VMAT-Based Planning Allows Sparing of a Spatial Dose Pattern Associated with Radiation Pneumonitis in Patients Treated with Radiotherapy for a Locally Advanced Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
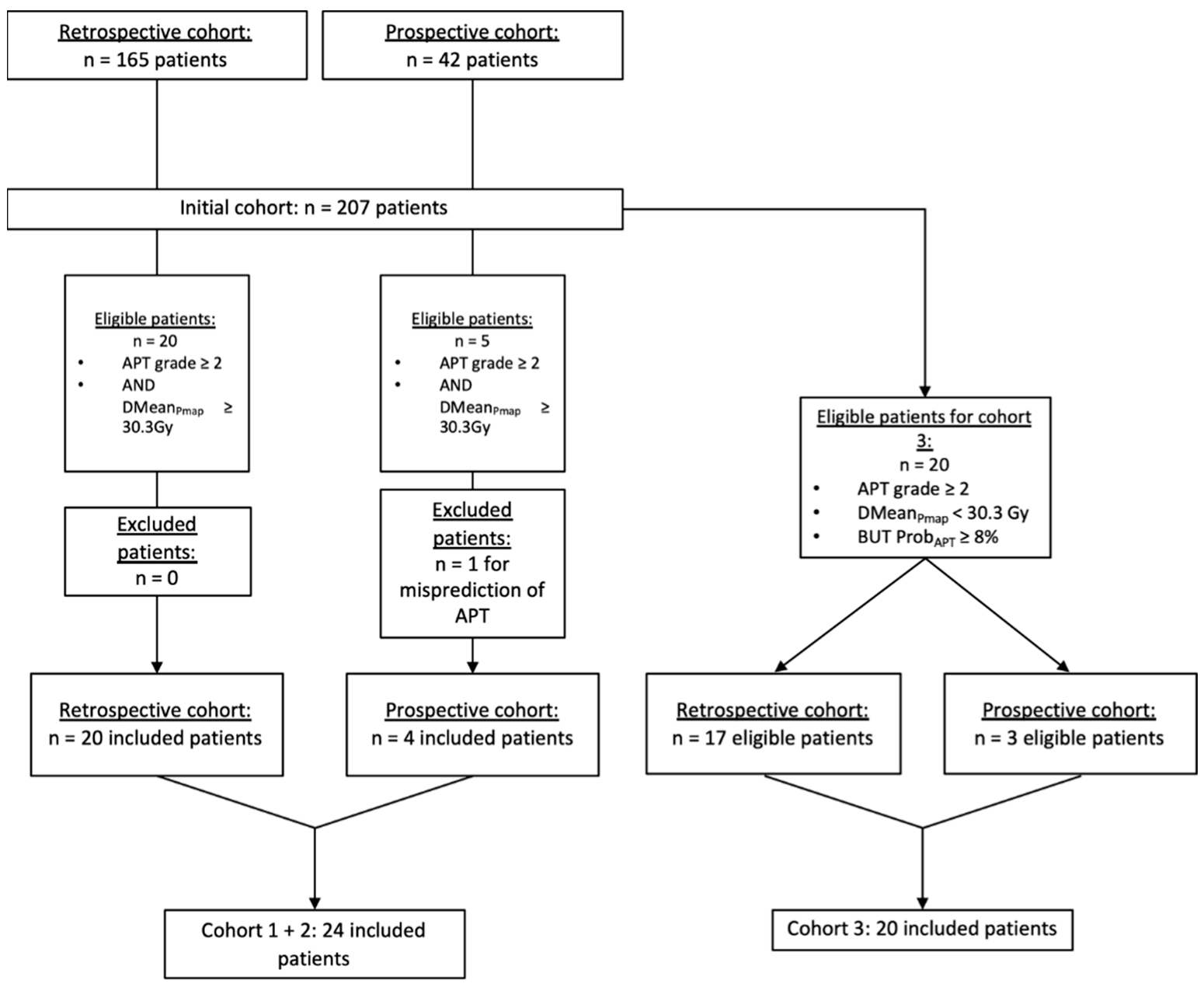
2.1. Population
2.2. Radiation Sensitive Sub-Region
2.3. Dosimetric Planning
- -
- Cohort 1: Patients who had a predicted risk of APT ≥ 8%, a DmeanPmap ≥ 30.3Gy, and in which dosimetric optimization was successful (DmeanPmap-Opti < 30.3Gy and respect to the PTV coverage and other dose constraints),
- -
- Cohort 2: Patients who had a predicted risk of APT ≥ 8%, a DmeanPmap ≥ 30.3Gy, and in which dosimetric optimization was unsuccessful (DmeanPmap-Opti ≥ 30.3Gy and/or non-respect of the PTV coverage and other dose constraints).
