Emerging Roles of the Nervous System in Gastrointestinal Cancer Development
Abstract
:Simple Summary
Abstract
1. Introduction
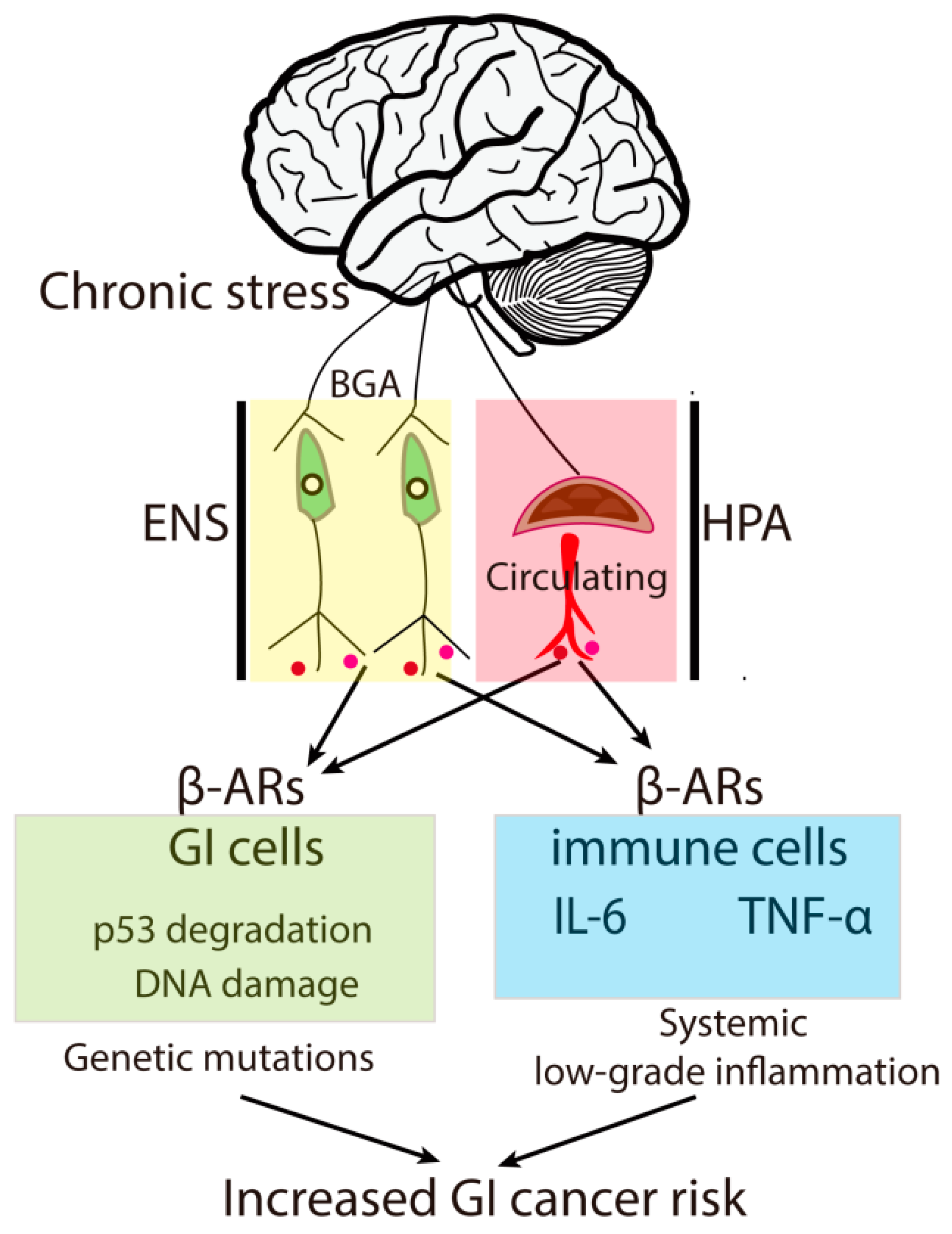
2. Chronic Stress Contributes to GI Tumor Risk
3. GI Tumorigenesis Initiates Active Neurogenesis and Neural Infiltration
4. Neural Infiltration Fuels GI Cancer Progression
5. The Roles of Neurotransmitter, Neurotrophin and Neuropeptide Receptor Pathways in Gastrointestinal Cancer Development
5.1. β-Adrenergic Receptors
5.2. Acetylcholine Receptors
5.3. Glutamate Receptors (GluRs)
5.4. Dopamine (DA) and 5-HT (Serotonin) Receptors
| Receptors | Expression in Cancer/Mechanism | Downstream Effectors/Mechanisms | Effects | Reference |
|---|---|---|---|---|
| β-adrenergic receptors (β-ARs) | ||||
| β1-AR | Upregulated in EC | ERK, COX2 | proliferation | [79,125] |
| Upregulated in metastatic GC | [126] | |||
| Expressed in PC | AKT, ERK, HIF-1α | [127] | ||
| Upregulated in CRC | [79] | |||
| β2-AR | Upregulated in GC | STAT3, AP-1, MUC4 | Proliferation, Chemoresistance, metastasis | [126,128,129] |
| Upregulated in PC | HIF-1α, ERK, PCBP2, AKR1B1, CDC42 | Proliferation, Angiogenesis, invasion | [83,85,87,127,130,131] | |
| Upregulated in CRC | EGFR-Akt/ERK | Proliferation, viability | [86,132] | |
| Upregulated in HCC | YB-1/β-catenin | metastasis | [76,133] | |
| β3-AR | Upregulated in CRC | [134] | ||
| Acetylcholine receptors (AChRs) | ||||
| α3nAChR | Expressed in ESCC | YAP1 | Proliferation, migration | [135] |
| α5nAChR | Expressed in GC | AKT | Chemoresistance | [136] |
| α7nAChR | Upregulated in EC | AKT/FOXO1/OTUD3/VEGF | lymphatic metastasis | [137] |
| Expressed in GC | E-cadherin, ZEB-1, fibronectin, AKT, MCL-1, BCL-2 | Migration, chemoresistance | [73,138,139] | |
| Upregulated in PC | MUC4, 29864419 | Stemness, metastasis | [91,140] | |
| Expressed in CRC | NF-κB, Fibronectin, Snail, ZEB1 | Migration | [141,142,143,144] | |
| Upregulated in CC | EMT, ERK | Proliferation, viability, migration | [74,145] | |
| Upregulated in HCC | TRAF6/NF-κB | Proliferation, chemoresistance | [72,93] | |
| m1AChR | Expressed in HCC | EMT, PI3K/AKT | Invasion | [146] |
| m3AChR | Upregulated in GC | EGFR, AKT, ERK | Proliferation, viability | [100,147,148] |
| Upregulated in PC | [149] | |||
| Upregulated in CRC | Calcium, MMP7, EGFR, p38, ERK, AKT | Proliferation, viability | [150,151,152,153] | |
| Upregulated in CC | Proliferation, metastasis | [154] | ||
| Glutamate receptors (GluRs) | ||||
| AMPA receptor (GluR1–4) | Downregulated in PC | Kras-MAPK | invasion | [108] |
| Downregulated in CRC (GluR4) /DNA methylation | [155] | |||
| NMDA receptors (NR1–3) | Upregulated in PC | AKT, ERK, CaMK II, HIF-1α | Proliferation, migration | [156,157] |
| Expressed in GC | proliferation | [113] | ||
| Upregulated in CRC (NR2D) | HIF-1α, AKT, ERK, CaMK II | migration, angiogenesis | [110,158] | |
| Upregulated in HCC | [159] | |||
| Kainate receptor | Downregulated in GC (GRIK2) /DNA methylation | Impaired Growth, migration | [160] | |
| Upregulated in GC (GRIK3) | [161] | |||
| metabotropic glutamate receptors (mGluRs) | Upregulated in PC (mGluR1) | PI3K/AKT/mTOR | Viability | [162,163] |
| Upregulated in CRC (mGluR4) | 5-FU resistance, recurrence | [109,164] | ||
| Expressed in HCC (mGluR5) | Calcium, MAPK | Chemoresistance | [165] | |
| Dopamine receptors (DRs) | ||||
| DRD1 | Upregulated in ESCC | [166] | ||
| Expressed in PC | Stemness, growth, migration | [167] | ||
| Upregulated in HCC | cAMP/PI3K/AKT/CREB | Proliferation, metastasis | [119] | |
| DRD2 | Upregulated in ESCC | lymph node metastasis | [166] | |
| Upregulated in GC | Proliferation | [120] | ||
| Upregulated in PC | Calcium, PKA | Proliferation, viability, migration | [121] | |
| DRD5 | Upregulated in EC | mTOR, AKT, Warburg effect | proliferation | [168] |
| Expressed in GC | mTOR | Impaired growth, autophagy | [169] | |
| Expressed in CRC | [169] | |||
| Upregulated in HCC | CD133, OCT4, and EpCam | Impaired growth, stemness, migration | [170] | |
| Serotonin (5-HT) receptor | ||||
| 5-HTRs | Expressed in CRC (5-HT1B, 5-HT3A, 5-HT3, and 5-HT4) | Calcium/CaMKIIα, NLRP3 inflammasome, MMP-12 | Growth, angiogenesis, viability | [122,171,172,173,174] |
| Expressed in PC (5-HT1B, 5-HT1D and 5-HT2B) | PI3K/AKT/mTOR, Warburg effect, uPAR/MMP-2, Integrin/Src/Fak | Growth, invasion | [123,124] | |
| Expressed in CC (5-HT1A, 5-HT2A, 5-HT2B, 5-HT4 and 5-HT6) | Growth | [175] | ||
| Expressed in HCC (5-HT1B and 5-HT2B) | AKT, FOXO3a | Proliferation | [176] |
5.5. Neurotrophin Receptors
5.6. Neuropeptide Receptors
6. Neural Infiltration Is an Integral Part of GI Tumor Microenvironment (TME)
6.1. Angiogenesis
6.2. Immune Regulation
6.3. Chemotactic Effects
7. Intervention of Neural Infiltration as Measurements of Cancer Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mullins, C.S.; Schafmayer, C.; Zeissig, S.; Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 2021, 41, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Carney, C.P.; Jones, L.; Woolson, R.F.; Noyes, R., Jr.; Doebbeling, B.N. Relationship between depression and pancreatic cancer in the general population. Psychosom. Med. 2003, 65, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Gregory, E.; Dugan, R.; David, G.; Song, Y.H. The biology and engineered modeling strategies of cancer-nerve crosstalk. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188406. [Google Scholar] [CrossRef]
- Kozlowska, A.; Kwiatkowski, P.; Oponowicz, A.; Majewski, M.; Kmiec, Z.; Godlewski, J. Myenteric plexuses atrophy in the vicinity of colorectal cancer tissue is not caused by apoptosis or necrosis. Folia Histochem. Cytobiol. 2016, 54, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Mou, H.; Liu, M.; Ran, Z.; Li, X.; Li, J.; Ou, Y. Multiomics characteristics of neurogenesis-related gene are dysregulated in tumor immune microenvironment. Npj. Genom. Med. 2021, 6, 37. [Google Scholar] [CrossRef]
- Yaman, I.; Cobanoglu, D.A.; Xie, T.; Ye, Y.; Amit, M. Advances in understanding cancer-associated neurogenesis and its implications on the neuroimmune axis in cancer. Pharmacol. Ther. 2022, 239, 108199. [Google Scholar] [CrossRef]
- Griffin, N.; Faulkner, S.; Jobling, P.; Hondermarck, H. Targeting neurotrophin signaling in cancer: The renaissance. Pharmacol. Res. 2018, 135, 12–17. [Google Scholar] [CrossRef]
- Baraldi, J.H.; Martyn, G.V.; Shurin, G.V.; Shurin, M.R. Tumor Innervation: History, Methodologies, and Significance. Cancers 2022, 14, 1979. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Al Omran, Y.; Aziz, Q. The brain-gut axis in health and disease. Adv. Exp. Med. Biol. 2014, 817, 135–153. [Google Scholar]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Shah, E.; Rezaie, A.; Riddle, M.; Pimentel, M. Psychological disorders in gastrointestinal disease: Epiphenomenon, cause or consequence? Ann. Gastroenterol. 2014, 27, 224–230. [Google Scholar]
- North, C.S.; Hong, B.A.; Alpers, D.H. Relationship of functional gastrointestinal disorders and psychiatric disorders: Implications for treatment. World J. Gastroenterol. 2007, 13, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Rosenstock, S.; Jacobsen, R.K.; Jorgensen, T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin. Gastroenterol. Hepatol. 2015, 13, 498–506.e491. [Google Scholar] [CrossRef]
