerov, A.A.; Ding, H. Simultaneous blockade of interacting CK2 and EGFR pathways by tumor-targeting nanobioconjugates increases therapeutic efficacy against glioblastoma multiforme. J. Control. Release 2016, 244, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Slaughter, T.; Doherty, C.; Chang, E.H. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood–brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int. J. Cancer 2019, 145, 2535–2546. [Google Scholar] [CrossRef] [Green Version]
- Van Woensel, M.; Mathivet, T.; Wauthoz, N.; Rosière, R.; Garg, A.D.; Agostinis, P.; Mathieu, V.; Kiss, R.; Lefranc, F.; Boon, L. Sensitization of glioblastoma tumor micro-environment to chemo-and immunotherapy by Galectin-1 intranasal knock-down strategy. Sci. Rep. 2017, 7, 1217. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ji, Q.; Cheng, Y.; Liu, M.; Zhang, B.; Mei, Q.; Liu, D.; Zhou, S. Biomimetic GBM-targeted drug delivery system boosting ferroptosis for immunotherapy of orthotopic drug-resistant GBM. J. Nanobiotechnol. 2022, 20, 161. [Google Scholar]
- Mangraviti, A.; Tzeng, S.Y.; Kozielski, K.L.; Wang, Y.; Jin, Y.; Gullotti, D.; Pedone, M.; Buaron, N.; Liu, A.; Wilson, D.R.; et al. Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo. ACS Nano 2015, 9, 1236–1249. [Google Scholar] [CrossRef] [Green Version]
- Oraee-Yazdani, S.; Akhlaghpasand, M.; Shokri, G.; Rostami, F.; Golmohammadi, M.; Jamshidi-Adegani, F.; Arefian, E.; Hafizi, M.; Zomorrod, M.S.; Oraee-Yazdani, M.; et al. Intracerebral administration of autologous mesenchymal stem cells as HSV-TK gene vehicle for treatment of glioblastoma multiform: Safety and feasibility assessment. Mol. Neurobiol. 2021, 58, 4425–4436. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Ding, F.; Liu, X.; Wu, Y.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. A Virus-Mimicking Nucleic Acid Nanogel Reprograms Microglia and Macrophages for Glioblastoma Therapy. Adv. Mater. 2021, 33, 2006116. [Google Scholar] [CrossRef]
- Hermida, M.A.; Kumar, J.D.; Schwarz, D.; Laverty, K.G.; Di Bartolo, A.; Ardron, M.; Bogomolnijs, M.; Clavreul, A.; Brennan, P.M.; Wiegand, U.K. Three dimensional in vitro models of cancer: Bioprinting multilineage glioblastoma models. Adv. Biol. Regul. 2020, 75, 100658. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Reisman, N.; Shtilerman, Y.; Ben-Shushan, D.; Pozzi, S.; Madi, A.; Tiram, G.; Eldar-Boock, A.; Ferber, S. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 2021, 7, eabi9119. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef]
- Parra-Cantu, C.; Li, W.; Quiñones-Hinojosa, A.; Zhang, Y.S. 3D bioprinting of glioblastoma models. J. 3D Print. Med. 2020, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-G.; Jeong, Y.H.; Kim, Y.; Choi, Y.-J.; Moon, H.E.; Park, S.H.; Kang, K.S.; Bae, M.; Jang, J.; Youn, H. A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng. 2019, 3, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Bansal, R.; Lammers, T.; Zhang, Y.S.; Michel Schiffelers, R.; Prakash, J. 3D-bioprinted mini-brain: A glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 2019, 31, 1806590. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ma, C.; Vasudevaraja, V.; Serrano, J.; Tong, J.; Peng, Y.; Delorenzo, M.; Shen, G.; Frenster, J.; Morales, R.-T.T. Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy. Elife 2020, 9, e52253. [Google Scholar] [CrossRef]








| Virus Type | Virus Name | Modifications | Efficacy | Clinical Phase | Status | Trial Name (ClinicalTrial.gov) |
|---|---|---|---|---|---|---|
| HSV-1 | G207 | γ134.5 loci deletion. ICP6 truncation. | Tumor cells are eliminated, necrosis occurs, and no toxin is produced. | I | Completed | NCT00028158 |