- -
- Cohort 3: Patients who had a predicted risk of APT ≥ 8% and a DmeanPmap < 30.3Gy. In Cohort 3, as the DmeanPmap was already inferior to 30.3Gy, the success of the optimization was defined by a ≥ 20% decrease of the DmeanPmap without compromising the PTV coverage.
2.4. Dose Map Comparisons
2.5. Prediction Based on the Pmap Model
3. Results
3.1. Population
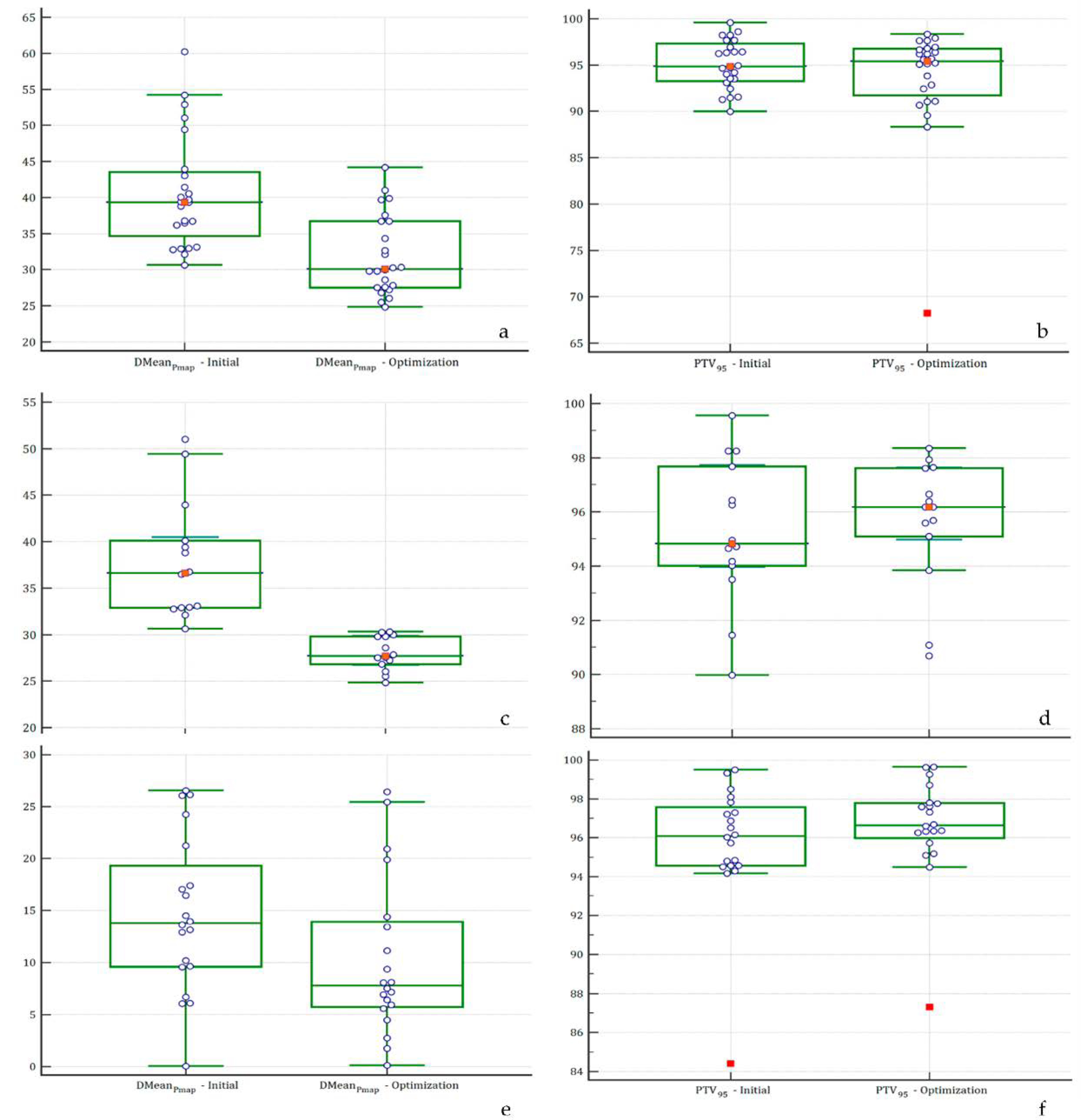
3.2. Dosimetric Planning and Dose Map Comparisons
3.3. Model Prediction
| Cohort | Cohort 1 + 2 n: 24 Patients | Cohort 1 n: 14 Patients | Cohort 3 n: 20 Patients | Overall Cohort: Cohort 1 + 2 + 3 n: 44 Patients | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose Map | Initial | Optimization | p | Initial | Optimization | p | Initial | Optimization | p | Initial | Optimization | p | ||||||||||
| Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | Mean | CI 95% | |||||||
| PTV | PTV95 (%) | 94.8 | 93.5-96.6 | 95.4 | 92.7–96.4 | 0.52 | 94.8 | 94.0–97.7 | 96.2 | 95.0–97.6 | 0.65 | 95.8 | 94.3–97.2 | 96.6 | 95.4–97.8 | 0.38 | 95.4 | 94.5–96.3 | 94.9 | 93.4–96.5 | 0.61 | |
| OAR | Pmap | DMean (Gy) | 39.4 | 36.4–41.9 | 30.1 | 27.8–34.9 | 0.0001 | 36.6 | 32.9–40.5 | 27.7 | 26.7–29.8 | <0.0001 | 14.6 | 11.1–18.1 | 10.3 | 6.8–13.8 | 0.08 | 28.8 | 24.2–33.4 | 22.1 | 18.3–26.0 | 0.03 |
| Spinal cord | DMax (Gy) | 37.5 | 33.9–38.4 | 38.4 | 35.3–39.4 | 0.24 | 38.2 | 35.7–38.5 | 38.6 | 35.1–40.1 | 0.25 | 32.5 | 28.8–36.2 | 31.9 | 27.7–32.2 | 0.84 | 33.9 | 31.8–35.9 | 34.1 | 31.7–36.6 | 0.88 | |
| LungH | DMean (Gy) | 21.1 | 18.6–23.5 | 22.5 | 20.1–23.1 | 0.54 | 21.1 | 17.2–23.7 | 21.8 | 19.4–23 | 0.93 | 21.2 | 18.3–24.1 | 21.4 | 17.8–25.1 | 0.92 | 20.8 | 19.3–22.4 | 21.7 | 19.8–23.5 | 0.48 | |
| DMax (Gy) | 68 | 62.7–69.5 | 67.5 | 63.4–69.9 | 0.48 | 66.5 | 61.9–69.4 | 68.1 | 62.8–70.3 | 0.29 | 65.4 | 61.7–69.0 | 68.0 | 66.6–69.5 | 0.16 | 65.5 | 63.5–67.5 | 67.4 | 66.4–68.4 | 0.09 | ||
| V5% (%) | 64.4 | 57.3– 72.0 | 71.3 | 63.9–80.4 | 0.22 | 64.9 | 57.6–80.9 | 69.1 | 59.1–92.4 | 0.58 | 68.2 | 61.6–74.7 | 63.6 | 53.4–73.7 | 0.43 | 67.1 | 62.5–71.7 | 68.2 | 62.3–74.0 | 0.77 | ||
| V10% (%) | 56.2 | 49.3–61.6 | 59.8 | 52.2–66.8 | 0.27 | 56.2 | 50.3–68.8 | 58.5 | 52.2–82.1 | 0.68 | 59.9 | 53.6–66.2 | 56.0 | 46.5–65.5 | 0.48 | 58.1 | 53.9–62.3 | 58.6 | 53.5–63.8 | 0.88 | ||
