The Role of Pathology-Based Methods in Qualitative and Quantitative Approaches to Cancer Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Multiplex Immunohistochemistry/Immunofluorescence (mIHC/IF) Techniques
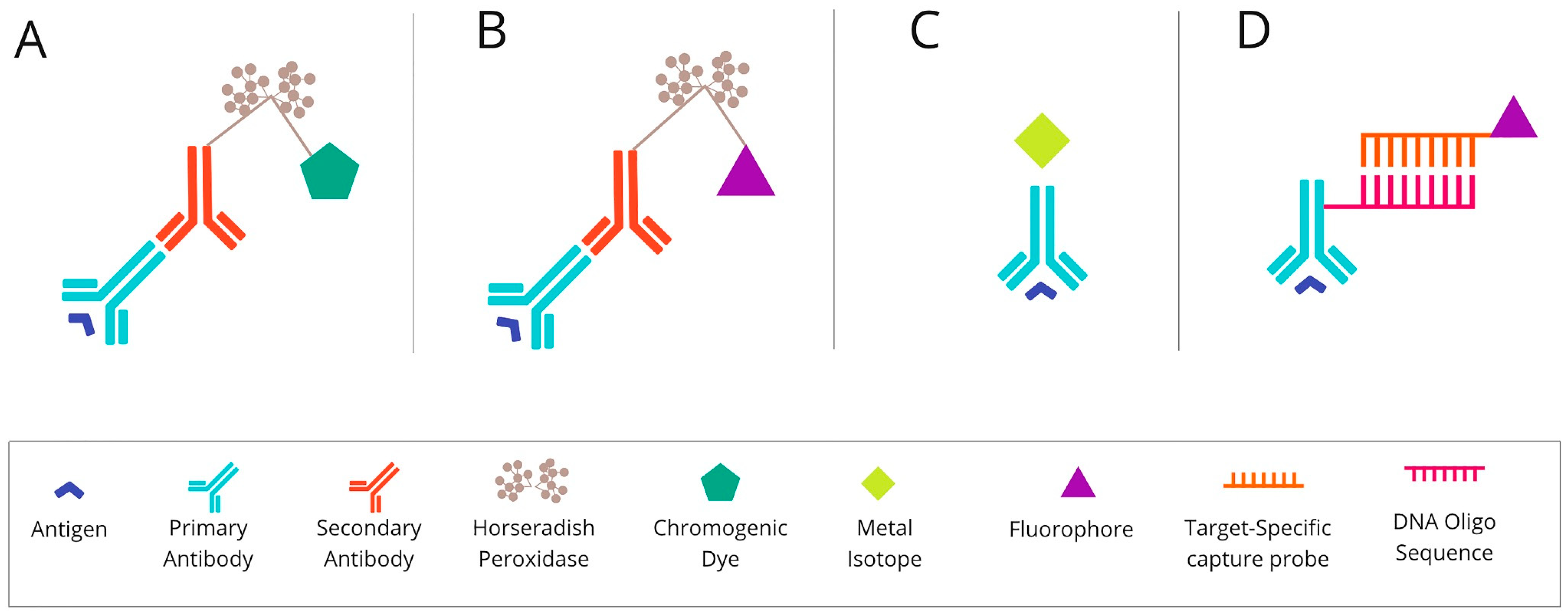
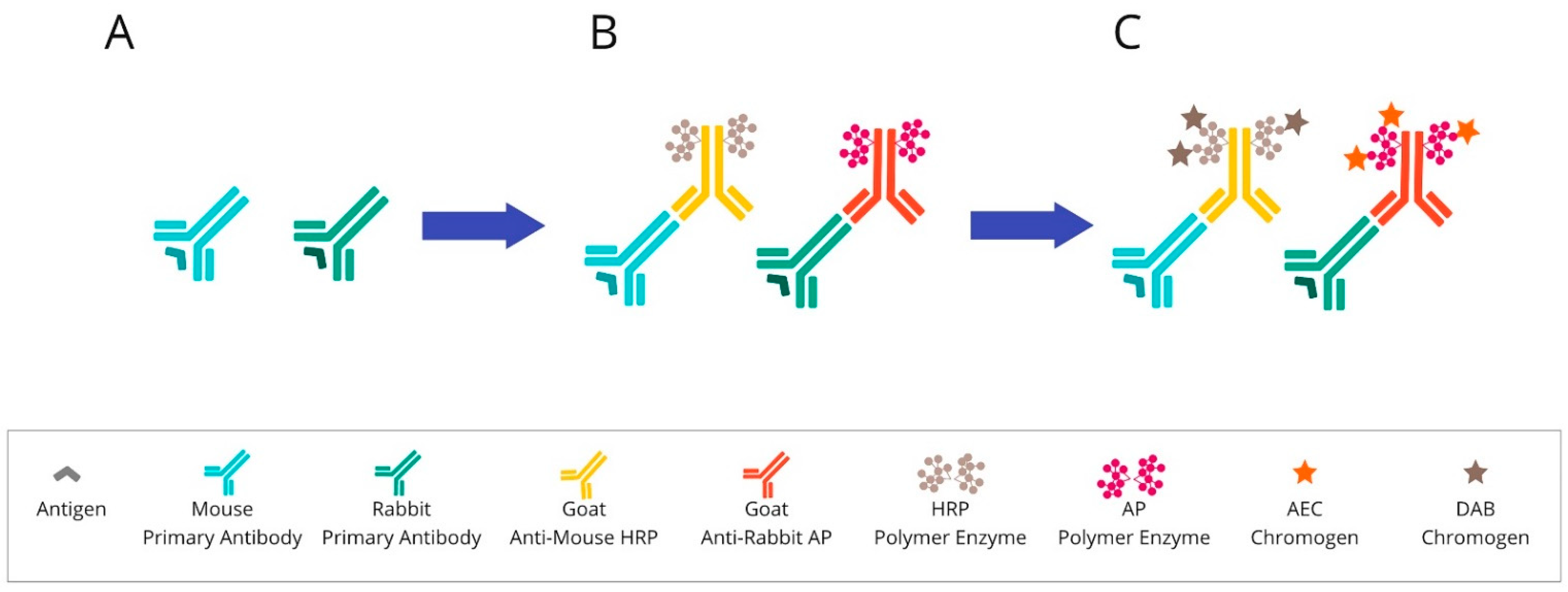
2.1. Chromogenic-Based mIHC Is Widely Based on Immunohistochemical (Standard Single-Antibody Chromogenic Immunohistochemistry) Technologies
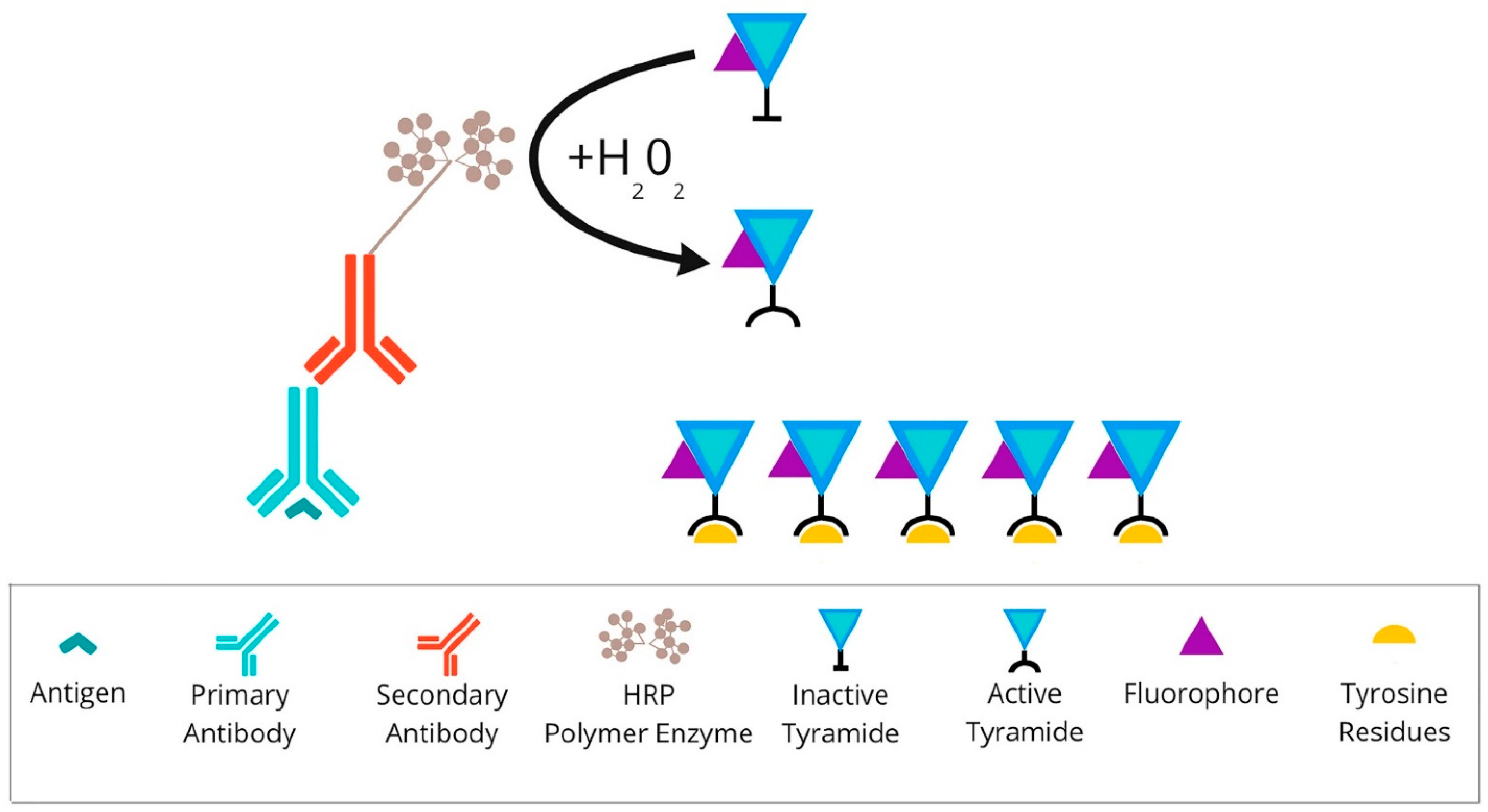
2.2. Fluorescence-Based mIHC/IF Is a Method That Provides Simultaneous Detection of Multiple Fluorescently Tagged Proteins of Interest in FFPE Tissue Sections
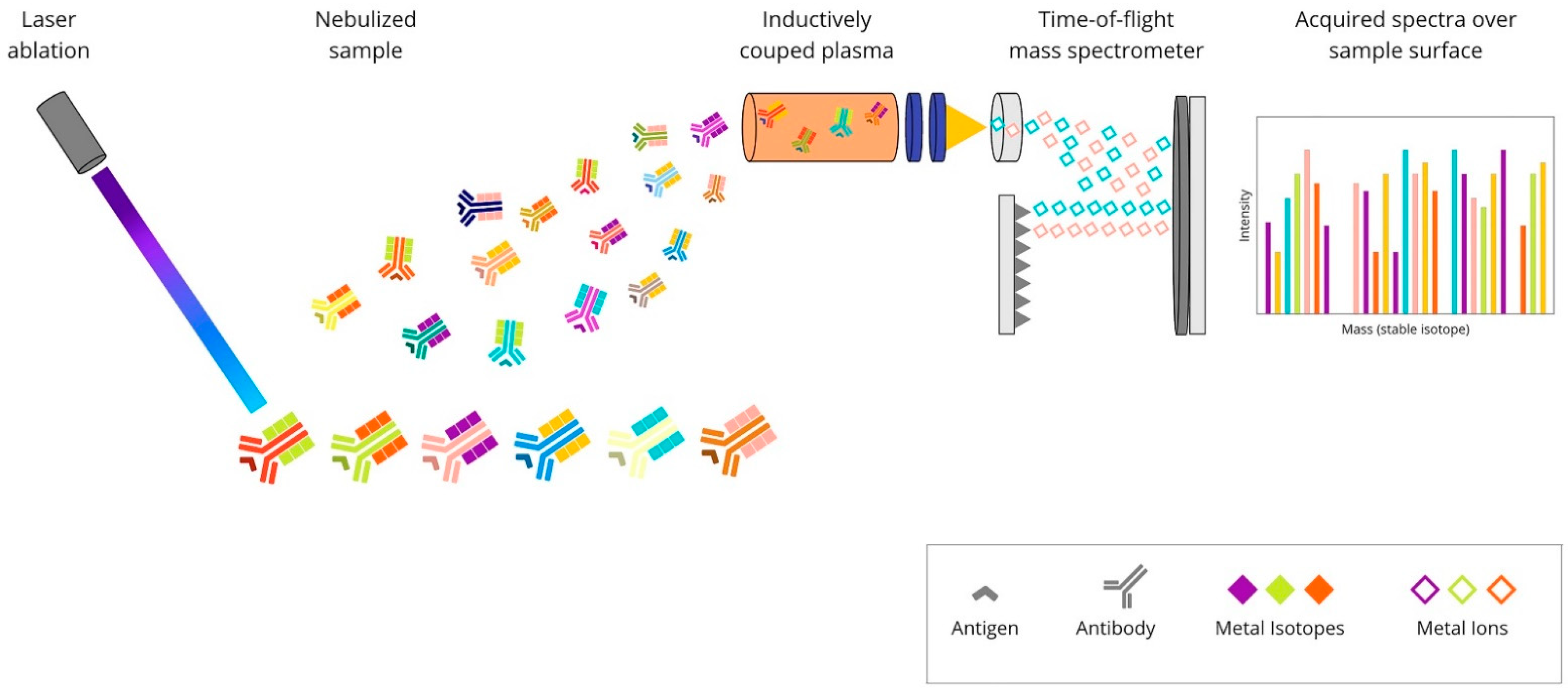
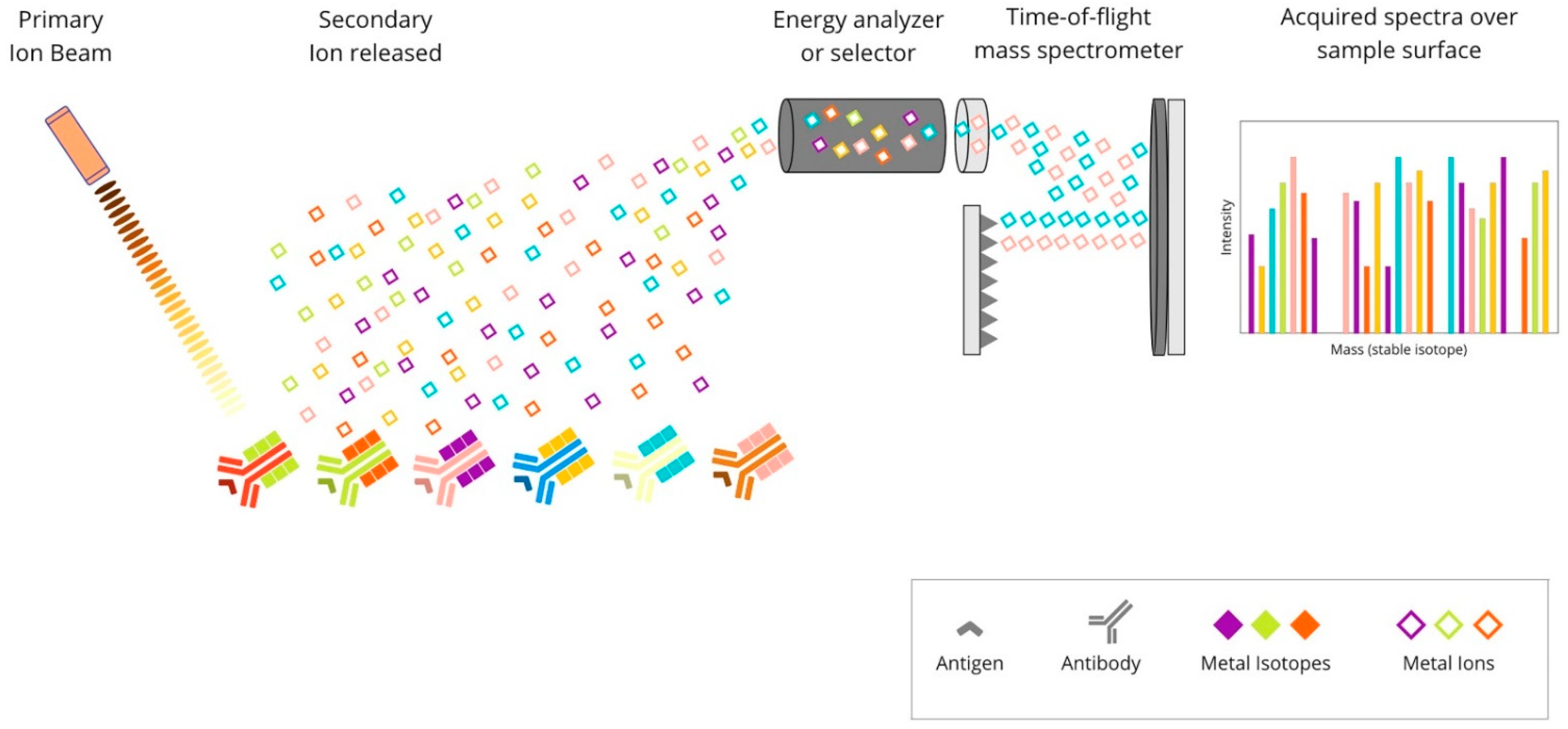
2.3. Metal-Based mIHC/IF Are Methods That Utilise Antibodies Conjugated with Isotopically Pure Metal-Chelator Tags
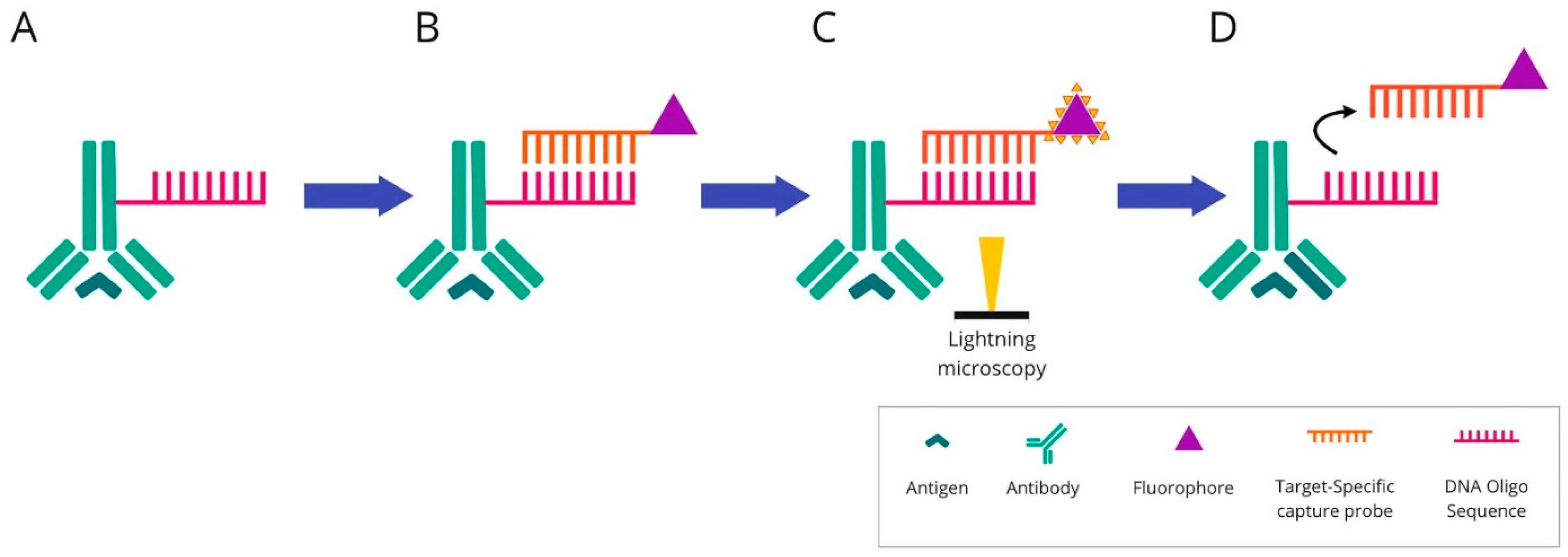
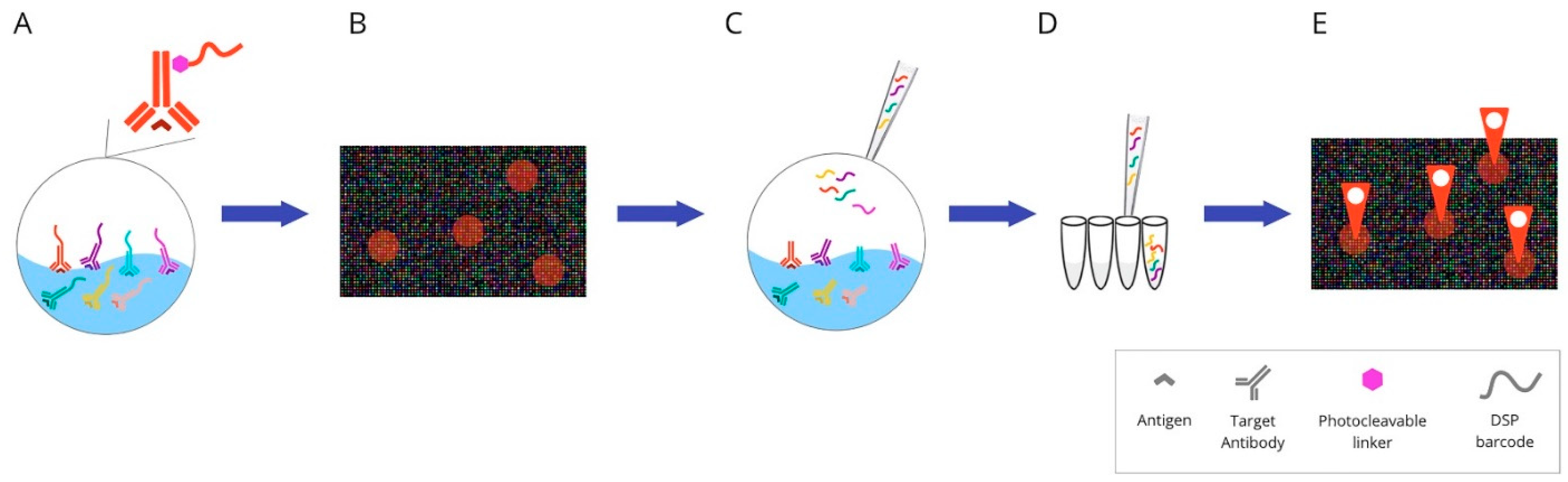
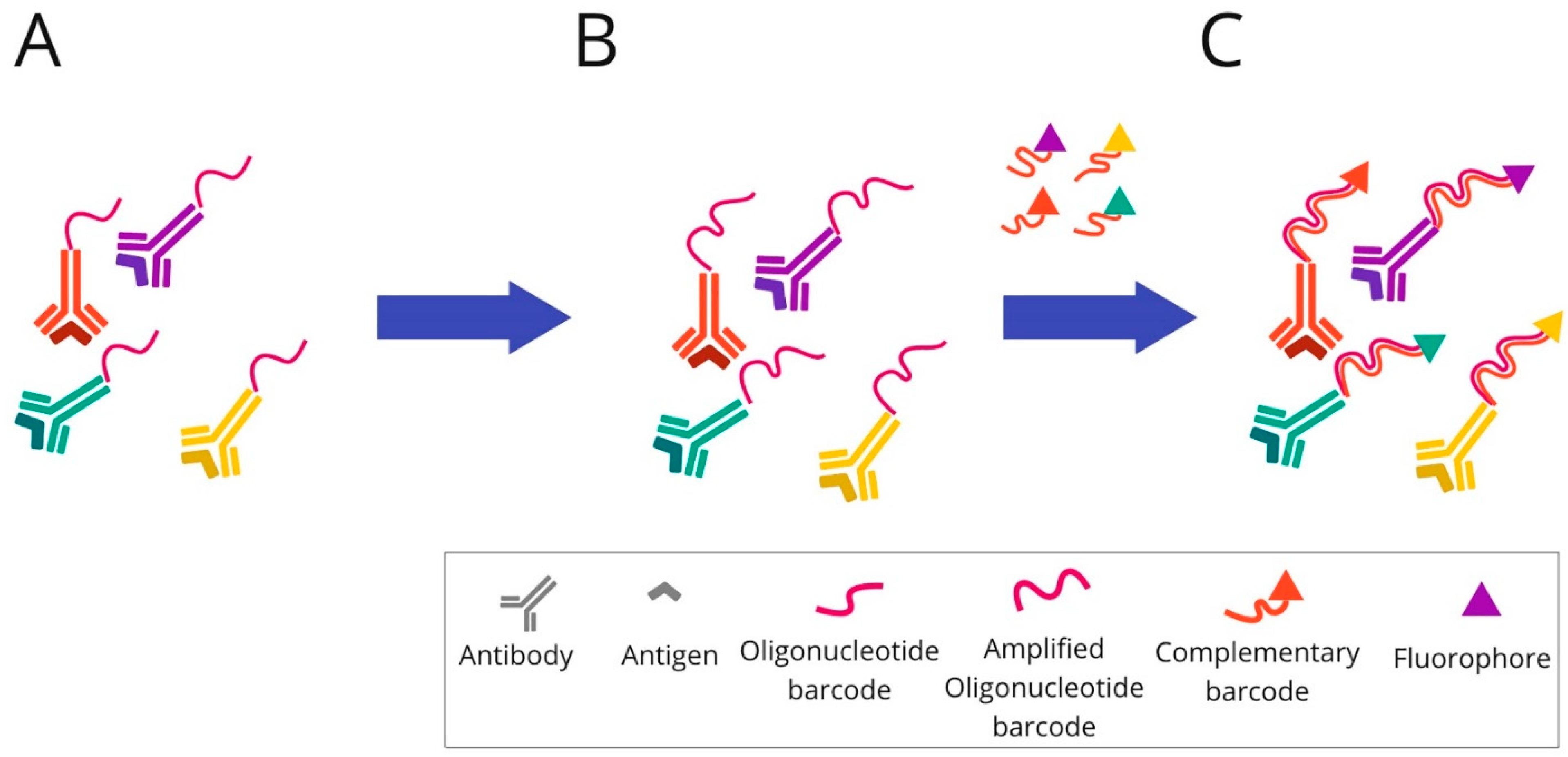
2.4. DNA Barcoding-Based mIHC/IF Are Techniques for Multiplexed Imaging Based on DNA Barcoding Using Oligonucleotide Detection Technologies
3. Conventional IHC vs. Multiplexed Imaging Techniques in PD-L1 Assessment
4. Digital Imaging in Quantitative Pathological Assessment
4.1. Slide Scanners
4.2. Open-Source Software
4.3. Artificial Intelligence (AI)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picardo, S.L.; Doi, J.; Hansen, A.R. Structure and Optimization of Checkpoint Inhibitors. Cancers 2019, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [Green Version]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Yu, J.; Zhang, Y.; Pang, X.; Ma, C.; Shen, M.; Ruan, S.; Wasan, H.S.; Qiu, S. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2083. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [Green Version]
- Gadiot, J.; Hooijkaas, A.I.; Kaiser, A.D.M.; Van Tinteren, H.; Van Boven, H.; Blank, C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011, 117, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Ramos, M.F.K.P.; Dias, A.R.; Ribeiro, R.; Cardili, L.; Zilberstein, B.; Cecconello, I.; Ribeiro, U.; de Mello, E.S.; de Castria, T.B. Scoring systems for PD-L1 expression and their prognostic impact in patients with resectable gastric cancer. Virchows Archiv. 2021, 478, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Erber, R.; Hartmann, A. Understanding PD-L1 Testing in Breast Cancer: A Practical Approach. Breast Care 2020, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rimm, D.L.; Han, G.; Taube, J.M.; Yi, E.S.; Bridge, J.A.; Flieder, D.B.; Homer, R.; West, W.W.; Wu, H.; Roden, A.C.; et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol. 2017, 3, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Batenchuk, C.; Albitar, M.; Zerba, K.; Sudarsanam, S.; Chizhevsky, V.; Jin, C.; Burns, V. A real-world, comparative study of FDA-approved diagnostic assays PD-L1 IHC 28-8 and 22C3 in lung cancer and other malignancies. J. Clin. Pathol. 2018, 71, 1078–1083. [Google Scholar] [CrossRef]
- Fujimoto, D.; Sato, Y.; Uehara, K.; Ishida, K.; Fukuoka, J.; Morimoto, T.; Kawachi, H.; Mori, R.; Ito, M.; Teraoka, S.; et al. Predictive Performance of Four Programmed Cell Death Ligand 1 Assay Systems on Nivolumab Response in Previously Treated Patients with Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Gaule, P.; Smithy, J.; Toki, M.; Rehman, J.; Patell-Socha, F.; Cougot, D.; Collin, P.; Morrill, P.; Neumeister, V.; Rimm, D.L. A Quantitative Comparison of Antibodies to Programmed Cell Death 1 Ligand 1. JAMA Oncol. 2017, 3, 256–259. [Google Scholar] [CrossRef]
- Hendry, S.; Byrne, D.J.; Wright, G.M.; Young, R.J.; Sturrock, S.; Cooper, W.A.; Fox, S.B. Comparison of Four PD-L1 Immunohistochemical Assays in Lung Cancer. J. Thorac. Oncol. 2018, 13, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, F.R.; McElhinny, A.; Stanforth, D.; Ranger-Moore, J.; Jansson, M.; Kulangara, K.; Richardson, W.; Towne, P.; Hanks, D.; Vennapusa, B.; et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017, 12, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, M.J.; Sharpe, A.; Midha, A.; Barker, C.; Scott, M.; Scorer, P.; Al-Masri, H.; Rebelatto, M.C.; Walker, J. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 3585–3591. [Google Scholar] [CrossRef] [Green Version]