- Huang, J.; Valdimarsdottir, U.; Fall, K.; Ye, W.; Fang, F. Pancreatic cancer risk after loss of a child: A register-based study in Sweden during 1991-2009. Am. J. Epidemiol. 2013, 178, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, B.; Valdimarsdottir, U.; Sundstrom, K.; Sparen, P.; Lambe, M.; Fall, K.; Fang, F. Loss of a parent and the risk of cancer in early life: A nationwide cohort study. Cancer Causes Control. 2014, 25, 499–506. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and anxiety in relation to cancer incidence and mortality: A systematic review and meta-analysis of cohort studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, X.; Lin, E.J.; Wang, C.; Choi, E.Y.; Riban, V.; Lin, B.; During, M.J. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 2010, 142, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Gan, Y.; Fan, Y.; Wu, Y.; Lin, H.; Song, Y.; Cai, X.; Yu, X.; Pan, W.; Yao, M.; et al. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci. Rep. 2015, 5, 7856. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Cai, W.; Lu, X.; He, W.; Li, D.; Zhong, H.; Yang, L.; Li, S.; Li, H.; Rafee, S.; et al. Chronic stress accelerates the process of gastric precancerous lesions in rats. J. Cancer 2021, 12, 4121–4133. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.R.; Ma, Q.; Hao, C.Q.; Guan, C.T.; Li, B.Y.; Wang, J.W.; Li, X.Q.; Liu, Z.K.; Wei, W.W. Analysis of psychological status and relevant factors of patients with esophageal and gastric cardia precancerous lesions in Linzhou of Henan. Zhonghua Yu Fang Yi Xue Za Zhi 2017, 51, 670–674. [Google Scholar]
- Baritaki, S.; de Bree, E.; Chatzaki, E.; Pothoulakis, C. Chronic Stress, Inflammation, and Colon Cancer: A CRH System-Driven Molecular Crosstalk. J. Clin. Med 2019, 8, 1669. [Google Scholar] [CrossRef] [Green Version]
- Riley, V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science 1981, 212, 1100–1109. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Preacher, K.J.; MacCallum, R.C.; Atkinson, C.; Malarkey, W.B.; Glaser, R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl. Acad. Sci. USA 2003, 100, 9090–9095. [Google Scholar] [CrossRef] [Green Version]
- Dentino, A.N.; Pieper, C.F.; Rao, M.K.; Currie, M.S.; Harris, T.; Blazer, D.G.; Cohen, H.J. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J. Am. Geriatr. Soc. 1999, 47, 6–11. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Garand, L.; Buckwalter, K.C.; Reimer, T.T.; Hong, S.Y.; Lubaroff, D.M. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M434–M439. [Google Scholar] [CrossRef]
- Miller, E.S.; Apple, C.G.; Kannan, K.B.; Funk, Z.M.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Chronic stress induces persistent low-grade inflammation. Am. J. Surg. 2019, 218, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.; Liu, L.; Zhang, C.; Zheng, T.; Wang, J.; Lin, M.; Zhao, Y.; Wang, X.; Levine, A.J.; Hu, W. Chronic restraint stress attenuates p53 function and promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 7013–7018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oertel, H. Innervation and Tumour Growth: A Preliminary Report. Can. Med. Assoc. J. 1928, 18, 135–139. [Google Scholar]
- Shapiro, D.M.; Warren, S. Cancer innervation. Cancer Res. 1949, 9, 707–711. [Google Scholar] [PubMed]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Zheng, B.; Wang, H.; Xia, Q.; Chen, L. Nervous system and primary liver cancer. Biochim. Biophys. Acta Rev. Cancer 2018, 1869, 286–292. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, L.; Tao, M.; Fu, W.; Xiu, D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin. J. Cancer Res. 2016, 28, 180–186. [Google Scholar] [CrossRef]
- Griffin, N.; Rowe, C.W.; Gao, F.; Jobling, P.; Wills, V.; Walker, M.M.; Faulkner, S.; Hondermarck, H. Clinicopathological Significance of Nerves in Esophageal Cancer. Am. J. Pathol. 2020, 190, 1921–1930. [Google Scholar] [CrossRef]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal. Transduct. Target. Ther. 2017, 2, 16036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Cheng, Y.; Zhu, Q.; Yu, Z.; Wu, X.; Huang, K.; Zhou, M.; Han, S.; Zhang, Q. The relationship between overexpression of glial cell-derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J. Int. Med. Res. 2008, 36, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceyhan, G.O.; Schafer, K.H.; Kerscher, A.G.; Rauch, U.; Demir, I.E.; Kadihasanoglu, M.; Bohm, C.; Muller, M.W.; Buchler, M.W.; Giese, N.A.; et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann. Surg. 2010, 251, 923–931. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arese, M.; Bussolino, F.; Pergolizzi, M.; Bizzozero, L.; Pascal, D. Tumor progression: The neuronal input. Ann. Transl. Med. 2018, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Schledwitz, A.; Xie, G.; Raufman, J.P. Exploiting unique features of the gut-brain interface to combat gastrointestinal cancer. J. Clin Investig. 2021, 131, e143776. [Google Scholar] [CrossRef]
- Rademakers, G.; Vaes, N.; Schonkeren, S.; Koch, A.; Sharkey, K.A.; Melotte, V. The role of enteric neurons in the development and progression of colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Villagrana, R.D.; Albores-Garcia, D.; Cervantes-Villagrana, A.R.; Garcia-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal. Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Cavel, O.; Shomron, O.; Shabtay, A.; Vital, J.; Trejo-Leider, L.; Weizman, N.; Krelin, Y.; Fong, Y.; Wong, R.J.; Amit, M.; et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012, 72, 5733–5743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [PubMed] [Green Version]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sivakumar, S.; Bednarsch, J.; Wiltberger, G.; Kather, J.N.; Niehues, J.; de Vos-Geelen, J.; Valkenburg-van Iersel, L.; Kintsler, S.; Roeth, A.; et al. Nerve fibers in the tumor microenvironment in neurotropic cancer-pancreatic cancer and cholangiocarcinoma. Oncogene 2021, 40, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Schonkeren, S.L.; Thijssen, M.S.; Vaes, N.; Boesmans, W.; Melotte, V. The Emerging Role of Nerves and Glia in Colorectal Cancer. Cancers 2021, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Bednarsch, J.; Kather, J.; Tan, X.; Sivakumar, S.; Cacchi, C.; Wiltberger, G.; Czigany, Z.; Ulmer, F.; Neumann, U.P.; Heij, L.R. Nerve Fibers in the Tumor Microenvironment as a Novel Biomarker for Oncological Outcome in Patients Undergoing Surgery for Perihilar Cholangiocarcinoma. Liver Cancer 2021, 10, 260–274. [Google Scholar] [CrossRef]
- Ferdoushi, A.; Griffin, N.; Marsland, M.; Xu, X.; Faulkner, S.; Gao, F.; Liu, H.; King, S.J.; Denham, J.W.; van Helden, D.F.; et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci. Rep. 2021, 11, 7390. [Google Scholar] [CrossRef]
- Gasparini, G.; Pellegatta, M.; Crippa, S.; Lena, M.S.; Belfiori, G.; Doglioni, C.; Taveggia, C.; Falconi, M. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers 2019, 11, 893. [Google Scholar] [CrossRef] [Green Version]
- Terada, T.; Matsunaga, Y. S-100-positive nerve fibers in hepatocellular carcinoma and intrahepatic cholangiocarcinoma: An immunohistochemical study. Pathol. Int. 2001, 51, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, L.; Jiang, S.; Yu, M. Nerve Dependence in Colorectal Cancer. Front. Cell Dev. Biol. 2022, 10, 766653. [Google Scholar] [CrossRef]
- Bednarsch, J.; Tan, X.; Czigany, Z.; Wiltberger, G.; Buelow, R.D.; Boor, P.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Heij, L.R. Limitations of Nerve Fiber Density as a Prognostic Marker in Predicting Oncological Outcomes in Hepatocellular Carcinoma. Cancers 2022, 14, 2237. [Google Scholar] [CrossRef]
- Holland, A.M.; Bon-Frauches, A.C.; Keszthelyi, D.; Melotte, V.; Boesmans, W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol. Life Sci. 2021, 78, 4713–4733. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Powley, T.L. Morphology and distribution of efferent vagal innervation of rat pancreas as revealed with anterograde transport of Dil. Brain Res. 1991, 553, 336–341. [Google Scholar] [CrossRef]
