Therapeutic Effect of Regional Chemotherapy in Diffuse Metastatic Cholangiocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Characterization of the Study Population
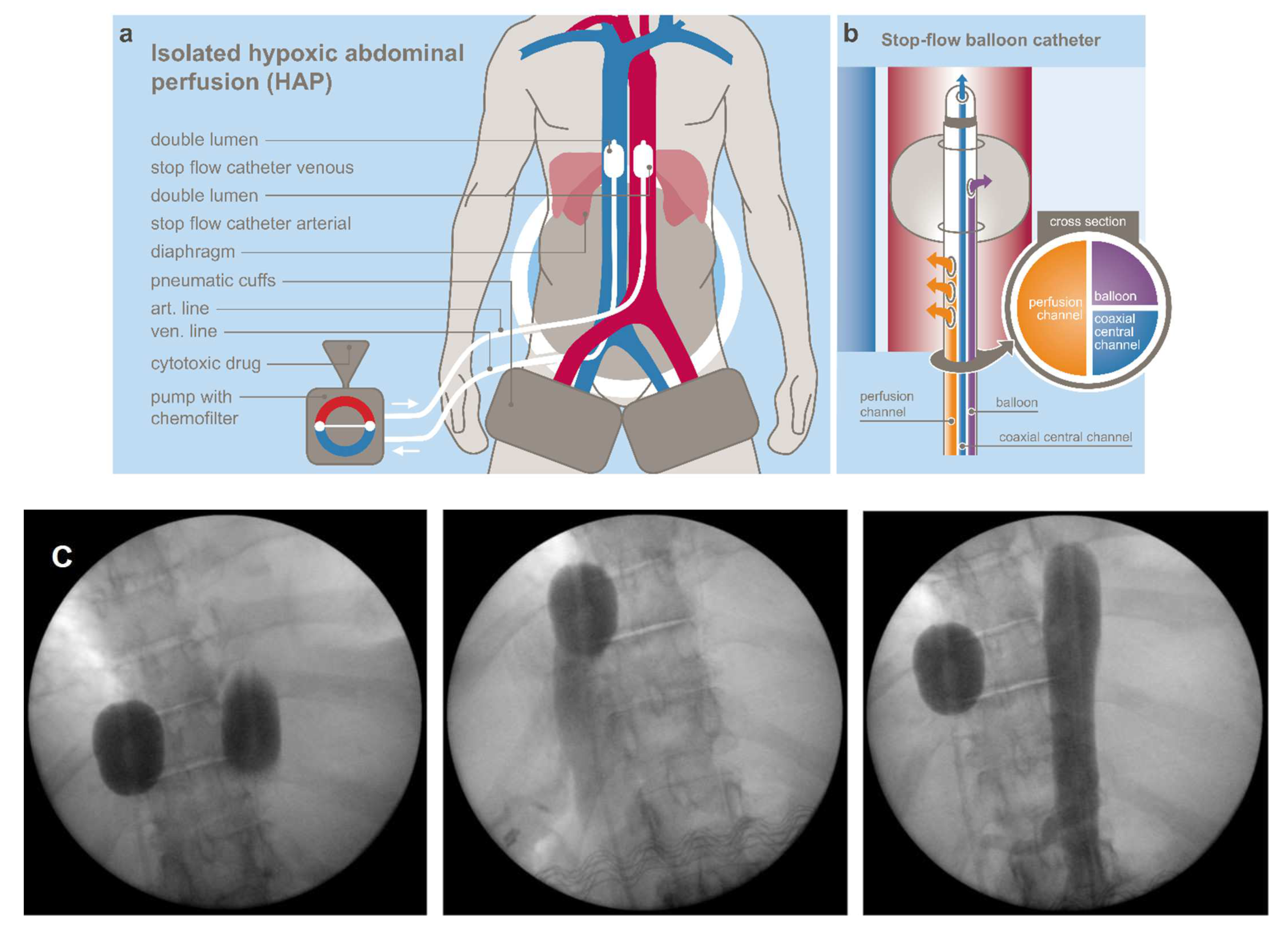
2.2. Cytotoxic Drugs and Methods
2.2.1. Regional Chemotherapy Techniques
2.2.2. Cytotoxic Drugs
2.2.3. Treatment Cycles
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.C.; Jarnagin, W.R. The Landmark Series: Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 4158–4170. [Google Scholar] [CrossRef]
- Jansen, H.; Pape, U.-F.; Utku, N. A review of systemic therapy in biliary tract carcinoma. J. Gastrointest. Oncol. 2020, 11, 770–789. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Davidson, B.R.; Goldin, R.; Heaton, N.; Karani, J.; Pereira, S.; Rosenberg, W.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.H.; Song, H.-N.; Kim, R.B.; Oh, S.Y.; Lim, H.Y.; Park, J.O.; Park, S.H.; Kim, M.J.; Lee, S.I.; Ryou, S.H.; et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn. J. Clin. Oncol. 2015, 45, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brieau, B.; Dahan, L.; De Rycke, Y.; Boussaha, T.; Vasseur, P.; Tougeron, D.; Lecomte, T.; Coriat, R.; Bachet, J.B.; Claudez, P.; et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcita-bine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer 2015, 12, 3290–3297. [Google Scholar] [CrossRef]
- Fornaro, L.; Vivaldi, C.; Cereda, S.; Leone, F.; Aprile, G.; Lonardi, S.; Silvestris, N.; Santini, D.; Milella, M.; Caparello, C.; et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: A multicenter survey and pooled analysis with published data. J. Exp. Clin. Cancer Res. 2015, 34, 156. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, S.; Kirstein, M.M.; Brüning, R.; Zeile, M.; Ferrucci, P.F.; Prevoo, W.; Radeleff, B.; Trillaud, H.; Tselikas, L.; Vicente, E.; et al. Percutaneous hepatic perfusion (chemosaturation) with melphalan in patients with intra-hepatic cholangiocarcinoma: European multicentre study on safety, short-term effects and survival. Eur. Radiol. 2019, 29, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, P.; Cocorocchio, E.; Bonomo, G.; Varano, G.; Della Vigna, P.; Orsi, F. A New Option for the Treatment of Intrahepatic Cholangiocarcinoma: Percutaneous Hepatic Perfusion with CHEMOSAT Delivery System. Cells 2021, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K.; Kooka, Y.; Suzuki, Y.; Suzuki, A.; Oikawa, T.; Ushio, A.; Kasai, Y.; Sawara, K.; Miyamoto, Y.; Oikawa, K.; et al. Efficacy of Hepatic Arterial Infusion Chemotherapy Using 5-Fluorouracil and Systemic Pegylated Interferon α-2b for Advanced Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2014, 21, 3638–3645. [Google Scholar] [CrossRef] [PubMed]