- Tretiakova, M.; Fulton, R.; Kocherginsky, M.; Long, T.; Ussakli, C.; Antic, T.; Gown, A. Concordance study of PD-L1 expression in primary and metastatic bladder carcinomas: Comparison of four commonly used antibodies and RNA expression. Mod. Pathol. 2018, 31, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, M.; Jung, H.; Nam, G.-H.; Kim, I.-S. The right Timing, right combination, right sequence, and right delivery for Cancer immunotherapy. J. Control. Release 2021, 331, 321–334. [Google Scholar] [CrossRef]
- Patel, K.R.; Martinez, A.; Stahl, J.M.; Logan, S.J.; Perricone, A.J.; Ferris, M.J.; Buchwald, Z.S.; Chowdhary, M.; Delman, K.A.; Monson, D.K.; et al. Increase in PD-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. OncoImmunology 2018, 7, e1442168. [Google Scholar] [CrossRef] [PubMed]
- Vilain, R.E.; Menzies, A.M.; Wilmott, J.S.; Kakavand, H.; Madore, J.; Guminski, A.; Liniker, E.; Kong, B.Y.; Cooper, A.J.; Howle, J.R.; et al. Dynamic Changes in PD-L1 Expression and Immune Infiltrates Early During Treatment Predict Response to PD-1 Blockade in Melanoma. Clin. Cancer Res. 2017, 23, 5024–5033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, E.R.; Uraoka, N.; Jiang, M.; Cook, P.; Gibbons, D.; Forget, M.-A.; Bernatchez, C.; Haymaker, C.; Wistuba, I.I.; Rodriguez-Canales, J. Validation of multiplex immunofluorescence panels using multispectral microscopy for immune-profiling of formalin-fixed and paraffin-embedded human tumor tissues. Sci. Rep. 2017, 7, 13380. [Google Scholar] [CrossRef] [Green Version]
- Hofman, P.; Badoual, C.; Henderson, F.; Berland, L.; Hamila, M.; Long-Mira, E.; Lassalle, S.; Roussel, H.; Hofman, V.; Tartour, E.; et al. Multiplexed Immunohistochemistry for Molecular and Immune Profiling in Lung Cancer-Just About Ready for Prime-Time? Cancers 2019, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.R.; Bathany, C.; Tsuei, M.; White, J.B.; Barald, K.F.; Takayama, S. Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev. Mol. Diagn. 2015, 15, 1171–1186. [Google Scholar] [CrossRef]
- Bussolati, G.; Leonardo, E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J. Clin. Pathol. 2008, 61, 1184–1192. [Google Scholar] [CrossRef]
- McNamara, G.; Difilippantonio, M.J.; Ried, T. Microscopy and Image Analysis. In Current Protocols in Human Genetics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; Chapter 4; pp. 4.4.1–4.4.34. [Google Scholar]
- Kim, S.-W.; Roh, J.; Park, C.-S. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips. J. Pathol. Transl. Med. 2016, 50, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Masucci, G.V.; Cesano, A.; Eggermont, A.; Fox, B.A.; Wang, E.; Marincola, F.M.; Ciliberto, G.; Dobbin, K.; Puzanov, I.; Taube, J.; et al. The need for a network to establish and validate predictive biomarkers in cancer immunotherapy. J. Transl. Med. 2017, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Thurin, M.; Cesano, A.; Marincola, F. Biomarkers for Immunotherapy of Cancer Methods and Protocols: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2020. [Google Scholar]
- Park, Y.; Na Seo, A.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, H.S. Expression of the immune checkpoint receptors PD-1, LAG3, and TIM3 in the immune context of stage II and III gastric cancer by using single and chromogenic multiplex immunohistochemistry. OncoImmunology 2021, 10, 1954761. [Google Scholar] [CrossRef]
- Yoshimura, K.; Tsujikawa, T.; Mitsuda, J.; Ogi, H.; Saburi, S.; Ohmura, G.; Arai, A.; Shibata, S.; Thibault, G.; Chang, Y.H.; et al. Spatial Profiles of Intratumoral PD-1+ Helper T Cells Predict Prognosis in Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 769534. [Google Scholar] [CrossRef] [PubMed]
- Ilié, M.; Beaulande, M.; Long-Mira, E.; Bontoux, C.; Zahaf, K.; Lalvée, S.; Hamila, M.; Benzaquen, J.; Cohen, C.; Berthet, J.-P.; et al. Analytical validation of automated multiplex chromogenic immunohistochemistry for diagnostic and predictive purpose in non-small cell lung cancer. Lung Cancer 2022, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Remark, R.; Merghoub, T.; Grabe, N.; Litjens, G.; Damotte, D.; Wolchok, J.D.; Merad, M.; Gnjatic, S. In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci. Immunol. 2016, 1, aaf6925. [Google Scholar] [CrossRef]
- Akturk, G.; Sweeney, R.; Remark, R.; Merad, M.; Gnjatic, S. Multiplexed Immunohistochemical Consecutive Staining on Single Slide (MICSSS): Multiplexed Chromogenic IHC Assay for High-Dimensional Tissue Analysis. Methods Mol. Biol. 2020, 2055, 497–519. [Google Scholar] [CrossRef]