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, X.; Wang, T.; Hu, X.; Hui, X.; Yan, M.; Gao, Q.; Chen, T.; Li, J.; Yao, M.; et al. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology 2011, 53, 493–503. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.M.; Wang, Y.H.; Feng, M.X.; Liu, X.J.; Zhang, Y.L.; Huang, S.; Wu, Z.; Xue, F.; Qin, W.X.; et al. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J. Hepatol. 2014, 60, 1225–1234. [Google Scholar] [CrossRef] [Green Version]
- Deborde, S.; Yu, Y.; Marcadis, A.; Chen, C.H.; Fan, N.; Bakst, R.L.; Wong, R.J. An In Vivo Murine Sciatic Nerve Model of Perineural Invasion. J. Vis. Exp. 2018, 134, 56857. [Google Scholar] [CrossRef] [PubMed]
- Gohrig, A.; Detjen, K.M.; Hilfenhaus, G.; Korner, J.L.; Welzel, M.; Arsenic, R.; Schmuck, R.; Bahra, M.; Wu, J.Y.; Wiedenmann, B.; et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014, 74, 1529–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.S.; Wang, Y.; Li, Q.; Zhang, S.L.; Shi, Y.R. [In vitro interaction of human pancreatic cancer cells and rat dorsal root ganglia: A co-culture model]. Zhonghua Zhong Liu Za Zhi 2012, 34, 259–263. [Google Scholar]
- Wu, F.Q.; Fang, T.; Yu, L.X.; Lv, G.S.; Lv, H.W.; Liang, D.; Li, T.; Wang, C.Z.; Tan, Y.X.; Ding, J.; et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. J. Hepatol. 2016, 65, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Somi, M.H.; Mansoori, B.; Khaze Shahgoli, V.; Derakhshani, A.; Mokhtarzadeh, A.; Shanehbandi, D.; Baradaran, B. Small interfering RNA targeting alpha7 nicotinic acetylcholine receptor sensitizes hepatocellular carcinoma cells to sorafenib. Life Sci. 2020, 244, 117332. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Huang, C.Y.; Cheng, W.L.; Hung, C.S.; Huang, M.T.; Tai, C.J.; Liu, Y.N.; Chen, C.L.; Chang, Y.J. Alpha 7-nicotinic acetylcholine receptor mediates the sensitivity of gastric cancer cells to 5-fluorouracil. Tumour Biol. 2015, 36, 9537–9544. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kang, X.; Liu, G.; Zhang, B.; Hu, X.; Feng, Y. Alpha7-Nicotinic Acetylcholine Receptor Promotes Cholangiocarcinoma Progression and Epithelial-Mesenchymal Transition Process. Dig. Dis. Sci. 2019, 64, 2843–2853. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhao, H.; Li, Q.; Liu, Y.; Zuo, Y.; Xu, Q.; Zuo, H.; Li, Y. Chronic stress model simulated by salbutamol promotes tumorigenesis of gastric cancer cells through beta2-AR/ERK/EMT pathway. J. Cancer 2022, 13, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, L.; Wan, C.; Xiao, M.; Ni, W.; Jiang, F.; Fan, Y.; Lu, C.; Ni, R. A novel beta2-AR/YB-1/beta-catenin axis mediates chronic stress-associated metastasis in hepatocellular carcinoma. Oncogenesis 2020, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Ahn, S.; Shukla, A.K.; Lefkowitz, R.J. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 179–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rains, S.L.; Amaya, C.N.; Bryan, B.A. Beta-adrenergic receptors are expressed across diverse cancers. Oncoscience 2017, 4, 95–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krizanova, O.; Babula, P.; Pacak, K. Stress, catecholaminergic system and cancer. Stress 2016, 19, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sayans, M.; Somoza-Martin, J.M.; Barros-Angueira, F.; Diz, P.G.; Gandara Rey, J.M.; Garcia-Garcia, A. Beta-adrenergic receptors in cancer: Therapeutic implications. Oncol. Res. 2010, 19, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. Beta2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 34, 863–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Lei, J.; Ma, J.; Chen, X.; Sheng, L.; Jiang, Z.; Nan, L.; Xu, Q.; Duan, W.; Wang, Z.; et al. Beta2-adrenogenic signaling regulates NNK-induced pancreatic cancer progression via upregulation of HIF-1alpha. Oncotarget 2016, 7, 17760–17772. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Wu, W.K.; Chu, K.M.; Koo, M.W.; Wong, H.P.; Lam, E.K.; Tai, E.K.; Cho, C.H. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol. Sci. 2007, 96, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, T.; Ma, J.; Ma, Q.; Guo, K.; Guo, J.; Li, X.; Li, W.; Liu, J.; Huang, C.; Wang, F.; et al. Beta2-AR-HIF-1alpha: A novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr. Mol. Med. 2013, 13, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.C.; Li, J.M.; Lee, K.F.; Huang, Y.C.; Wang, K.C.; Lai, H.C.; Cheng, C.C.; Kuo, Y.H.; Shi, C.S. Selective beta2-AR Blockage Suppresses Colorectal Cancer Growth Through Regulation of EGFR-Akt/ERK1/2 Signaling, G1-Phase Arrest, and Apoptosis. J. Cell Physiol. 2016, 231, 459–472. [Google Scholar] [CrossRef]
- Wan, C.; Gong, C.; Zhang, H.; Hua, L.; Li, X.; Chen, X.; Chen, Y.; Ding, X.; He, S.; Cao, W.; et al. Beta2-adrenergic receptor signaling promotes pancreatic ductal adenocarcinoma (PDAC) progression through facilitating PCBP2-dependent c-myc expression. Cancer Lett. 2016, 373, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Chan, R.L.; Lu, L.; Shen, J.; Zhang, L.; Wu, W.K.; Wang, L.; Hu, T.; Li, M.X.; Cho, C.H. Cigarette smoking and gastrointestinal diseases: The causal relationship and underlying molecular mechanisms (review). Int. J. Mol. Med. 2014, 34, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Kabbani, N.; Nordman, J.C.; Corgiat, B.A.; Veltri, D.P.; Shehu, A.; Seymour, V.A.; Adams, D.J. Are nicotinic acetylcholine receptors coupled to G proteins? Bioessays 2013, 35, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Momi, N.; Ponnusamy, M.P.; Kaur, S.; Rachagani, S.; Kunigal, S.S.; Chellappan, S.; Ouellette, M.M.; Batra, S.K. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through alpha7nAChR-mediated MUC4 upregulation. Oncogene 2013, 32, 1384–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaal, C.; Padmanabhan, J.; Chellappan, S. The Role of nAChR and Calcium Signaling in Pancreatic Cancer Initiation and Progression. Cancers 2015, 7, 1447–1471. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Wu, M.; Zhang, S.; Chen, Y.; Lu, C. Alpha7nAChR-mediated recruitment of PP1gamma promotes TRAF6/NF-kappaB cascade to facilitate the progression of Hepatocellular Carcinoma. Mol. Carcinog. 2018, 57, 1626–1639. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohnishi, H.; Sugiura, Y.; Honda, K.; Suematsu, M.; Kawasaki, T.; Deguchi, T.; Fujii, T.; Orihashi, K.; Hippo, Y.; et al. Non-neuronal acetylcholine as an endogenous regulator of proliferation and differentiation of Lgr5-positive stem cells in mice. FEBS J. 2014, 281, 4672–4690. [Google Scholar] [CrossRef] [PubMed]
- Middelhoff, M.; Nienhuser, H.; Valenti, G.; Maurer, H.C.; Hayakawa, Y.; Takahashi, R.; Kim, W.; Jiang, Z.; Malagola, E.; Cuti, K.; et al. Prox1-positive cells monitor and sustain the murine intestinal epithelial cholinergic niche. Nat. Commun 2020, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Raufman, J.P.; Samimi, R.; Shah, N.; Khurana, S.; Shant, J.; Drachenberg, C.; Xie, G.; Wess, J.; Cheng, K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res. 2008, 68, 3573–3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodaira, M.; Kajimura, M.; Takeuchi, K.; Lin, S.; Hanai, H.; Kaneko, E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J. Gastroenterol. 1999, 34, 163–171. [Google Scholar] [CrossRef]
- Ukegawa, J.I.; Takeuchi, Y.; Kusayanagi, S.; Mitamura, K. Growth-promoting effect of muscarinic acetylcholine receptors in colon cancer cells. J. Cancer Res. Clin. Oncol. 2003, 129, 272–278. [Google Scholar] [CrossRef]
- Park, Y.S.; Cho, N.J. EGFR and PKC are involved in the activation of ERK1/2 and p90 RSK and the subsequent proliferation of SNU-407 colon cancer cells by muscarinic acetylcholine receptors. Mol. Cell Biochem. 2012, 370, 191–198. [Google Scholar] [CrossRef]