| V13% (%) | 51.5 | 40.1–57.5 | 52.7 | 49.1–60.9 | 0.32 | 52.2 | 48.1–62.6 | 51.3 | 48.5–67.2 | 0.78 | 54.5 | 48.1–60.8 | 51.9 | 43.0–60.7 | 0.62 | 52.2 | 48.3–56.1 | 53.4 | 48.8–58.0 | 0.69 | ||
| V20% (%) | 39.2 | 34.3 – 46.0 | 40.5 | 36.9–45.7 | 0.39 | 39.2 | 33.3–46.1 | 37.5 | 36.4–47 | 0.68 | 41.6 | 35.3–47.8 | 41.4 | 34.2–48.6 | 0.97 | 40.1 | 36.8–43.5 | 41.8 | 38.1–45.6 | 0.50 | ||
| V30% (%) | 28.1 | 25.8–32.4 | 28.8 | 24.8–34.4 | 0.65 | 26.4 | 20.5–33.8 | 27.6 | 23.9–35.4 | 0.55 | 28.3 | 23.0–33.6 | 29.8 | 24.0–35.6 | 0.68 | 28.3 | 25.4–31.3 | 30.1 | 27.1–33.2 | 0.40 | ||
| LungC | DMean (Gy) | 7.7 | 5.8–10.3 | 8.2 | 6.3–10.5 | 0.85 | 7.2 | 5.4–11.2 | 9.1 | 6.3–13.2 | 0.52 | 8.7 | 7.4–10.0 | 7.7 | 6.4–9.1 | 0.26 | 8.4 | 7.5–9.4 | 8.2 | 7.1–9.3 | 0.74 | |
| DMax (Gy) | 48.2 | 34.0–56.6 | 49.8 | 43.1 – 62.0 | 0.71 | 47.6 | 22.0–55.3 | 51.2 | 40.9–62.6 | 0.58 | 51.7 | 44.0–59.5 | 53.6 | 46.3–61.0 | 0.71 | 47.9 | 42.4–53.4 | 50.4 | 45.2–55.5 | 0.51 | ||
| V5% (%) | 55.4 | 43.7–62.7 | 58 | 46.4–64.7 | 0.93 | 57.3 | 36.9 – 76.0 | 61.1 | 46.5–91.8 | 0.82 | 57.3 | 48.8–65.8 | 49.5 | 40.0–59.1 | 0.21 | 56.4 | 50.8–62.1 | 53.4 | 46.6–60.2 | 0.49 | ||
| V10% (%) | 32.4 | 15.1–38.9 | 34.5 | 17.4–38.8 | 0.95 | 28.5 | 12.1 – 43.0 | 37.5 | 17.4–44.3 | 0.46 | 36.1 | 29.3–42.8 | 30.9 | 24.8–37.1 | 0.25 | 58.1 | 53.9–62.3 | 58.6 | 53.5–63.8 | 0.88 | ||
| V13% (%) | 18.4 | 8.9–28.5 | 19.4 | 11.5–27.2 | 0.86 | 15.3 | 5.3–33.7 | 19.4 | 12.2–34.8 | 0.35 | 25.8 | 19.6–32.1 | 21.2 | 16.9–25.5 | 0.21 | 52.2 | 48.3–56.1 | 53.4 | 48.8–58.0 | 0.69 | ||
| V20% (%) | 4.6 | 1.9–9.7 | 5.4 | 2.4–11.9 | 0.6 | 4.3 | 0.3–9.4 | 6.1 | 2.2–17.1 | 0.27 | 9.5 | 6.3–12.7 | 8.4 | 6.0–10.8 | 0.56 | 40.1 | 36.8–43.5 | 41.8 | 38.1–45.6 | 0.50 | ||
| V30% (%) | 1 | 0.1–3.1 | 1.2 | 0.4–4.5 | 0.66 | 0.9 | 0.0–3.5 | 1.2 | 0.4–5.1 | 0.38 | 2.9 | 1.4–4.4 | 2.7 | 1.4–4.0 | 0.81 | 28.3 | 25.4–31.3 | 30.1 | 27.1–33.2 | 0.40 | ||
| 2Lungs | DMean (Gy) | 15.0 | 12.6–16.7 | 16.0 | 14.2–16.6 | 0.38 | 15.2 | 12.2–16.7 | 16 | 12.6–17.7 | 0.31 | 15.6 | 13.1–18.0 | 13.6 | 11.7–15.5 | 0.20 | 14.9 | 13.7–16.2 | 14.6 | 13.5–15.8 | 0.72 | |
| V13% (%) | 35 | 29.2–42.9 | 38.2 | 33.7–43.1 | 0.29 | 33.5 | 28.3–44.1 | 40.1 | 30.5–45.5 | 0.41 | 38.6 | 34.5–42.8 | 34.3 | 29.1–39.5 | 0.19 | 36.4 | 33.6–39.2 | 36.8 | 33.2–40.3 | 0.88 | ||