- Akturk, G.; Sweeney, R.; Remark, R.; Merad, M.; Gnjatic, S. Multiplexed Immunohistochemical Consecutive Staining on Single Slide (MICSSS): Multiplexed Chromogenic IHC Assay for High-Dimensional Tissue Analysis, in Biomarkers for Immunotherapy of Cancer: Methods and Protocols; Thurin, M., Cesano, A., Marincola, F.M., Eds.; Springer: New York, NY, USA, 2020; pp. 497–519. [Google Scholar]
- Dharmapuri, S.; Cabal, R.; Ioannou, G.; Ozbey, S.; Paulsen, J.; Ang, C.; Sarpel, U.; Sung, M.W.; Kozuch, P.; Schwartz, M.E.; et al. A multiplexed immunohistochemical consecutive staining on single slide (MICSSS) analysis of the immune microenvironment of bile duct cancers (BDC) pre and post neoadjuvantchemotherapy (NACT). J. Clin. Oncol. 2022, 40, e16151. [Google Scholar] [CrossRef]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar] [CrossRef]
- Bobrow, M.N.; Harris, T.D.; Shaughnessy, K.J.; Litt, G.J. Catalyzed reporter deposition, a novel method of signal amplification application to immunoassays. J. Immunol. Methods 1989, 125, 279–285. [Google Scholar] [CrossRef]
- Faget, L.; Hnasko, T.S. Tyramide Signal Amplification for Immunofluorescent Enhancement. Methods Mol. Biol. 2015, 1318, 161–172. [Google Scholar] [CrossRef]
- Stack, E.C.; Wang, C.; Roman, K.A.; Hoyt, C.C. Multiplexed immunohistochemistry, imaging, and quantitation: A review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014, 70, 46–58. [Google Scholar] [CrossRef]
- Badoual, C.; Hans, S.; Rodriguez, J.; Peyrard, S.; Klein, C.; Agueznay, N.E.H.; Mosseri, V.; Laccourreye, O.; Bruneval, P.; Fridman, W.H.; et al. Prognostic Value of Tumor-Infiltrating CD4+ T-Cell Subpopulations in Head and Neck Cancers. Clin. Cancer Res. 2006, 12, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Granier, C.; Vinatier, E.; Colin, E.; Mandavit, M.; Dariane, C.; Verkarre, V.; Biard, L.; El Zein, R.; Lesaffre, C.; Galy-Fauroux, I.; et al. Multiplexed Immunofluorescence Analysis and Quantification of Intratumoral PD-1+ Tim-3+ CD8+ T Cells. J. Vis. Exp. 2018, 132, e56606. [Google Scholar] [CrossRef]
- Mori, H.; Bolen, J.; Schuetter, L.; Massion, P.; Hoyt, C.C.; VandenBerg, S.; Esserman, L.; Borowsky, A.D.; Campbell, M.J. Characterizing the Tumor Immune Microenvironment with Tyramide-Based Multiplex Immunofluorescence. J. Mammary Gland Biol. Neoplasia 2020, 25, 417–432. [Google Scholar] [CrossRef]
- Graeser, M.; Feuerhake, F.; Gluz, O.; Volk, V.; Hauptmann, M.; Jozwiak, K.; Christgen, M.; Kuemmel, S.; Grischke, E.-M.; Forstbauer, H.; et al. Immune cell composition and functional marker dynamics from multiplexed immunohistochemistry to predict response to neoadjuvant chemotherapy in the WSG-ADAPT-TN trial. J. Immunother. Cancer 2021, 9, e002198. [Google Scholar] [CrossRef]
- Yam, C.; Yen, E.-Y.; Chang, J.T.; Bassett, R.L.; Al-Atrash, G.; Garber, H.; Huo, L.; Yang, F.; Philips, A.V.; Ding, Q.-Q.; et al. Immune Phenotype and Response to Neoadjuvant Therapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2021, 27, 5365–5375. [Google Scholar] [CrossRef]
- Hernandez, S.; Rojas, F.; Laberiano, C.; Lazcano, R.; Wistuba, I.; Parra, E.R. Multiplex Immunofluorescence Tyramide Signal Amplification for Immune Cell Profiling of Paraffin-Embedded Tumor Tissues. Front. Mol. Biosci. 2021, 8, 667067. [Google Scholar] [CrossRef]
- Ijsselsteijn, M.E.; Brouwer, T.P.; Abdulrahman, Z.; Reidy, E.; Ramalheiro, A.; Heeren, A.M.; Vahrmeijer, A.; Jordanova, E.S.; De Miranda, N.F. Cancer immunophenotyping by seven-colour multispectral imaging without tyramide signal amplification. J. Pathol. Clin. Res. 2019, 5, 3–11. [Google Scholar] [CrossRef]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Buisseret, L.; Pommey, S.; Allard, B.; Garaud, S.; Bergeron, M.; Cousineau, I.; Ameye, L.; Bareche, Y.; Paesmans, M.; Crown, J.; et al. Clinical significance of CD73 in triple-negative breast cancer: Multiplex analysis of a phase III clinical trial. Ann. Oncol. 2018, 29, 1056–1062. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Laberiano-Fernández, C.; Salazar-Alejo, R.; Zhang, J.; Rendon, J.L.S.; Lee, J.; Soto, L.M.S.; Wistuba, I.I.; Parra, E.R. Impact of Region-of-Interest Size on Immune Profiling Using Multiplex Immunofluorescence Tyramide Signal Amplification for Paraffin-Embedded Tumor Tissues. Pathobiology 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Jiang, M.; Solis, L.; Mino, B.; Laberiano, C.; Hernandez, S.; Gite, S.; Verma, A.; Tetzlaff, M.; Haymaker, C.; et al. Procedural Requirements and Recommendations for Multiplex Immunofluorescence Tyramide Signal Amplification Assays to Support Translational Oncology Studies. Cancers 2020, 12, 255. [Google Scholar] [CrossRef] [Green Version]