- Yu, H.; Xia, H.; Tang, Q.; Xu, H.; Wei, G.; Chen, Y.; Dai, X.; Gong, Q.; Bi, F. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci. Rep. 2017, 7, 40802. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfitzinger, P.L.; Fangmann, L.; Wang, K.; Demir, E.; Gurlevik, E.; Fleischmann-Mundt, B.; Brooks, J.; D’Haese, J.G.; Teller, S.; Hecker, A.; et al. Indirect cholinergic activation slows down pancreatic cancer growth and tumor-associated inflammation. J. Exp. Clin. Cancer Res. 2020, 39, 289. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Hamada, T.; Zaidi, S.F.; Oshiro, M.; Lee, J.; Yamamoto, T.; Ishii, Y.; Sasahara, M.; Kadowaki, M. Nicotine suppresses acute colitis and colonic tumorigenesis associated with chronic colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G968–G978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, T.; Yu, F.; Fei, R.; Qian, J.; Chen, W. CHRNA7 inhibits cell invasion and metastasis of LoVo human colorectal cancer cells through PI3K/Akt signaling. Oncol. Rep. 2016, 35, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udupa, S.; Nguyen, S.; Hoang, G.; Nguyen, T.; Quinones, A.; Pham, K.; Asaka, R.; Nguyen, K.; Zhang, C.; Elgogary, A.; et al. Upregulation of the Glutaminase II Pathway Contributes to Glutamate Production upon Glutaminase 1 Inhibition in Pancreatic Cancer. Proteomics 2019, 19, e1800451. [Google Scholar] [CrossRef] [Green Version]
- Stepulak, A.; Rola, R.; Polberg, K.; Ikonomidou, C. Glutamate and its receptors in cancer. J. Neural Transm 2014, 121, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepulak, A.; Luksch, H.; Gebhardt, C.; Uckermann, O.; Marzahn, J.; Sifringer, M.; Rzeski, W.; Staufner, C.; Brocke, K.S.; Turski, L.; et al. Expression of glutamate receptor subunits in human cancers. Histochem. Cell Biol. 2009, 132, 435–445. [Google Scholar] [CrossRef]
- Herner, A.; Sauliunaite, D.; Michalski, C.W.; Erkan, M.; De Oliveira, T.; Abiatari, I.; Kong, B.; Esposito, I.; Friess, H.; Kleeff, J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int. J. Cancer 2011, 129, 2349–2359. [Google Scholar] [CrossRef]
- Chang, H.J.; Yoo, B.C.; Lim, S.B.; Jeong, S.Y.; Kim, W.H.; Park, J.G. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin. Cancer Res. 2005, 11, 3288–3295. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, H.J.; Wragg, J.W.; Ward, S.; Heath, V.L.; Ismail, T.; Bicknell, R. Glutamate dependent NMDA receptor 2D is a novel angiogenic tumour endothelial marker in colorectal cancer. Oncotarget 2016, 7, 20440–20454. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Duan, W.; Liu, Y. Ketamine inhibits aerobic glycolysis in colorectal cancer cells by blocking the NMDA receptor-CaMK II-c-Myc pathway. Clin. Exp. Pharmacol. Physiol. 2020, 47, 848–856. [Google Scholar] [CrossRef]
- Yan, Z.; Li, P.; Xue, Y.; Tian, H.; Zhou, T.; Zhang, G. Glutamate receptor, ionotropic, Nmethyl Daspartateassociated protein 1 promotes colorectal cancer cell proliferation and metastasis, and is negatively regulated by miR2963p. Mol. Med. Rep. 2021, 24, 700. [Google Scholar] [CrossRef]
- Watanabe, K.; Kanno, T.; Oshima, T.; Miwa, H.; Tashiro, C.; Nishizaki, T. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem. Biophys. Res. Commun. 2008, 367, 487–490. [Google Scholar] [CrossRef]
- Xu, D.H.; Li, Q.; Hu, H.; Ni, B.; Liu, X.; Huang, C.; Zhang, Z.Z.; Zhao, G. Transmembrane protein GRINA modulates aerobic glycolysis and promotes tumor progression in gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hanahan, D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell 2013, 153, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fandriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab 1997, 82, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun 2020, 40, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Huang, W.; Tan, Z.; Li, M.; Zhang, L.; Ding, Q.; Wu, X.; Lu, J.; Liu, Y.; Dong, Q.; et al. Dopamine receptor D2 is correlated with gastric cancer prognosis. Oncol. Lett. 2017, 13, 1223–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandaghi, P.; Najafabadi, H.S.; Bauer, A.S.; Papadakis, A.I.; Fassan, M.; Hall, A.; Monast, A.; von Knebel Doeberitz, M.; Neoptolemos, J.P.; Costello, E.; et al. Expression of DRD2 Is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology 2016, 151, 1218–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Fu, B.; Zhang, X.; Zhou, Y.; Yang, M.; Cao, M.; Chen, Y.; Tan, Y.; Hu, R. Overproduction of Gastrointestinal 5-HT Promotes Colitis-Associated Colorectal Cancer Progression via Enhancing NLRP3 Inflammasome Activation. Cancer Immunol. Res. 2021, 9, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Li, J.; Dong, F.Y.; Yang, J.Y.; Liu, D.J.; Yang, X.M.; Wang, Y.H.; Yang, M.W.; Fu, X.L.; Zhang, X.X.; et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017, 153, 277–291.e219. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, N.; Ashour, A.A.; Alpay, S.N.; Ozpolat, B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS ONE 2014, 9, e105245. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, W.K.; Yu, L.; Sung, J.J.; Srivastava, G.; Zhang, S.T.; Cho, C.H. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J. Cell Biochem. 2008, 105, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Han, C.; Wei, B.; Qian, L.; Chen, C.; Guo, L.; Hu, M.; Yu, M.; et al. Catecholamine up-regulates MMP-7 expression by activating AP-1 and STAT3 in gastric cancer. Mol. Cancer 2010, 9, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.T.; Ma, Q.Y.; Zhang, D.; Shen, S.G.; Han, L.; Ma, Y.D.; Li, R.F.; Xie, K.P. HIF-1alpha links beta-adrenoceptor agonists and pancreatic cancer cells under normoxic condition. Acta Pharmacol. Sin. 2010, 31, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, M.; Takahashi, T.; Kurokawa, Y.; Kobayashi, T.; Saito, T.; Ishida, T.; Serada, S.; Fujimoto, M.; Naka, T.; Wada, N.; et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric. Cancer 2021, 24, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kaira, K.; Shimizu, A.; Sato, T.; Takahashi, N.; Ogawa, H.; Yoshinari, D.; Yokobori, T.; Asao, T.; Takeyoshi, I.; et al. Clinical significance of beta2-adrenergic receptor expression in patients with surgically resected gastric adenocarcinoma. Tumour Biol. 2016, 37, 13885–13892. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.B.; Jin, D.D.; Jiao, Y.J.; Ni, W.K.; Liu, J.X.; Qu, L.S.; Lu, C.H.; Ni, R.Z.; Jiang, F.; Chen, W.C. Beta2-AR regulates the expression of AKR1B1 in human pancreatic cancer cells and promotes their proliferation via the ERK1/2 pathway. Mol. Biol. Rep. 2018, 45, 1863–1871. [Google Scholar] [CrossRef]
- Gong, C.; Hu, B.; Chen, H.; Zhu, J.; Nie, J.; Hua, L.; Chen, L.; Fang, Y.; Hang, C.; Lu, Y. Beta2-adrenergic receptor drives the metastasis and invasion of pancreatic ductal adenocarcinoma through activating Cdc42 signaling pathway. J. Mol. Histol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kaira, K.; Motegi, Y.; Yokobori, T.; Takada, T.; Kato, R.; Osone, K.; Takahashi, R.; Suga, K.; Ozawa, N.; et al. Prognostic significance of beta2-adrenergic receptor expression in patients with surgically resected colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xing, W.; Hong, J.; Wang, M.; Huang, Y.; Zhu, C.; Yuan, Y.; Zeng, W. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann. Surg. Oncol. 2012, 19, 3556–3565. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Notarnicola, M.; Caruso, M.G.; Tutino, V.; Scilimati, A. Upregulation of beta3-adrenergic receptor mRNA in human colon cancer: A preliminary study. Oncology 2008, 75, 224–229. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, W.; Xue, L.; Zhang, W.; Zhan, Q. Nicotine activates YAP1 through nAChRs mediated signaling in esophageal squamous cell cancer (ESCC). PLoS ONE 2014, 9, e90836. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Sun, H.; Wu, H.; Zhang, H.; Zhang, X.; Xiao, D.; Ma, X.; Wang, Y. Nicotine Inhibits Cisplatin-Induced Apoptosis via Regulating alpha5-nAChR/AKT Signaling in Human Gastric Cancer Cells. PLoS ONE 2016, 11, e0149120. [Google Scholar]