| V20% (%) | 22.9 | 21.3–26.3 | 26.6 | 21.7–28.5 | 0.22 | 22.2 | 20.9–26 | 24.7 | 20.9–32.3 | 0.33 | 24.7 | 21.8–27.6 | 23.1 | 19.9–26.4 | 0.45 | 24.1 | 22.4–25.9 | 24.8 | 22.7–26.8 | 0.63 | ||
| V30 (%) | 15 | 13.2–19.4 | 18.4 | 14.9–19.5 | 0.43 | 13.7 | 11.2–19.5 | 18.2 | 12.5–21.1 | 0.38 | 14.8 | 12.5–17.1 | 14.8 | 12.4–17.2 | 1.00 | 15.5 | 14.2–16.9 | 16.2 | 14.8–17.7 | 0.47 | ||
| Heart | DMean (Gy) | 9.8 | 5.5–12.3 | 10.9 | 7.6–16.4 | 0.4 | 11.3 | 6.2–18.9 | 12.1 | 6.8–18.3 | 0.78 | 10.1 | 7.3–12.9 | 8.6 | 5.3–11.9 | 0.47 | 9.9 | 8.1–11.8 | 10.2 | 8.1–12.2 | 0.87 | |
| V30% (%) | 5.6 | 1.7 – 13.0 | 9.4 | 4.3–11.5 | 0.45 | 5 | 1.7–14.2 | 8 | 4–11.9 | 0.52 | 9.3 | 5.9–12.7 | 8.6 | 4.8–12.4 | 0.75 | 9.6 | 6.3–12.8 | 9.5 | 7.0–12.0 | 1.00 | ||
| V40% (%) | 3.5 | 0.7–4.9 | 4.2 | 1.9–7.1 | 0.26 | 2.8 | 0.7–4.9 | 3.6 | 1.7–7.2 | 0.55 | 5.1 | 3.3–6.8 | 5.1 | 2.9–7.2 | 1.0 | 4.5 | 3.2–5.8 | 5.5 | 3.9–7.1 | 0.32 | ||
| Results of the Prediction Model | Cohort 1 + 2 n: 24 Patients | Cohort 1 n: 14 Patients | Cohort 3 n: 20 Patients | Cohort 1 + 2 + 3 n: 44 Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial Planning | Optimized Planning | p | Initial Planning | Optimized Planning | p | Initial Planning | Optimized Planning | p | Initial Planning | Optimized Planning | p | ||
| Risk probability of APT ≥ grade 2 | Mean (%, SD) | 90.8 (17.5) | 60.9 (42.9) | 0.003 | 93.9 (2.7) | 43.9 (44.6) | 0.0003 | 84.2 (26.0) | 35.4 (17.6) | 0.0001 | 87.8 (23.8) | 45.3 (19.1) | <0.0001 |
| Median (%, CI 95%) | 94.5 (92.9–95.4) | 91.4 (7.9–93.7) | 0.002 | 94.2 (91.9–95.5) | 9.9 (7.5–93.6) | 0.004 | 93.0 (89.8–94.1) | 7.4 (5.2–89.1) | 0.0001 | 93.9 (92.6–94.7) | 9.1 (7.3–90.6) | <0.0001 | |
| Patients classified at high risk of APT (%) | 100.0% | 58.3% | 0.001 | 100% | 50% | 0.006 | 100.0% | 45.0% | 0.0004 | 100% | 56.8% | <0.0001 | |
| Mean IC | 96.0 | 94.0 | 0.25 | 92.2 | 96.4 | 0.83 | 98.3 | 98.6 | 0.89 | 97.0 | 96.1 | 0.89 | |
| Mean HIV | 94.0 | 94.0 | 0.77 | 94.3 | 95.5 | 0.36 | 93.5 | 94.2 | 0.59 | 93.8 | 93.9 | 0.87 | |

- -
- A successful case (Patient #1):
- o
- Patient’s characteristics: male patient, 74 years old; COPD: no; MEVS: 78.0% of the theorical value; history of smoking; chemotherapy: neoadjuvant and concurrent; AJCC stage: IIIA.