- Ornatsky, O.I.; Kinach, R.; Bandura, D.R.; Lou, X.; Tanner, S.D.; Baranov, V.I.; Nitz, M.; Winnik, M.A. Development of analytical methods for multiplex bio-assay with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2008, 23, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bandura, D.R.; Baranov, V.I.; Ornatsky, O.I.; Antonov, A.; Kinach, R.; Lou, X.; Pavlov, S.; Vorobiev, S.; Dick, J.E.; Tanner, S.D. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Anal. Chem. 2009, 81, 6813–6822. [Google Scholar] [CrossRef] [PubMed]
- Devine, R.D.; Behbehani, G.K. Mass Cytometry, Imaging Mass Cytometry, and Multiplexed Ion Beam Imaging Use in a Clinical Setting. Clin. Lab. Med. 2021, 41, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Elaldi, R.; Hemon, P.; Petti, L.; Cosson, E.; Desrues, B.; Sudaka, A.; Poissonnet, G.; Van Obberghen-Schilling, E.; Pers, J.-O.; Braud, V.M.; et al. High Dimensional Imaging Mass Cytometry Panel to Visualize the Tumor Immune Microenvironment Contexture. Front. Immunol. 2021, 12, 666233. [Google Scholar] [CrossRef] [PubMed]
- Bouzekri, A.; Esch, A.; Ornatsky, O. Multidimensional profiling of drug-treated cells by Imaging Mass Cytometry. FEBS Open Bio 2019, 9, 1652–1669. [Google Scholar] [CrossRef] [Green Version]
- Ijsselsteijn, M.E.; Van Der Breggen, R.; Sarasqueta, A.F.; Koning, F.; De Miranda, N.F.C.C. A 40-Marker Panel for High Dimensional Characterization of Cancer Immune Microenvironments by Imaging Mass Cytometry. Front. Immunol. 2019, 10, 2534. [Google Scholar] [CrossRef]
- Martinez-Morilla, S.; Villarroel-Espindola, F.; Wong, P.F.; Toki, M.I.; Aung, T.N.; Pelekanou, V.; Bourke-Martin, B.; Schalper, K.A.; Kluger, H.M.; Rimm, D.L. Biomarker Discovery in Patients with Immunotherapy-Treated Melanoma with Imaging Mass Cytometry. Clin. Cancer Res. 2021, 27, 1987–1996. [Google Scholar] [CrossRef]
- Xie, S.; Shan, X.-F.; Yau, V.; Zhang, J.-Y.; Zhang, X.-Y.; Yan, Y.-P.; Cai, Z.-G. Hyperion imaging system reveals heterogeneous tumor microenvironment of oral squamous cell carcinoma patients at T1N0M0 stage. Ann. Transl. Med. 2020, 8, 1513. [Google Scholar] [CrossRef]
- Aoki, T.; Chong, L.C.; Takata, K.; Milne, K.; Hav, M.; Colombo, A.; Chavez, E.A.; Nissen, M.; Wang, X.; Miyata-Takata, T.; et al. Single-Cell Transcriptome Analysis Reveals Disease-Defining T-cell Subsets in the Tumor Microenvironment of Classic Hodgkin Lymphoma. Cancer Discov. 2020, 10, 406–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keren, L.; Bosse, M.; Thompson, S.; Risom, T.; Vijayaragavan, K.; McCaffrey, E.; Marquez, D.; Angoshtari, R.; Greenwald, N.F.; Fienberg, H.; et al. MIBI-TOF: A multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv. 2019, 5, eaax5851. [Google Scholar] [CrossRef] [Green Version]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.-R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goltsev, Y.; Samusik, N.; Kennedy-Darling, J.; Bhate, S.; Hale, M.; Vazquez, G.; Black, S.; Nolan, G.P. Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging. Cell 2018, 174, 968–981.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, S.; Phillips, D.; Hickey, J.W.; Kennedy-Darling, J.; Venkataraaman, V.G.; Samusik, N.; Goltsev, Y.; Schürch, C.M.; Nolan, G.P. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nat. Protoc. 2021, 16, 3802–3835. [Google Scholar] [CrossRef]
- Medrano, R.; Han, F.; Ben Cheikh, B.; Leinert, P.; Leinert, W.P.; Braubach, O.; Schreiber, R. 309 Visualizing the immunotherapy-induced spatial reorganization of the tumor-immune microenvironment by CODEX multiplex imaging. J. Immunother. Cancer 2021, 9, A334. [Google Scholar] [CrossRef]
- Quek, C.; Silva, I.P.; Al-Eryani, G.; Mayer, A.; Bartonicek, N.; Harvey, K.; Buckley, C.; Braubach, O.; Thompson, J.F.; Stretch, J.R.; et al. Abstract 2761: CODEX highly multiplex image mapping to CITEseq datasets reveal the spatial dynamics of the TME during the development of acquired resistant in immunotherapy treated melanoma. Cancer Res. 2021, 81, 2761. [Google Scholar] [CrossRef]