- Aigner, K.; Vashist, Y.K.; Selak, E.; Gailhofer, S.; Aigner, K.R. Efficacy of Regional Chemotherapy Approach in Peritoneal Metastatic Gastric Cancer. J. Clin. Med. 2021, 10, 5322. [Google Scholar] [CrossRef] [PubMed]
- Aigner, K.R.; Gailhofer, S.; Selak, E.; Aigner, K. Intra-arterial infusion chemotherapy versus isolated upper abdominal perfusion for ad-vanced pancreatic cancer: A retrospective cohort study on 454 patients. J. Cancer Res. Clin. Oncol. 2019, 145, 2855–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, K.R.; Selak, E.; Gailhofer, S. Isolated thoracic perfusion with chemofiltration for progressive malignant pleural mesothelioma. Onco Targets Ther. 2017, 10, 3049–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadagni, S.; Aigner, K.; Zoras, O.; Masedu, F.; Fiorentini, G.; Ricevuto, E.; Deraco, M.; Clementi, M. Isolated thoracic perfusion in lung metastases from breast cancer: A retrospective observational study. Updates Surg. 2019, 71, 165–177. [Google Scholar] [CrossRef]
- Guadagni, S.; Clementi, M.; Valenti, M.; Fiorentini, G.; Cantore, M.; Kanavos, E.; Caterino, G.; Di Giuro, G.; Amicucci, G. Hypoxic abdominal stop-flow perfusion in the treatment of advanced pancreatic cancer: A phase II evaluation/trial. Eur. J. Surg. Oncol. (EJSO) 2007, 33, 72–78. [Google Scholar] [CrossRef]
- Laface, C.; Laforgia, M.; Molinari, P.; Foti, C.; Ambrogio, F.; Gadaleta, C.D.; Ranieri, G. Intra-Arterial Infusion Chemotherapy in Advanced Pancreatic Cancer: A Comprehensive Review. Cancers 2022, 14, 450. [Google Scholar] [CrossRef]
- Sartorelli, A.C.; Hodnick, W.F.; Belcourt, M.F.; Tomasz, M.; Haffty, B.; Fischer, J.J.; Rockwell, S. Mitomycin C: A prototype bioreductive agent. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 1994, 6, 501–508. [Google Scholar]
- Teicher, B.A.; Lazo, J.S.; Sartorelli, A.C. Classification of antineoplastic agents by their selective toxicities to-ward oxygenated and hypoxic tumor cells. Cancer Res. 1981, 41, 73–81. [Google Scholar] [PubMed]
- Aigner, K.R.; Knapp, A. Toxicity Profiles with Systemic Versus Regional Chemotherapy, 2nd ed.; Induction Chemotherapy, Springer: Berlin/Heidelberg, Germany, 2016; p. 506. [Google Scholar]