- o
- 3a (initial dose map): PTV95 = 96.4%; DMeanPmap = 36.5 Gy; ProbAPT = 91.3%.
- o
- 3b (optimized dose map): PTV95 = 97.7%; DMeanPmap = 24.8 Gy; ProbAPT = 7.5%.
- -
- An unsuccessful case (Patient #6):
- o
- Patient’s characteristics: male patient, 54 years old; COPD: no; MEVS: 46.8% of the theorical value; history of smoking; chemotherapy: neoadjuvant and concurrent; AJCC stage: IIIB.
- o
- 3c (initial dose map): PTV95 = 93.6%; DMeanPmap = 60.2 Gy; ProbAPT = 97.7%.
- o
- 3d (optimized dose map): PTV95 = 68.3%; DMeanPmap = 44.2 Gy; ProbAPT = 96.3%.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Grills, I.S.; Yan, D.; Martinez, A.A.; Vicini, F.A.; Wong, J.W.; Kestin, L.L. Potential for Reduced Toxicity and Dose Escalation in the Treatment of Inoperable Non-Small-Cell Lung Cancer: A Comparison of Intensity-Modulated Radiation Therapy (IMRT), 3D Conformal Radiation, and Elective Nodal Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 875–890. [Google Scholar] [CrossRef]
- Christian, J.A.; Bedford, J.L.; Webb, S.; Brada, M. Comparison of Inverse-Planned Three-Dimensional Conformal Radiotherapy and Intensity-Modulated Radiotherapy for Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Tripoli, A.; Molino, L.; Cacciola, A.; Lillo, S.; Parisi, S.; Umina, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; et al. How Much Daily Image-Guided Volumetric Modulated Arc Therapy Is Useful for Proctitis Prevention With Respect to Static Intensity Modulated Radiotherapy Supported by Topical Medications Among Localized Prostate Cancer Patients? Anticancer Res. 2021, 41, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Valenti, V.; Puliafito, I.; Illari, S.I.; Marchese, V.A.; Borzì, G.R. Volumetric Modulated Arc Therapy Capabilities for Treating Lower-Extremity Skin Affected by Several Merkel Cell Carcinoma Nodules: When Technological Advances Effectively Achieve the Palliative Therapeutic Goal While Minimising the Risk of Potential Toxicities. Med. Kaunas Lith. 2021, 57, 1379. [Google Scholar] [CrossRef]
- Khalil, A.A.; Hoffmann, L.; Moeller, D.S.; Farr, K.P.; Knap, M.M. New Dose Constraint Reduces Radiation-Induced Fatal Pneumonitis in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Intensity-Modulated Radiotherapy. Acta Oncol. Stockh. Swed. 2015, 54, 1343–1349. [Google Scholar] [CrossRef] [Green Version]