- Phillips, D.; Schürch, C.M.; Khodadoust, M.S.; Kim, Y.H.; Nolan, G.P.; Jiang, S. Highly Multiplexed Phenotyping of Immunoregulatory Proteins in the Tumor Microenvironment by CODEX Tissue Imaging. Front. Immunol. 2021, 12, 687673. [Google Scholar] [CrossRef]
- Schürch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R.; et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell 2020, 183, 838. [Google Scholar] [CrossRef]
- Merritt, C.R.; Ong, G.T.; Church, S.E.; Barker, K.; Danaher, P.; Geiss, G.; Hoang, M.; Jung, J.; Liang, Y.; McKay-Fleisch, J.; et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020, 38, 586–599. [Google Scholar] [CrossRef]
- Zollinger, D.R.; Lingle, S.E.; Sorg, K.; Beechem, J.M.; Merritt, C.R. GeoMx™ RNA Assay: High Multiplex, Digital, Spatial Analysis of RNA in FFPE Tissue. Methods Mol. Biol. 2020, 2148, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.-F.; Xue, W.; Koh, S.-P.; Chiu, Y.-M.; Ng, L.P.-W.; Wong, S.-C.C. NanoString, a novel digital color-coded barcode technology: Current and future applications in molecular diagnostics. Expert Rev. Mol. Diagn. 2017, 17, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Beechem, J. High-Plex Spatially Resolved RNA and Protein Detection Using Digital Spatial Profiling: A Technology Designed for Immuno-oncology Biomarker Discovery and Translational Research. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2055, pp. 563–583. [Google Scholar]
- Zugazagoitia, J.; Gupta, S.; Liu, Y.; Fuhrman, K.; Gettinger, S.; Herbst, R.S.; Schalper, K.A.; Rimm, D.L. Biomarkers Associated with Beneficial PD-1 Checkpoint Blockade in Non–Small Cell Lung Cancer (NSCLC) Identified Using High-Plex Digital Spatial Profiling. Clin. Cancer Res. 2020, 26, 4360–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulasinghe, A.; Taheri, T.; O’Byrne, K.; Hughes, B.G.M.; Kenny, L.; Punyadeera, C. Highly Multiplexed Digital Spatial Profiling of the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma Patients. Front. Oncol. 2020, 10, 607349. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Labrie, M.; Yong, G.; Camp, T.; Ma, H.; Grout, M.; Xu, W.; Beasley, G.; Schuchter, L.M.; Mcgettigan, S.; et al. Multiomics profiling of longitudinal melanoma specimens unravels molecular mechanisms of resistance to sequential targeted and cancer immunotherapies. J. Clin. Oncol. 2020, 38, e22015. [Google Scholar] [CrossRef]
- Toki, M.I.; Merritt, C.R.; Wong, P.F.; Smithy, J.W.; Kluger, H.M.; Syrigos, K.N.; Ong, G.T.; Warren, S.E.; Beechem, J.M.; Rimm, D.L. High-Plex Predictive Marker Discovery for Melanoma Immunotherapy–Treated Patients Using Digital Spatial Profiling. Clin. Cancer Res. 2019, 25, 5503–5512. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; Van De Wiel, B.; Kvistborg, P.; Krijgsman, O.; Braber, M.V.D.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef]
- Schlam, I.; Church, S.E.; Hether, T.D.; Chaldekas, K.; Hudson, B.M.; White, A.M.; Maisonet, E.; Harris, B.T.; Swain, S.M. The tumor immune microenvironment of primary and metastatic HER2− positive breast cancers utilizing gene expression and spatial proteomic profiling. J. Transl. Med. 2021, 19, 480. [Google Scholar] [CrossRef]
- Stewart, R.L.; Matynia, A.P.; Factor, R.E.; Varley, K.E. Spatially-resolved quantification of proteins in triple negative breast cancers reveals differences in the immune microenvironment associated with prognosis. Sci. Rep. 2020, 10, 6598. [Google Scholar] [CrossRef] [Green Version]
- Bergholtz, H.; Carter, J.M.; Cesano, A.; Cheang, M.C.U.; Church, S.E.; Divakar, P.; Fuhrman, C.A.; Goel, S.; Gong, J.; Guerriero, J.L.; et al. Best Practices for Spatial Profiling for Breast Cancer Research with the GeoMx® Digital Spatial Profiler. Cancers 2021, 13, 4456. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Zugazagoitia, J.; Martinez-Morilla, S.; Fuhrman, K.; Rimm, D.L. Digital quantitative assessment of PD-L1 using digital spatial profiling. Lab. Investig. 2020, 100, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Manesse, M.; Patel, K.K.; Bobrow, M.; Downing, S.R. The InSituPlex® Staining Method for Multiplexed Immunofluorescence Cell Phenotyping and Spatial Profiling of Tumor FFPE Samples. Methods Mol. Biol. 2019, 2055, 585–592. [Google Scholar] [CrossRef]