- Wang, M.; Li, Y.; Xiao, Y.; Yang, M.; Chen, J.; Jian, Y.; Chen, X.; Shi, D.; Ouyang, Y.; Kong, L.; et al. Nicotine-mediated OTUD3 downregulation inhibits VEGF-C mRNA decay to promote lymphatic metastasis of human esophageal cancer. Nat. Commun. 2021, 12, 7006. [Google Scholar] [CrossRef]
- Lien, Y.C.; Wang, W.; Kuo, L.J.; Liu, J.J.; Wei, P.L.; Ho, Y.S.; Ting, W.C.; Wu, C.H.; Chang, Y.J. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann. Surg. Oncol. 2011, 18, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chin-Sheng, H.; Kuo, L.J.; Wei, P.L.; Lien, Y.C.; Lin, F.Y.; Liu, H.H.; Ho, Y.S.; Wu, C.H.; Chang, Y.J. NNK enhances cell migration through alpha7-nicotinic acetylcholine receptor accompanied by increased of fibronectin expression in gastric cancer. Ann. Surg. Oncol. 2012, 19 (Suppl. S3), S580–S588. [Google Scholar] [CrossRef]
- Nimmakayala, R.K.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Chugh, S.; Karmakar, S.; Rauth, S.; Vengoji, R.; Atri, P.; Talmon, G.A.; et al. Cigarette Smoke Induces Stem Cell Features of Pancreatic Cancer Cells via PAF1. Gastroenterology 2018, 155, 892–908.e896. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.N.; Liu, E.S.; Shin, V.Y.; Wu, W.K.; Cho, C.H. The modulating role of nuclear factor-kappaB in the action of alpha7-nicotinic acetylcholine receptor and cross-talk between 5-lipoxygenase and cyclooxygenase-2 in colon cancer growth induced by 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. J. Pharmacol. Exp. Ther. 2004, 311, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.L.; Kuo, L.J.; Huang, M.T.; Ting, W.C.; Ho, Y.S.; Wang, W.; An, J.; Chang, Y.J. Nicotine enhances colon cancer cell migration by induction of fibronectin. Ann. Surg. Oncol. 2011, 18, 1782–1790. [Google Scholar] [CrossRef]
- Novotny, A.; Ryberg, K.; Heiman Ullmark, J.; Nilsson, L.; Khorram-Manesh, A.; Nordgren, S.; Delbro, D.S.; Nylund, G. Is acetylcholine a signaling molecule for human colon cancer progression? Scand J. Gastroenterol. 2011, 46, 446–455. [Google Scholar] [CrossRef]
- Wei, P.L.; Chang, Y.J.; Ho, Y.S.; Lee, C.H.; Yang, Y.Y.; An, J.; Lin, S.Y. Tobacco-specific carcinogen enhances colon cancer cell migration through alpha7-nicotinic acetylcholine receptor. Ann. Surg. 2009, 249, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.K.; Jensen, K.; Hall, C.; O’Brien, A.; Ehrlich, L.; White, T.; Meng, F.; Zhou, T.; Greene, J., Jr.; Bernuzzi, F.; et al. Nicotine Promotes Cholangiocarcinoma Growth in Xenograft Mice. Am. J. Pathol. 2017, 187, 1093–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wu, L.L.; Huan, H.B.; Wen, X.D.; Yang, D.P.; Chen, D.F.; Xia, F. Activation of muscarinic acetylcholine receptor 1 promotes invasion of hepatocellular carcinoma by inducing epithelial-mesenchymal transition. Anticancer Drugs 2020, 31, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Xia, Y.; Yin, K.; Li, Z.; Li, B.; Wang, W.; Xu, H.; Yang, L.; Xu, Z. Muscarinic acetylcholine receptor 3 mediates vagus nerve-induced gastric cancer. Oncogenesis 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. 2016, 37, 2105–2117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiu, D.; Zhan, J.; He, X.; Guo, L.; Wang, J.; Tao, M.; Fu, W.; Zhang, H. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco Targets Ther. 2016, 9, 6719–6726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering, N.A.; Liu, V.; Kim, R.; Weixler, B.; Droeser, R.A.; Arndt, M.; Pozios, I.; Beyer, K.; Kreis, M.E.; Seeliger, H. Blockage of Cholinergic Signaling via Muscarinic Acetylcholine Receptor 3 Inhibits Tumor Growth in Human Colorectal Adenocarcinoma. Cancers 2021, 13, 3220. [Google Scholar] [CrossRef]
- Frucht, H.; Jensen, R.T.; Dexter, D.; Yang, W.L.; Xiao, Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin. Cancer Res. 1999, 5, 2532–2539. [Google Scholar] [PubMed]
- Xie, G.; Cheng, K.; Shant, J.; Raufman, J.P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G755–G763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.; Zimniak, P.; Raufman, J.P. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003, 63, 6744–6750. [Google Scholar] [CrossRef]
- Feng, Y.J.; Zhang, B.Y.; Yao, R.Y.; Lu, Y. Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells. Hepatobiliary Pancreat Dis. Int. 2012, 11, 418–423. [Google Scholar] [CrossRef]
- Vega-Benedetti, A.F.; Loi, E.; Moi, L.; Restivo, A.; Cabras, F.; Deidda, S.; Pretta, A.; Ziranu, P.; Orru, S.; Scartozzi, M.; et al. Colorectal cancer promoter methylation alteration affects the expression of glutamate ionotropic receptor AMPA type subunit 4 alternative isoforms potentially relevant in colon tissue. Hum. Cell 2022, 35, 310–319. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Lin, L.Z.; Tian, R.; Akerman, B. NMDA receptors are important regulators of pancreatic cancer and are potential targets for treatment. Clin. Pharmacol. 2017, 9, 79–86. [Google Scholar] [PubMed] [Green Version]
- Chen, X.; Wu, Q.; You, L.; Chen, S.; Zhu, M.; Miao, C. Propofol attenuates pancreatic cancer malignant potential via inhibition of NMDA receptor. Eur. J. Pharmacol. 2017, 795, 150–159. [Google Scholar] [CrossRef]
- Duan, W.; Hu, J.; Liu, Y. Ketamine inhibits colorectal cancer cells malignant potential via blockage of NMDA receptor. Exp. Mol. Pathol. 2019, 107, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, Y.V.; Golovynska, I.; Dziubenko, N.V.; Kuznietsova, H.M.; Petriv, N.; Skrypkina, I.; Golovynskyi, S.; Stepanova, L.I.; Stohnii, Y.; Garmanchuk, L.V.; et al. NMDA receptor expression during cell transformation process at early stages of liver cancer in rodent models. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G142–G153. [Google Scholar] [CrossRef]
- Wu, C.S.; Lu, Y.J.; Li, H.P.; Hsueh, C.; Lu, C.Y.; Leu, Y.W.; Liu, H.P.; Lin, K.H.; Hui-Ming Huang, T.; Chang, Y.S. Glutamate receptor, ionotropic, kainate 2 silencing by DNA hypermethylation possesses tumor suppressor function in gastric cancer. Int. J. Cancer 2010, 126, 2542–2552. [Google Scholar] [CrossRef]
- Gong, B.; Li, Y.; Cheng, Z.; Wang, P.; Luo, L.; Huang, H.; Duan, S.; Liu, F. GRIK3: A novel oncogenic protein related to tumor TNM stage, lymph node metastasis, and poor prognosis of GC. Tumour Biol. 2017, 39, 1010428317704364. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Li, Y.; Wei, Y.; Xu, Y.; Wang, R.; Fu, Z.; Zheng, S.; Zhou, Q.; Zhou, Y.; Chen, R.; et al. Anticancer effect of HOTTIP regulates human pancreatic cancer via the metabotropic glutamate receptor 1 pathway. Oncol. Lett. 2018, 16, 1937–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiao, L.; Yu, H. Expression levels of long non-coding RNA HOXA distal transcript antisense RNA and metabotropic glutamate receptor 1 in pancreatic carcinoma, and their prognostic values. Oncol. Lett. 2018, 15, 9464–9470. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.C.; Jeon, E.; Hong, S.H.; Shin, Y.K.; Chang, H.J.; Park, J.G. Metabotropic glutamate receptor 4-mediated 5-Fluorouracil resistance in a human colon cancer cell line. Clin. Cancer Res. 2004, 10, 4176–4184. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.M.; Hou, T.Z.; Zhang, Y.N.; Zhao, S.D.; Wu, Y.L.; Zhang, H. Blocked metabotropic glutamate receptor 5 enhances chemosensitivity in hepatocellular carcinoma and attenuates chemotoxicity in the normal liver by regulating DNA damage. Cancer Gene Ther. 2022. [Google Scholar] [CrossRef]