- Wijsman, R.; Dankers, F.; Troost, E.G.C.; Hoffmann, A.L.; van der Heijden, E.H.F.M.; de Geus-Oei, L.-F.; Bussink, J. Comparison of Toxicity and Outcome in Advanced Stage Non-Small Cell Lung Cancer Patients Treated with Intensity-Modulated (Chemo-)Radiotherapy Using IMRT or VMAT. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 122, 295–299. [Google Scholar] [CrossRef]
- Ling, D.C.; Hess, C.B.; Chen, A.M.; Daly, M.E. Comparison of Toxicity Between Intensity-Modulated Radiotherapy and 3-Dimensional Conformal Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2016, 17, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Bourbonne, V.; Delafoy, A.; Lucia, F.; Quéré, G.; Pradier, O.; Schick, U. Toxicity after Volumetric Modulated Arc Therapy for Lung Cancer: A Monocentric Retrospective Study. Transl. Lung Cancer Res. 2021, 10, 156–166. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Palma, G.; Monti, S.; Xu, T.; Scifoni, E.; Yang, P.; Hahn, S.M.; Durante, M.; Mohan, R.; Liao, Z.; Cella, L. Spatial Dose Patterns Associated With Radiation Pneumonitis in a Randomized Trial Comparing Intensity-Modulated Photon Therapy With Passive Scattering Proton Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1124–1132. [Google Scholar] [CrossRef]
- Palma, G.; Monti, S.; Cella, L. Voxel-Based Analysis in Radiation Oncology: A Methodological Cookbook. Phys. Medica PM Int. J. Devoted Appl. Phys. Med. Biol. Off. J. Ital. Assoc. Biomed. Phys. AIFB 2020, 69, 192–204. [Google Scholar] [CrossRef]
- Siva, S.; Devereux, T.; Ball, D.L.; MacManus, M.P.; Hardcastle, N.; Kron, T.; Bressel, M.; Foroudi, F.; Plumridge, N.; Steinfort, D.; et al. Ga-68 MAA Perfusion 4D-PET/CT Scanning Allows for Functional Lung Avoidance Using Conformal Radiation Therapy Planning. Technol. Cancer Res. Treat. 2016, 15, 114–121. [Google Scholar] [CrossRef]
- Siva, S.; Thomas, R.; Callahan, J.; Hardcastle, N.; Pham, D.; Kron, T.; Hicks, R.J.; MacManus, M.P.; Ball, D.L.; Hofman, M.S. High-Resolution Pulmonary Ventilation and Perfusion PET/CT Allows for Functionally Adapted Intensity Modulated Radiotherapy in Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2015, 115, 157–162. [Google Scholar] [CrossRef]