- Downing, S.; Patel, K.; Buell, J.; Hebert, C. Abstract 5657: Use of a novel immunofluorescence multiplexing technology, InSituPlex™, for the simultaneous detection of immune cells in multiple cancer types. Cancer Res. 2018, 78, 5657. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Xia, Z.; Chatterjee, G.; Hwang, K.; Manesse, M. Abstract 1183: High-plex spatial profiling of whole FFPE tissue sections using InSituPlex® technology for discovery applications. Cancer Res. 2019, 79, 1183. [Google Scholar] [CrossRef]
- Taube, J.M.; Akturk, G.; Angelo, M.; Engle, E.L.; Gnjatic, S.; Greenbaum, S.; Greenwald, N.F.; Hedvat, C.V.; Hollmann, T.J.; Juco, J.; et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J. Immunother. Cancer 2020, 8, e000155. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A., Jr.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal Growth Factor Receptor in Non–Small-Cell Lung Carcinomas: Correlation Between Gene Copy Number and Protein Expression and Impact on Prognosis. J. Clin. Oncol. 2003, 21, 3798–3807. [Google Scholar] [CrossRef]
- Ilié, M.; Beaulande, M.; Ben Hadj, S.; Chamorey, E.; Schiappa, R.; Long-Mira, E.; Lassalle, S.; Butori, C.; Cohen, C.; Leroy, S.; et al. Chromogenic Multiplex Immunohistochemistry Reveals Modulation of the Immune Microenvironment Associated with Survival in Elderly Patients with Lung Adenocarcinoma. Cancers 2018, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Farkas, D.L.; Du, C.; Fisher, G.W.; Lau, C.; Niu, W.; Wachman, E.S.; Levenson, R.M. Non-invasive image acquisition and advanced processing in optical bioimaging. Comput. Med Imaging Graph. 1998, 22, 89–102. [Google Scholar] [CrossRef]
- Bodenmiller, B. Multiplexed Epitope-Based Tissue Imaging for Discovery and Healthcare Applications. Cell Syst. 2016, 2, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Gohil, S.H.; Iorgulescu, J.B.; Braun, D.A.; Keskin, D.B.; Livak, K.J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, J.; Dimitrion, P.; Yao, Y.; Zhou, L.; Ozog, D.; Mi, Q.-S. Research Techniques Made Simple: Use of Imaging Mass Cytometry for Dermatological Research and Clinical Applications. J. Investig. Dermatol. 2021, 141, 705–712.e1. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Ornatsky, O.I.; Siddiqui, I.; Loboda, A.; Baranov, V.I.; Hedley, D.W. Imaging Mass Cytometry. Cytom. A 2017, 91, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Angelo, M.; Bendall, S.C.; Finck, R.; Hale, M.B.; Hitzman, C.; Borowsky, A.D.; Levenson, R.M.; Lowe, J.B.; Liu, S.D.; Zhao, S.; et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 2014, 20, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Parra, E.R.; Francisco-Cruz, A.; Wistuba, I.I. State-of-the-Art of Profiling Immune Contexture in the Era of Multiplexed Staining and Digital Analysis to Study Paraffin Tumor Tissues. Cancers 2019, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, K. Multiplexing Immunohistochemistry. Mater. Methods 2019, 9. [Google Scholar] [CrossRef]
- Andersen, N.L.; Brügmann, A.; Lelkaitis, G.; Nielsen, S.; Lippert, M.F.; Vyberg, M. Virtual Double Staining: A Digital Approach to Immunohistochemical Quantification of Estrogen Receptor Protein in Breast Carcinoma Specimens. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 620–626. [Google Scholar] [CrossRef]
- Humphries, M.P.; Bingham, V.; Sidi, F.A.; Craig, S.; McQuaid, S.; James, J.; Salto-Tellez, M. Improving the Diagnostic Accuracy of the PD-L1 Test with Image Analysis and Multiplex Hybridization. Cancers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Wang, H.; Xu, Z.; Wang, Y.; Li, S.; Liu, J.; Chen, Y.; Luo, H.; Wu, L.; et al. Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer. J. Immunother. Cancer 2021, 9, e002356. [Google Scholar] [CrossRef]
- Wimberly, H.; Brown, J.R.; Schalper, K.; Haack, H.; Silver, M.R.; Nixon, C.; Bossuyt, V.; Pusztai, L.; Lannin, D.R.; Rimm, D.L. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol. Res. 2015, 3, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, W.; Yang, X.; Wang, H.; Liu, X.; Chi, K.; Zhou, L.; Huang, X.; Zhao, S.; Ding, T.; et al. FP07.03 Landscape Heterogeneity of PD-L1 Expression and Immune Cells Predicts Prognosis of Metastatic Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2021, 16, S203–S204. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Parwani, A.V.; Li, Z. Evaluation of Immune Reaction and PD-L1 Expression Using Multiplex Immunohistochemistry in HER2-Positive Breast Cancer: The Association with Response to Anti-HER2 Neoadjuvant Therapy. Clin. Breast Cancer 2018, 18, e237–e244. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Nguyen, P.; Engle, E.L.; Kaunitz, G.J.; Cottrell, T.R.; Berry, S.; Green, B.; Soni, A.; Cuda, J.D.; Stein, J.E.; et al. Multidimensional, quantitative assessment of PD-1/PD-L1 expression in patients with Merkel cell carcinoma and association with response to pembrolizumab. J. Immunother. Cancer 2018, 6, 99. [Google Scholar] [CrossRef]