- Li, L.; Miyamoto, M.; Ebihara, Y.; Mega, S.; Takahashi, R.; Hase, R.; Kaneko, H.; Kadoya, M.; Itoh, T.; Shichinohe, T.; et al. DRD2/DARPP-32 expression correlates with lymph node metastasis and tumor progression in patients with esophageal squamous cell carcinoma. World J. Surg. 2006, 30, 1672–1679, discussion 1680–1671. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Xue, Z.; Feng, Y.; Xie, Y.; Deng, B.; Yao, Y.; Tian, X.; An, Q.; Yang, L.; Yao, Q.; et al. N-arylpiperazine-containing compound (C2): An enhancer of sunitinib in the treatment of pancreatic cancer, involving D1DR activation. Toxicol. Appl. Pharmacol. 2019, 384, 114789. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, D.; Cao, Z.; Ma, H. Dopamine Pathway Mediated by DRD5 Facilitates Tumor Growth via Enhancing Warburg Effect in Esophageal Cancer. Front. Oncol. 2021, 11, 655861. [Google Scholar] [CrossRef]
- Leng, Z.G.; Lin, S.J.; Wu, Z.R.; Guo, Y.H.; Cai, L.; Shang, H.B.; Tang, H.; Xue, Y.J.; Lou, M.Q.; Zhao, W.; et al. Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy 2017, 13, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, J.; Luo, Z.; Zhang, S.; Xue, S.; Wang, K.; Shi, Y.; Zhang, C.; Chen, H.; Li, Z. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis. Onco Targets Ther. 2015, 8, 1543–1552. [Google Scholar]
- Ataee, R.; Ajdary, S.; Zarrindast, M.; Rezayat, M.; Hayatbakhsh, M.R. Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. J. Cancer Res. Clin. Oncol. 2010, 136, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Ataee, R.; Ajdary, S.; Rezayat, M.; Shokrgozar, M.A.; Shahriari, S.; Zarrindast, M.R. Study of 5HT3 and HT4 receptor expression in HT29 cell line and human colon adenocarcinoma tissues. Arch. Iran. Med. 2010, 13, 120–125. [Google Scholar] [PubMed]
- Nocito, A.; Dahm, F.; Jochum, W.; Jang, J.H.; Georgiev, P.; Bader, M.; Graf, R.; Clavien, P.A. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008, 68, 5152–5158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataee, R.; Ajdary, S.; Zarrindast, M.; Rezayat, M.; Shokrgozar, M.A.; Ataee, A. Y25130 hydrochloride, a selective 5HT3 receptor antagonist has potent antimitogenic and apoptotic effect on HT29 colorectal cancer cell line. Eur. J. Cancer Prev. 2010, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Alpini, G.; Invernizzi, P.; Gaudio, E.; Venter, J.; Kopriva, S.; Bernuzzi, F.; Onori, P.; Franchitto, A.; Coufal, M.; Frampton, G.; et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008, 68, 9184–9193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soll, C.; Riener, M.O.; Oberkofler, C.E.; Hellerbrand, C.; Wild, P.J.; DeOliveira, M.L.; Clavien, P.A. Expression of serotonin receptors in human hepatocellular cancer. Clin. Cancer Res. 2012, 18, 5902–5910. [Google Scholar] [CrossRef] [Green Version]
- Blondy, S.; Christou, N.; David, V.; Verdier, M.; Jauberteau, M.O.; Mathonnet, M.; Perraud, A. Neurotrophins and their involvement in digestive cancers. Cell Death Dis. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Lee, J.J.; Kim, M.S.; Son, B.H.; Cho, Y.K.; Kim, H.P. DNA methylation-dependent regulation of TrkA, TrkB, and TrkC genes in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2011, 406, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Kitazawa, R.; Maeda, S.; Kitazawa, S. Methylation adjacent to negatively regulating AP-1 site reactivates TrkA gene expression during cancer progression. Oncogene 2005, 24, 5108–5118. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Kaz, A.M.; Kanngurn, S.; Welsch, P.; Morris, S.M.; Wang, J.; Lutterbaugh, J.D.; Markowitz, S.D.; Grady, W.M. NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer. PLoS Genet. 2013, 9, e1003552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Huang, H.; Yu, Y.; Chen, W.; Zhang, S.; Zhang, Y. Nerve growth factor regulates liver cancer cell polarity and motility. Mol. Med. Rep. 2021, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; He, X.; Huang, H.; He, Y.; Lan, J.; Yang, J.; Liu, W.; Zhang, T. Nerve growth factor orchestrates NGAL and matrix metalloproteinases activity to promote colorectal cancer metastasis. Clin. Transl. Oncol. 2022, 24, 34–47. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, J.; Qin, T.; Wang, Z.; Han, L. NGF from pancreatic stellate cells induces pancreatic cancer proliferation and invasion by PI3K/AKT/GSK signal pathway. J. Cell Mol. Med. 2020, 24, 5901–5910. [Google Scholar] [CrossRef] [Green Version]
- Okugawa, Y.; Tanaka, K.; Inoue, Y.; Kawamura, M.; Kawamoto, A.; Hiro, J.; Saigusa, S.; Toiyama, Y.; Ohi, M.; Uchida, K.; et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br. J. Cancer 2013, 108, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, Y.; Nagao, S.; Na, L.; Yanai, K.; Umebayashi, M.; Nakamura, K.; Nagai, S.; Fujimura, A.; Yamasaki, A.; Nakayama, K.; et al. TrkB/BDNF Signaling Could Be a New Therapeutic Target for Pancreatic Cancer. Anticancer Res. 2021, 41, 4047–4052. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Suh, K.W.; Hong, S.; Jin, W. TrkC promotes colorectal cancer growth and metastasis. Oncotarget 2017, 8, 41319–41333. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Friess, H.; Ceyhan, G.O. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim. Biophys. Acta 2016, 1866, 37–50. [Google Scholar]
- Okumura, T.; Tsunoda, S.; Mori, Y.; Ito, T.; Kikuchi, K.; Wang, T.C.; Yasumoto, S.; Shimada, Y. The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res. 2006, 12, 5096–5103. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.D.; Yuan, Y.; Liu, X.H.; Gong, D.J.; Bai, C.G.; Wang, F.; Luo, J.H.; Xu, Z.Y. Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 2009, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Kojima, H.; Okumura, T.; Yamaguchi, T.; Miwa, T.; Shimada, Y.; Nagata, T. Enhanced cancer stem cell properties of a mitotically quiescent subpopulation of p75NTR-positive cells in esophageal squamous cell carcinoma. Int. J. Oncol. 2017, 51, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Pan, Y.; He, L.; Zhai, H.; Li, X.; Zhao, L.; Sun, L.; Liu, J.; Hong, L.; Song, J.; et al. p75 neurotrophin receptor inhibits invasion and metastasis of gastric cancer. Mol. Cancer Res. 2007, 5, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Wu, Z.; Tan, B.; Liu, Z.; Zu, Z.; Wu, X.; Bi, Y.; Hu, X. Ibuprofen promotes p75 neurotrophin receptor expression through modifying promoter methylation and N6-methyladenosine-RNA-methylation in human gastric cancer cells. Bioengineered 2022, 13, 14595–14604. [Google Scholar] [CrossRef]
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS ONE 2016, 11, e0165586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Kleeff, J.; Kayed, H.; Wang, L.; Korc, M.; Buchler, M.W.; Friess, H. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol. Carcinog. 2002, 35, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Huo, L.; Bai, Y.; Fan, X.; Ni, B.; Fang, L.; Hu, J.; Peng, J.; Wang, L.; et al. Epigenetic inactivation and tumor-suppressor behavior of NGFR in human colorectal cancer. Mol. Cancer Res. 2015, 13, 107–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuanlong, H.; Haifeng, J.; Xiaoyin, Z.; Jialin, S.; Jie, L.; Li, Y.; Huahong, X.; Jiugang, S.; Yanglin, P.; Kaichun, W.; et al. The inhibitory effect of p75 neurotrophin receptor on growth of human hepatocellular carcinoma cells. Cancer Lett. 2008, 268, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Tokusashi, Y.; Asai, K.; Tamakawa, S.; Yamamoto, M.; Yoshie, M.; Yaginuma, Y.; Miyokawa, N.; Aoki, T.; Kino, S.; Kasai, S.; et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int. J. Cancer 2005, 114, 39–45. [Google Scholar] [CrossRef]
- Griffin, N.; Gao, F.; Jobling, P.; Oldmeadow, C.; Wills, V.; Walker, M.M.; Faulkner, S.; Hondermarck, H. The neurotrophic tyrosine kinase receptor 1 (TrkA) is overexpressed in oesophageal squamous cell carcinoma. Pathology 2021, 53, 470–477. [Google Scholar] [CrossRef]