- Bucknell, N.; Hardcastle, N.; Jackson, P.; Hofman, M.; Callahan, J.; Eu, P.; Iravani, A.; Lawrence, R.; Martin, O.; Bressel, M.; et al. Single-Arm Prospective Interventional Study Assessing Feasibility of Using Gallium-68 Ventilation and Perfusion PET/CT to Avoid Functional Lung in Patients with Stage III Non-Small Cell Lung Cancer. BMJ Open 2020, 10, e042465. [Google Scholar] [CrossRef]
- Bourbonne, V.; Lucia, F.; Jaouen, V.; Bert, J.; Rehn, M.; Pradier, O.; Visvikis, D.; Schick, U. Development and Prospective Validation of a Spatial Dose Pattern Based Model Predicting Acute Pulmonary Toxicity in Patients Treated with Volumetric Arc-Therapy for Locally Advanced Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 164, 43–49. [Google Scholar] [CrossRef]
- Kong, F.-M.S.; Ritter, T.; Quint, D.J.; Senan, S.; Gaspar, L.E.; Komaki, R.U.; Hurkmans, C.W.; Timmerman, R.; Bezjak, A.; Bradley, J.D.; et al. Consideration of Dose Limits for Organs at Risk of Thoracic Radiotherapy: Atlas for Lung, Proximal Bronchial Tree, Esophagus, Spinal Cord, Ribs, and Brachial Plexus. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1442–1457. [Google Scholar] [CrossRef] [Green Version]
- Bhandare, N.; Jackson, A.; Eisbruch, A.; Pan, C.C.; Flickinger, J.C.; Antonelli, P.; Mendenhall, W.M. Radiation Therapy and Hearing Loss. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S50–S57. [Google Scholar] [CrossRef] [Green Version]
- Rudra, S.; Al-Hallaq, H.A.; Feng, C.; Chmura, S.J.; Hasan, Y. Effect of RTOG Breast/Chest Wall Guidelines on Dose-Volume Histogram Parameters. J. Appl. Clin. Med. Phys. 2014, 15, 4547. [Google Scholar] [CrossRef]
- Yanagi, T.; Kamada, T.; Tsuji, H.; Imai, R.; Serizawa, I.; Tsujii, H. Dose-Volume Histogram and Dose-Surface Histogram Analysis for Skin Reactions to Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcoma. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2010, 95, 60–65. [Google Scholar] [CrossRef]
- Graham, M.V.; Purdy, J.A.; Emami, B.; Harms, W.; Bosch, W.; Lockett, M.A.; Perez, C.A. Clinical Dose-Volume Histogram Analysis for Pneumonitis after 3D Treatment for Non-Small Cell Lung Cancer (NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 323–329. [Google Scholar] [CrossRef]