| rQNestin34.5 | ICP6 deletion. γ134.5 expression under nestin promoter. | Enhanced oncolytic activity in vitro and in an in vivo model. | I | Recruiting | NCT03152318 | |
| HSV-1716 | γ134.5 loci partial deletion. | Infection and death of tumor cells. | I | Terminated | NCT02031965 | |
| M032 | 2 copies of γ134.5 deletion. Human IL-12 expression. | Causes the tumor cell to synthesize and secrete the immunity-stimulating protein interleukin-12 (IL-12) | I | Active, not Recruiting | NCT02062827 | |
| C134 | γ134.5 loci deletion. HCMV’s IRS1 protein expression. | Tumor volume reduction and improved surveillance. Improved replication and increased in vivo survival. | I | Recruiting | NCT03657576 | |
| Adeno virus | DNX-2401 | 24 base pair deletion in E1A gene. Engineered RGD-4 C binding motif. | Improved virus replication in cancer cells via Rb-pathway deficiency. Improved specificity by targeting tumor cells via αvβ3 and αvβ5. | II | Completed | NCT02798406 |
| DNX-2440 | 24 base pair deletion in E1A gene. Engineered RGD-4 C binding motif. OX40L insertion. | Improved virus replication in cancer cells via Rb-pathway-deficiency. Improved specificity by targeting tumor cells via αvβ3 and αvβ5. Stimulation of immune responses. | I | Recruiting | NCT03714334 | |
| Vaccinia virus | TG6002 | TK deletion. ribonucleotide reductase genes deletion. FCU1 expression. | Survival in s.c. and i.c. over an extended period. Synergy with 5FC in an i.c. model. | I | Unknown | NCT03294486 |
| Parvo virus | Parvo virus H-1 (ParvOryx) | Non-engineered virus. | Tumors in complete remission. Cathepsin B activation induces cell death in H-1PV with a complete remission of the tumors. Selective infection, no toxicity, in vivo reduction in tumor volume | II | Completed | NCT01301430 |
| Reovirus | REOLYSIN | Non-engineered reovirus type 3. | Safe, well tolerated (no significant toxicity) | I | Completed | NCT00528684 |
| Polio/Rhinovirus Recombinant (PVS-RIPO) | PVS-RIPO consists of the genome of the live attenuated serotype 1 (Sabin) vaccine strain of poliovirus containing an internal ribosomal entry site (IRES) of human rhinovirus type-2. | Attenuation of neurovirulence. Attenuation determinants mapping to the coding regions for the type 1 (Sabin) capsid13 and RNA-dependent RNA polymerase. | I | Active, not Recruiting | NCT03043391 | |
| NDV WT | ---------- | Apoptosis induction. Tumor volume should be decreased. NDV causes idiopathic cardiomyopathy (ICD). The combination of TMZ has synergistic effects. Reduces the number of tumors and prolongs survival time. | Safe, well tolerated | I | Withdrawn | NCT01174537 |
| Trial Name (ClinicalTrial.gov) | Phase | Virus Used/Mode of Action | Associated Treatments | Primary Endpoint | Results |
|---|---|---|---|---|---|
| NCT03636477 | I | Ad-RTS-hIL-12 | Veledimex + Nivolumab | Safety and feasibility | Active, not recruiting |
| NCT02062827 | I | M032-HSV-1 | - | Highest safe dose/MTD | Ongoing, Recruiting |
| NCT03330197 | I/II | Ad-RTS-hIL-12 | Veledimex | Safety and tolerability | Ongoing, Recruiting |
| NCT01811992 | I | Ad-hCMV-TK and Ad-hCMV-Flt3L | - | Maximum Tolerated Dose (MTD) | Active, not recruiting |
| NCT03714334 | I | DNX-2440 | - | Incidence of Treatment-Emergent Adverse Events | Ongoing, Recruiting |
| Type of Nanomaterial | Therapeutic Compounds | Mechanism of Immunotherapy | Ref. |
|---|---|---|---|
| pH-sensitive polymeric micelles | - Anti-PD-1antibodies - Epirubicin | - Inducing immunogenic cell death - Eliminating the immunosuppressive myeloid-derived suppressor cells - Reducing the expression of PD-L1 | [215] |