- Kamiya, A.; Inokuchi, M.; Otsuki, S.; Sugita, H.; Kato, K.; Uetake, H.; Sugihara, K.; Takagi, Y.; Kojima, K. Prognostic value of tropomyosin-related kinases A, B, and C in gastric cancer. Clin. Transl. Oncol. 2016, 18, 599–607. [Google Scholar] [CrossRef]
- Schneider, M.B.; Standop, J.; Ulrich, A.; Wittel, U.; Friess, H.; Andren-Sandberg, A.; Pour, P.M. Expression of nerve growth factors in pancreatic neural tissue and pancreatic cancer. J. Histochem. Cytochem. 2001, 49, 1205–1210. [Google Scholar] [CrossRef] [Green Version]
- Dang, C.; Zhang, Y.; Ma, Q.; Shimahara, Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol. 2006, 21, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Y.; Dang, C.; Ma, Q.; Lee, W.; Chen, W. siRNA directed against TrkA sensitizes human pancreatic cancer cells to apoptosis induced by gemcitabine through an inactivation of PI3K/Akt-dependent pathway. Oncol. Rep. 2007, 18, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Xu, Y.F.; Guo, S.; Liu, Y.; Ning, S.L.; Lu, X.F.; Yang, H.; Chen, Y.X. Clinical significance of nerve growth factor and tropomyosin-receptor-kinase signaling pathway in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2014, 20, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Mohri, Y.; Nishioka, J.; Ohi, M.; Yokoe, T.; Miki, C.; Tonouchi, H.; Nobori, T.; Kusunoki, M. Neurotrophic receptor, tropomyosin-related kinase B, as a chemoresistant marker in oesophageal cancer. Clin. Oncol. 2009, 21, 362–363. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Mohri, Y.; Nishioka, J.; Kobayashi, M.; Ohi, M.; Miki, C.; Tonouchi, H.; Nobori, T.; Kusunoki, M. Neurotrophic receptor, tropomyosin-related kinase B as an independent prognostic marker in gastric cancer patients. J. Surg. Oncol. 2009, 99, 307–310. [Google Scholar] [CrossRef]
- Zhao, M.X.; Ding, S.G.; Liu, L.N.; Wang, Y.; Zhang, J.; Zhang, H.J.; Zhang, Y. [Predicative value of expression of TrkB and TRIM29 in biopsy tissues from preoperative gastroscopy in lymph node metastasis of gastric cancer]. Zhonghua Yi Xue Za Zhi 2012, 92, 376–379. [Google Scholar] [PubMed]
- Jin, Z.; Lu, Y.; Wu, X.; Pan, T.; Yu, Z.; Hou, J.; Wu, A.; Li, J.; Yang, Z.; Li, C.; et al. The cross-talk between tumor cells and activated fibroblasts mediated by lactate/BDNF/TrkB signaling promotes acquired resistance to anlotinib in human gastric cancer. Redox Biol. 2021, 46, 102076. [Google Scholar] [CrossRef]
- Sclabas, G.M.; Fujioka, S.; Schmidt, C.; Li, Z.; Frederick, W.A.; Yang, W.; Yokoi, K.; Evans, D.B.; Abbruzzese, J.L.; Hess, K.R.; et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin. Cancer Res. 2005, 11, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Wang, X.; Yang, Z.; Ou, G. Overexpression of TrkB promotes the progression of colon cancer. APMIS 2010, 118, 188–195. [Google Scholar] [CrossRef]
- De Farias, C.B.; Heinen, T.E.; dos Santos, R.P.; Abujamra, A.L.; Schwartsmann, G.; Roesler, R. BDNF/TrkB signaling protects HT-29 human colon cancer cells from EGFR inhibition. Biochem. Biophys. Res. Commun. 2012, 425, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, S.W.; Yuan, W.; Tang, J.; Sang, Y. Downregulation of SUN2 promotes metastasis of colon cancer by activating BDNF/TrkB signalling by interacting with SIRT1. J. Pathol. 2021, 254, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Park, G.B.; Choi, S.; Yoon, Y.S.; Kim, D. TrkB/C-induced HOXC6 activation enhances the ADAM8-mediated metastasis of chemoresistant colon cancer cells. Mol. Med. Rep. 2021, 23, 423. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.T.; Yang, Z.F.; Lau, C.K.; Tam, K.H.; Fan, S.T.; Poon, R.T. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: Implication in hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 3123–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Huang, Z.; Luo, Y.; Zou, Q.; Bai, L.; Tang, G.; Wang, X.; Cao, G.; Huang, M.; Xiang, J.; et al. Genome-wide analysis identifies critical DNA methylations within NTRKs genes in colorectal cancer. J. Transl. Med. 2021, 19, 73. [Google Scholar] [CrossRef]
- Genevois, A.L.; Ichim, G.; Coissieux, M.M.; Lambert, M.P.; Lavial, F.; Goldschneider, D.; Jarrosson-Wuilleme, L.; Lepinasse, F.; Gouysse, G.; Herceg, Z.; et al. Dependence receptor TrkC is a putative colon cancer tumor suppressor. Proc. Natl. Acad. Sci. USA 2013, 110, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- Botticelli, L.; Micioni Di Bonaventura, E.; Ubaldi, M.; Ciccocioppo, R.; Cifani, C.; Micioni Di Bonaventura, M.V. The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals 2021, 14, 293. [Google Scholar] [CrossRef]
- Arora, S.; Anubhuti. Role of neuropeptides in appetite regulation and obesity--A review. Neuropeptides 2006, 40, 375–401. [Google Scholar] [CrossRef]
- Wei, P.; Keller, C.; Li, L. Neuropeptides in gut-brain axis and their influence on host immunity and stress. Comput. Struct. Biotechnol. J. 2020, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.; Allen, W.L.; Turkington, R.; Jithesh, P.V.; Proutski, I.; Stewart, G.; Lenz, H.J.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Identification of galanin and its receptor GalR1 as novel determinants of resistance to chemotherapy and potential biomarkers in colorectal cancer. Clin. Cancer Res. 2012, 18, 5412–5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagayoshi, K.; Ueki, T.; Tashiro, K.; Mizuuchi, Y.; Manabe, T.; Araki, H.; Oda, Y.; Kuhara, S.; Tanaka, M. Galanin plays an important role in cancer invasiveness and is associated with poor prognosis in stage II colorectal cancer. Oncol. Rep. 2015, 33, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.; Bae, K.; Lee, M.K.; Kim, J.H.; Yoon, K.A. Galanin is an epigenetically silenced tumor suppressor gene in gastric cancer cells. PLoS ONE 2018, 13, e0193275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Fang, P.; Chai, C.; Shao, L.; Mao, H.; Qiao, D.; Kong, G.; Dong, X.; Shi, M.; Zhang, Z.; et al. Galanin expression is down-regulated in patients with gastric cancer. J. Int. Med. Res. 2019, 47, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, X.; Meng, Y.; Chen, H.; Griffin, N.; Gao, X.; Shan, F. Methionine enkephalin (MENK) inhibits human gastric cancer through regulating tumor associated macrophages (TAMs) and PI3K/AKT/mTOR signaling pathway inside cancer cells. Int. Immunopharmacol. 2018, 65, 312–322. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Jiao, X.; Geng, J.; Wang, R.; Liu, N.; Gao, X.; Griffin, N.; Gao, Y.; Shan, F. The novel mechanism of anticancer effect on gastric cancer through inducing G0/G1 cell cycle arrest and caspase-dependent apoptosis in vitro and in vivo by methionine enkephalin. Cancer Manag. Res. 2018, 10, 4773–4787. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, S.; Li, Z. Plasma neuropeptide Y (NPY) levels in patients with gastric and colorectal carcinomas. Zhonghua Zhong Liu Za Zhi 1998, 20, 213–215. [Google Scholar] [PubMed]
- Jeppsson, S.; Srinivasan, S.; Chandrasekharan, B. Neuropeptide Y (NPY) promotes inflammation-induced tumorigenesis by enhancing epithelial cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G103–G111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front. Physiol. 2012, 3, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hondermarck, H.; Jobling, P. The Sympathetic Nervous System Drives Tumor Angiogenesis. Trends Cancer 2018, 4, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Macklin, K.D.; Maus, A.D.; Pereira, E.F.; Albuquerque, E.X.; Conti-Fine, B.M. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1998, 287, 435–439. [Google Scholar]