- Bourbonne, V.; Da-Ano, R.; Jaouen, V.; Lucia, F.; Dissaux, G.; Bert, J.; Pradier, O.; Visvikis, D.; Hatt, M.; Schick, U. Radiomics Analysis of 3D Dose Distributions to Predict Toxicity of Radiotherapy for Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 155, 144–150. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, J.; Sun, J.; Li, B.; Wang, Z.; Wei, L.; Yin, Y.; Sun, H.; Fu, Z.; Zhang, X.; et al. Functional and Biologic Metrics for Predicting Radiation Pneumonitis in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Chemoradiotherapy. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2012, 14, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.-Y.; Siva, S.; Steinfort, D.P.; Callahan, J.; Eu, P.; Irving, L.B.; Hicks, R.J.; Hofman, M.S. Correlation of 68Ga Ventilation-Perfusion PET/CT with Pulmonary Function Test Indices for Assessing Lung Function. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1718–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadalà, R.E.; Santacaterina, A.; Sindoni, A.; Platania, A.; Arcudi, A.; Ferini, G.; Mazzei, M.M.; Marletta, D.; Rifatto, C.; Risoleti, E.V.I.; et al. Stereotactic Body Radiotherapy in Non-Operable Lung Cancer Patients. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2016, 18, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourbonne, V.; Lucia, F.; Jaouen, V.; Bert, J.; Pradier, O.; Visvikis, D.; Schick, U. VMAT-Based Planning Allows Sparing of a Spatial Dose Pattern Associated with Radiation Pneumonitis in Patients Treated with Radiotherapy for a Locally Advanced Lung Cancer. Cancers 2022, 14, 3702. https://doi.org/10.3390/cancers14153702
Bourbonne V, Lucia F, Jaouen V, Bert J, Pradier O, Visvikis D, Schick U. VMAT-Based Planning Allows Sparing of a Spatial Dose Pattern Associated with Radiation Pneumonitis in Patients Treated with Radiotherapy for a Locally Advanced Lung Cancer. Cancers. 2022; 14(15):3702. https://doi.org/10.3390/cancers14153702
Chicago/Turabian StyleBourbonne, Vincent, Francois Lucia, Vincent Jaouen, Julien Bert, Olivier Pradier, Dimitris Visvikis, and Ulrike Schick. 2022. "VMAT-Based Planning Allows Sparing of a Spatial Dose Pattern Associated with Radiation Pneumonitis in Patients Treated with Radiotherapy for a Locally Advanced Lung Cancer" Cancers 14, no. 15: 3702. https://doi.org/10.3390/cancers14153702