- Sidi, F.A.; Bingham, V.; Craig, S.G.; McQuaid, S.; James, J.; Humphries, M.P.; Salto-Tellez, M. PD-L1 Multiplex and Quantitative Image Analysis for Molecular Diagnostics. Cancers 2020, 13, 29. [Google Scholar] [CrossRef]
- Webster, J.D.; Dunstan, R.W. Whole-slide imaging and automated image analysis: Considerations and opportunities in the practice of pathology. Vet. Pathol. 2014, 51, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Mantri, M.; Taran, S.; Sunder, G. DICOM Integration Libraries for Medical Image Interoperability: A Technical Review. IEEE Rev. Biomed. Eng. 2020, 15, 247–259. [Google Scholar] [CrossRef]
- Kahn, C.E.; Carrino, J.A.; Flynn, M.J.; Peck, D.J.; Horii, S.C. DICOM and Radiology: Past, Present, and Future. J. Am. Coll. Radiol. 2007, 4, 652–657. [Google Scholar] [CrossRef]
- Genereaux, B.W.; Dennison, D.K.; Ho, K.; Horn, R.; Silver, E.L.; O’Donnell, K.; Kahn, C.E., Jr. DICOMweb™: Background and Application of the Web Standard for Medical Imaging. J. Digit. Imaging 2018, 31, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Prodduturi, N.; Jiang, J.; Flotte, T.J.; Hart, S.N. Dicom_wsi: A Python Implementation for Converting Whole-Slide Images to Digital Imaging and Communications in Medicine Compliant Files. J. Pathol. Inform. 2021, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.; Lipp, P.; Kaestner, L. ATOM-an OMERO add-on for automated import of image data. BMC Res. Notes 2011, 4, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Besson, S.; Blackburn, C.; Carroll, M.; Ferguson, R.K.; Flynn, H.; Gillen, K.; Leigh, R.; Lindner, D.; Linkert, M.; et al. Metadata management for high content screening in OMERO. Methods 2016, 96, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiner, M.C.; Rueden, C.T.; Eliceiri, K.W. SCIFIO: An extensible framework to support scientific image formats. BMC Bioinform. 2016, 17, 521. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Chubb, L.; Pantanowitz, L.; Parwani, A. Standardization in digital pathology: Supplement 145 of the DICOM standards. J. Pathol. Inform. 2011, 2, 23. [Google Scholar] [CrossRef]
- Kalinski, T.; Zwönitzer, R.; Roßner, M.; Hofmann, H.; Roessner, A.; Guenther, T. Digital Imaging and Communications in Medicine (DICOM) as standard in digital pathology. Histopathology 2012, 61, 132–134. [Google Scholar] [CrossRef]
- Godinho, T.M.; Lebre, R.; Silva, L.B.; Costa, C. An efficient architecture to support digital pathology in standard medical imaging repositories. J. Biomed. Inform. 2017, 71, 190–197. [Google Scholar] [CrossRef]
- Clunie, D.; Hosseinzadeh, D.; Wintell, M.; De Mena, D.; Lajara, N.; García-Rojo, M.; Bueno, G.; Saligrama, K.; Stearrett, A.; Toomey, D.; et al. Digital Imaging and Communications in Medicine Whole Slide Imaging Connectathon at Digital Pathology Association Pathology Visions 2017. J. Pathol. Inform. 2018, 9, 6. [Google Scholar] [CrossRef]
- Madabhushi, A.; Lee, G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med. Image Anal. 2016, 33, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Jahn, S.W.; Plass, M.; Moinfar, F. Digital Pathology: Advantages, Limitations and Emerging Perspectives. J. Clin. Med. 2020, 9, 3697. [Google Scholar] [CrossRef]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [Green Version]
- Lyons, C.; Lawler, D. Aperio Cellular IF Algorithm Validation. Pathologist 2016. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, M.; Maxwell, P.; Salto-Tellez, M. QuPath: The global impact of an open source digital pathology system. Comput. Struct. Biotechnol. J. 2021, 19, 852–859. [Google Scholar] [CrossRef]
- Girish, V.; Vijayalakshmi, A. Affordable image analysis using NIH Image/ImageJ. Indian J. Cancer 2004, 41, 47. [Google Scholar]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43 (Suppl. S1), S25–S30. [Google Scholar] [CrossRef]
- Abramoff, M.; Magalhães, P.; Ram, S.J. Image Processing with Image. J. Biophotonics Int. 2003, 11, 36–42. [Google Scholar]