- Schuller, H.M.; Al-Wadei, H.A.; Ullah, M.F.; Plummer, H.K., 3rd. Regulation of pancreatic cancer by neuropsychological stress responses: A novel target for intervention. Carcinogenesis 2012, 33, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xu, Q.; Zuo, Y.; Liu, L.; Liu, S.; Chen, L.; Wang, K.; Lei, Y.; Zhao, X.; Li, Y. Isoprenaline/beta2-AR activates Plexin-A1/VEGFR2 signals via VEGF secretion in gastric cancer cells to promote tumor angiogenesis. BMC Cancer 2017, 17, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakroborty, D.; Sarkar, C.; Basu, B.; Dasgupta, P.S.; Basu, S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009, 69, 3727–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakroborty, D.; Sarkar, C.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004, 10, 4349–4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Nagy, J.A.; Pal, S.; Vasile, E.; Eckelhoefer, I.A.; Bliss, V.S.; Manseau, E.J.; Dasgupta, P.S.; Dvorak, H.F.; Mukhopadhyay, D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat. Med. 2001, 7, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Yang, Y.; Peng, W.T.; Sun, J.C.; Sun, W.Y.; Wei, W. G protein-coupled receptor kinase 2 regulating beta2-adrenergic receptor signaling in M2-polarized macrophages contributes to hepatocellular carcinoma progression. Onco Targets Ther. 2019, 12, 5499–5513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, R.; Zhang, Y.; Wang, S.; Xiang, T.; Chen, W. Alpha7 nicotinic acetylcholine receptor in tumor-associated macrophages inhibits colorectal cancer metastasis through the JAK2/STAT3 signaling pathway. Oncol. Rep. 2017, 38, 2619–2628. [Google Scholar] [CrossRef]
- Ganor, Y.; Levite, M. The neurotransmitter glutamate and human T cells: Glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J. Neural Transm. 2014, 121, 983–1006. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Chen, M.; Mohammadpour, H.; MacDonald, C.R.; Bucsek, M.J.; Hylander, B.L.; Barbi, J.J.; Repasky, E.A. Chronic Adrenergic Stress Contributes to Metabolic Dysfunction and an Exhausted Phenotype in T Cells in the Tumor Microenvironment. Cancer Immunol Res. 2021, 9, 651–664. [Google Scholar] [CrossRef]
- Partecke, L.I.; Speerforck, S.; Kading, A.; Seubert, F.; Kuhn, S.; Lorenz, E.; Schwandke, S.; Sendler, M.; Kessler, W.; Trung, D.N.; et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 2016, 16, 423–433. [Google Scholar] [CrossRef]
- Yang, M.W.; Tao, L.Y.; Jiang, Y.S.; Yang, J.Y.; Huo, Y.M.; Liu, D.J.; Li, J.; Fu, X.L.; He, R.; Lin, C.; et al. Perineural Invasion Reprograms the Immune Microenvironment through Cholinergic Signaling in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020, 80, 1991–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Watanabe, T.; Okuno, K.; Yasutomi, M. Perineural invasion as a predictor of recurrence of gastric cancer. Cancer 1994, 73, 550–555. [Google Scholar] [CrossRef]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural invasion in pancreatic cancer: The past, present and future. Cancers 2010, 2, 1513–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Shan, T.; Cui, X.; Li, W.; Lin, W.; Li, Y.; Chen, X.; Wu, T. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014, 105, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, V.; Dietrich, A.; Kasparek, M.S.; Benhaqi, P.; Schneider, M.R.; Schemann, M.; Seeliger, H.; Kreis, M.E. Extrinsic intestinal denervation modulates tumor development in the small intestine of Apc(Min/+) mice. J. Exp. Clin. Cancer Res. 2015, 34, 39. [Google Scholar] [CrossRef] [Green Version]
- Sadighparvar, S.; Darband, S.G.; Ghaderi-Pakdel, F.; Mihanfar, A.; Majidinia, M. Parasympathetic, but not sympathetic denervation, suppressed colorectal cancer progression. Eur. J. Pharmacol. 2021, 913, 174626. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liao, P.; Li, W.; Hu, J.; Chen, C.; Zhang, Y.; Wang, Y.; Chen, L.; Song, K.; Liu, J.; et al. Clinical Use of Propranolol Reduces Biomarkers of Proliferation in Gastric Cancer. Front. Oncol. 2021, 11, 628613. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Utomo, R.Y. Functional network analysis reveals potential repurposing of beta-blocker atenolol for pancreatic cancer therapy. Daru 2020, 28, 685–699. [Google Scholar] [CrossRef]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Nkontchou, G.; Aout, M.; Mahmoudi, A.; Roulot, D.; Bourcier, V.; Grando-Lemaire, V.; Ganne-Carrie, N.; Trinchet, J.C.; Vicaut, E.; Beaugrand, M. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev. Res. 2012, 5, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.; Goldstein, J.; Margalit, O.; Shacham-Shmueli, E.; Lawrence, Y.R.; Yang, Y.X.; Reiss, K.A.; Golan, T.; Mamtani, R.; Halpern, N.; et al. Assessing the effects of beta-blockers on pancreatic cancer risk: A nested case-control study. Pharmacoepidemiol. Drug Saf. 2020, 29, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, Q.Y.; Hu, H.T.; Zhang, M. Beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, V.Y.; Jin, H.C.; Ng, E.K.; Yu, J.; Leung, W.K.; Cho, C.H.; Sung, J.J. Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathways. Toxicol. Appl. Pharmacol. 2008, 233, 254–261. [Google Scholar] [CrossRef]
- Lv, G.B.; Wang, T.T.; Zhu, H.L.; Wang, H.K.; Sun, W.; Zhao, L.F. Vortioxetine induces apoptosis and autophagy of gastric cancer AGS cells via the PI3K/AKT pathway. FEBS Open Bio 2020, 10, 2157–2165. [Google Scholar] [CrossRef]
- Curtis, J.J.; Vo, N.T.K.; Seymour, C.B.; Mothersill, C.E. 5-HT2A and 5-HT3 receptors contribute to the exacerbation of targeted and non-targeted effects of ionizing radiation-induced cell death in human colon carcinoma cells. Int. J. Radiat. Biol. 2020, 96, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, S.; Kong, J.S.; Kim, M.J.; Park, S.; Lee, S.S.; Kim, A. Overexpression of dopamine receptor D2 promotes colorectal cancer progression by activating the beta-catenin/ZEB1 axis. Cancer Sci. 2021, 112, 3732–3743. [Google Scholar] [CrossRef]

| Receptors | Expression in Cancer/Mechanism | Downstream Effectors | Effects | Reference |
|---|---|---|---|---|
| p75NTR | Expressed in ESCC | Bmi-1 | Self-renewal, proliferation, chemoresistance | [188,189,190] |
| Downregulated in GC/DNA methylation | uPA, MMP-9, NF-κB | Impaired proliferation, invasion and metastasis | [126,191,192] | |
| Expressed in PC | Neural invasion, proliferation | [193,194] | ||
| Downregulated in CRC/DNA methylation | Impaired proliferation, invasion and viability | [195] | ||
| Downregulated in HCC | Impaired proliferation | [196,197] | ||
| TrkA | Upregulated in ESCC | [198] | ||
| Expressed in GC | [199] | |||
| Expressed in PC | PI3K/AKT | Chemoresistance | [200,201,202] | |
| Expressed in CRC | MAPK/ERK, MMP2, MMP9 | metastasis | [182] | |
| Expressed in CC | [203] | |||
| Upregulated in HCC/DNA demethlyation | Proliferation | [178] | ||
| TrkB | Expressed in ESCC | Chemoresistance | [204] | |
| Expressed in GC | Nrf2 | lymph node metastasis, chemoresistance | [205,206,207] | |
| Expressed in PC | Invasion | [185,208] | ||
| Upregulated in CRC | ERK | Proliferation, invasion, viability | [209,210,211,212] | |
| Upregulated in HCC/DNA demethlyation | RhoA, VEGF | Angiogenesis, proliferation, chemoresistance | [72,178,213] | |
| TrkC | Expressed in GC | [199] | ||
| Upregulated in PC | [149] | |||
| Downregulated in CRC/DNA methylation | Impaired viability | [180,214,215] | ||
| Upregulated in HCC/DNA demethlyation | Proliferation | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Yan, X.; Hu, B.; Zhang, X. Emerging Roles of the Nervous System in Gastrointestinal Cancer Development. Cancers 2022, 14, 3722. https://doi.org/10.3390/cancers14153722
Wan C, Yan X, Hu B, Zhang X. Emerging Roles of the Nervous System in Gastrointestinal Cancer Development. Cancers. 2022; 14(15):3722. https://doi.org/10.3390/cancers14153722
Chicago/Turabian StyleWan, Chunhua, Xiaoqin Yan, Baoying Hu, and Xinhua Zhang. 2022. "Emerging Roles of the Nervous System in Gastrointestinal Cancer Development" Cancers 14, no. 15: 3722. https://doi.org/10.3390/cancers14153722