| Polycaprolactone (PCL):PEG:PCL polymer hydrogel | Anti-PD-1 antibody | Increasing IFN-γ and TNF-α levels | [216] |
| High-density lipoprotein (HDL)-mimicking nanodiscs | - CpG - Docetaxel | - Increasing the response of CD8+ T cell - Inducing cancer cell death and releasing the tumor antigens and damage-associated molecular pattern molecules (DAMPs) into the TME - Activation of macrophages and DCs | [217] |
| Smart redox responsive DOX loaded mesoporous silica nanoparticles | - DOX - Asp-Glu-Val-Asp (DEVD) peptide | - Activation of cytotoxic CD8+ T lymphocytes - Inhibition of CD4+ T cells - Upregulating the levels of antitumor cytokines | [218] |
| Polyglycerol-nanodiamond composites | - DOX | - Inducing autophagy to GBM cells - Improving the activation of DCs | [219] |
| Angiopep LipoPCB (TMZ+BAP/siTGF-β) smart nanoformulation | - TMZ - siRNA | - Down-regulating the expression of TGFβ - Enhancing the efficacy of FDA-approved drug TMZ - Regulating the proliferation of other T cells, including T effect cells (Teff), T regulation cells (Treg), and cytotoxic lymphocyte (CTL). | [220] |
| Poly (β-l-malic acid) (PMLA) nanoparticles functionalized with anti-transferrin receptor (a-TfR) mAb | - Cetuximab - siRNAs | - Reducing the expression of PDL-1 - Downregulating the expression of serine/threonine-protein kinase CK2 and the wild-type/mutated epidermal growth factor receptor (EGFR/EGFRvIII) - Suppressing cancer stem cell marker expression (such as c-Myc, CD133, and nestin) | [221] |
| Cationic liposome functionalized with a single chain antibody fragment recognizing the transferrin receptor (TfRscFv) | - Human wildtype TP53 (wtp53) plasmid - Anti- PD-1 antibody | - Inhibiting tumor growth - Inducing tumor cell apoptosis - Enhancing intratumoral T-cell infiltration | [222] |
| Chitosan nanoparticles | - siRNA targeting Gal-1 (siGal-1) | - Reducing myeloid suppressor cells and regulatory T cells - Increasing CD4+ and CD8+ T cells. | [223] |
| Hydrogels contain tumor-homing immune nanoregulator (THINR) | - Mitoxantrone - siRNA targeted indoleamine 2,3-dioxygenase-1 - Chemokine ligand 10 (CXCL10) | - Triggering immunogenic cell death - Inducing DC maturation -Suppressing the IDO1 - Enhancing the recruitment of activated T cells | [196] |
| Microglial membrane coated Fe3O4 nanoparticles connected with siRNA | siRNA against PD-L1 | - Decreasing the expression of PD-L1 protein - Increasing the ratio of effector T cells and regulatory T cells - Inducing GBM cells ferroptosis - Inducing DC cell maturation - Increasing the polarization of M2-type microglia to M1-type | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekrirad, Z.; Barzegar Behrooz, A.; Ghaemi, S.; Khosrojerdi, A.; Zarepour, A.; Zarrabi, A.; Arefian, E.; Ghavami, S. Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy. Cancers 2022, 14, 3698. https://doi.org/10.3390/cancers14153698
Fekrirad Z, Barzegar Behrooz A, Ghaemi S, Khosrojerdi A, Zarepour A, Zarrabi A, Arefian E, Ghavami S. Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy. Cancers. 2022; 14(15):3698. https://doi.org/10.3390/cancers14153698
Chicago/Turabian StyleFekrirad, Zahra, Amir Barzegar Behrooz, Shokoofeh Ghaemi, Arezou Khosrojerdi, Atefeh Zarepour, Ali Zarrabi, Ehsan Arefian, and Saeid Ghavami. 2022. "Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy" Cancers 14, no. 15: 3698. https://doi.org/10.3390/cancers14153698
APA StyleFekrirad, Z., Barzegar Behrooz, A., Ghaemi, S., Khosrojerdi, A., Zarepour, A., Zarrabi, A., Arefian, E., & Ghavami, S. (2022). Immunology Meets Bioengineering: Improving the Effectiveness of Glioblastoma Immunotherapy. Cancers, 14(15), 3698. https://doi.org/10.3390/cancers14153698