- El-Diwany, R.; Pawlik, T.M.; Ejaz, A. Intrahepatic Cholangiocarcinoma. Surg. Oncol. Clin. 2019, 28, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Oneda, E.; Abu Hilal, M.; Zaniboni, A. Biliary Tract Cancer: Current Medical Treatment Strategies. Cancers 2020, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Balci, D.; Sakamoto, Y.; Li, J.; Di Benedetto, F.; Kirimker, E.O.; Petrowsky, H. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int. J. Surg. 2020, 82, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ruzzenente, A.; Bagante, F.; Olthof, P.B.; Aldrighetti, L.; Alikhanov, R.; Cescon, M.; Koerkamp, B.G.; Jarnagin, W.R.; Nadalin, S.; Pratschke, J.; et al. Surgery for Bismuth-Corlette Type 4 Perihilar Cholangiocarcinoma: Results from a Western Multicenter Collaborative Group. Ann. Surg Oncol. 2021, 28, 7719–7729. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Eckel, F.; Schmid, R.M. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br. J. Cancer 2007, 96, 896–902. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, H.S.; Jo, J.H.; Cho, I.R.; Chung, M.J.; Bang, S.; Park, S.W.; Song, S.Y.; Park, J.Y. Combination Therapy with Capecitabine and Cisplatin as Second-Line Chemotherapy for Ad-vanced Biliary Tract Cancer. Chemotherapy 2017, 62, 361–366. [Google Scholar] [CrossRef] [PubMed]




| Variable | N | % |
|---|---|---|
| All | 36 | 100 |
| Sex | ||
| female | 15 | 41.7 |
| male | 21 | 58.3 |
| Metastatic site | ||
| liver | 22 | 61.1 |
| lymph nodes | 36 | 100.0 |
| lungs | 8 | 22.2 |
| peritoneum | 21 | 58.3 |
| others | 5 | 13.9 |
| Karnofsky index | ||
| 100–70 | 19 | 52.8 |
| 60–50 | 10 | 27.8 |
| 40–30 | 7 | 19.4 |
| ECOG | ||
| 0–1 | 19 | 52.8 |
| 2 | 10 | 27.8 |
| 3 | 7 | 19.4 |
| Liver resection | ||
| 10 | 27.8 | |
| SCTx | ||
| 14 | 38.9 | |
| Choledochal Stent | ||
| 12 | 33.3 | |
| RegCTx | ||
| Total Cycles | 189 | 100.0 |
| Art. Infusion | 90 | 47.6 |
| UAP | 74 | 39.2 |
| ITP | 12 | 6.3 |
| HAP | 13 | 6.9 |
| Number | N | % | Cumulative % |
|---|---|---|---|
| 1 | 2 | 5.6 | 5.6 |
| 2 | 7 | 19.4 | 25.0 |
| 3 | 3 | 8.3 | 33.3 |
| 4 | 7 | 19.4 | 52.8 |
| 5 | 4 | 11.1 | 63.9 |
| 6 | 4 | 11.1 | 75.0 |
| 7 | 1 | 2.8 | 77.8 |
| 8 | 2 | 5.6 | 83.3 |
| 9 | 2 | 5.6 | 88.9 |
| 11 | 1 | 2.8 | 91.7 |
| 12 | 1 | 2.8 | 94.4 |
| 14 | 2 | 5.6 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashist, Y.; Aigner, K.; Gailhofer, S.; Aigner, K.R. Therapeutic Effect of Regional Chemotherapy in Diffuse Metastatic Cholangiocarcinoma. Cancers 2022, 14, 3701. https://doi.org/10.3390/cancers14153701
Vashist Y, Aigner K, Gailhofer S, Aigner KR. Therapeutic Effect of Regional Chemotherapy in Diffuse Metastatic Cholangiocarcinoma. Cancers. 2022; 14(15):3701. https://doi.org/10.3390/cancers14153701
Chicago/Turabian StyleVashist, Yogesh, Kornelia Aigner, Sabine Gailhofer, and Karl R. Aigner. 2022. "Therapeutic Effect of Regional Chemotherapy in Diffuse Metastatic Cholangiocarcinoma" Cancers 14, no. 15: 3701. https://doi.org/10.3390/cancers14153701