- Schroeder, A.B.; Dobson, E.T.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lamprecht, M.R.; Sabatini, D.M.; Carpenter, A.E. CellProfiler™: Free, versatile software for automated biological image analysis. BioTechniques 2007, 42, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Dobson, E.T.; Cimini, B.; Klemm, A.H.; Wählby, C.; Carpenter, A.E.; Eliceiri, K.W. ImageJ and CellProfiler: Complements in Open-Source Bioimage Analysis. Curr. Protoc. 2021, 1, e89. [Google Scholar] [CrossRef] [PubMed]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Chaumont, F.; Dallongeville, S.; Olivo-Marin, J. ICY: A New Open-Source Community Image Processing Software. In Proceedings of the 2011 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March 2011–2 April 2011. [Google Scholar]
- de Chaumont, F.; Dallongeville, S.; Chenouard, N.; Hervé, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; Lecomte, T.; Le Montagner, Y.; et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, M.J.; Pajenda, S.; Ilhan-Mutlu, A.; Steindl, A.; Kiesel, B.; Widhalm, G.; Dieckmann, K.; Feldmann, K.; Hainfellner, J.; Marosi, C.; et al. Soluble PD-L1 is associated with local and systemic inflammation markers in primary and secondary brain tumours. ESMO Open 2020, 5, e000863. [Google Scholar] [CrossRef]
- Xia, D.; Casanova, R.; Machiraju, D.; McKee, T.; Weder, W.; Beck, A.H.; Soltermann, A. Computationally-Guided Development of a Stromal Inflammation Histologic Biomarker in Lung Squamous Cell Carcinoma. Sci. Rep. 2018, 8, 3941. [Google Scholar] [CrossRef] [Green Version]
- Starzer, A.M.; Berghoff, A.S.; Hamacher, R.; Tomasich, E.; Feldmann, K.; Hatziioannou, T.; Traint, S.; Lamm, W.; Noebauer-Huhmann, I.M.; Furtner, J.; et al. Tumor DNA methylation profiles correlate with response to anti-PD-1 immune checkpoint inhibitor monotherapy in sarcoma patients. J. Immunother. Cancer 2021, 9, e001458. [Google Scholar] [CrossRef]
- Naso, J.R.; Povshedna, T.; Wang, G.; Banyi, N.; MacAulay, C.; Ionescu, D.N.; Zhou, C. Automated PD-L1 Scoring for Non-Small Cell Lung Carcinoma Using Open-Source Software. Pathol. Oncol. Res. 2021, 27, 609717. [Google Scholar] [CrossRef]
- Inge, L.; Dennis, E. Development and applications of computer image analysis algorithms for scoring of PD-L1 immunohistochemistry. Immuno-Oncol. Technol. 2020, 6, 2–8. [Google Scholar] [CrossRef]
- Puladi, B.; Ooms, M.; Kintsler, S.; Houschyar, K.S.; Steib, F.; Modabber, A.; Hölzle, F.; Knüchel-Clarke, R.; Braunschweig, T. Automated PD-L1 Scoring Using Artificial Intelligence in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 4409. [Google Scholar] [CrossRef]
- Haragan, A.; Field, J.K.; Davies, M.P.; Escriu, C.; Gruver, A.; Gosney, J.R. Heterogeneity of PD-L1 expression in non-small cell lung cancer: Implications for specimen sampling in predicting treatment response. Lung Cancer 2019, 134, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-N.; Jang, J.; Heo, Y.J.; Kim, B.; Jung, H.; Jang, Y.; Kang, S.Y.; Kim, S.T.; Lee, J.; Kang, W.K.; et al. PD-L1 expression in gastric cancer determined by digital image analyses: Pitfalls and correlation with pathologist interpretation. Virchows Arch. 2020, 476, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kapil, A.; Meier, A.; Steele, K.; Rebelatto, M.; Nekolla, K.; Haragan, A.; Silva, A.; Zuraw, A.; Barker, C.; Scott, M.L.; et al. Domain Adaptation-Based Deep Learning for Automated Tumor Cell (TC) Scoring and Survival Analysis on PD-L1 Stained Tissue Images. IEEE Trans. Med. Imaging 2021, 40, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
| Method | Advantages | Disadvantages |
|---|---|---|
| mIHC | Low cost and automation of staining. The simplicity of usage and interpretation. Established guidelines and protocols. Standard light microscope for interpretation. | Co-expression studies require careful selection of the chromogen pairs, and due to the limited amount of tissue on one slide, only a restricted number of chromogens can be used. Semiquantitative method, unable to assess marker intensity. |
| MICSSS | It is a simple and relatively affordable technique, similar to standard chromogenic immunohistochemistry. Ability to preview the entire slide for each marker. Each marker is individually stained, excluding staining or signal interferences. Standard light microscope for interpretation. | Time-consuming method due to slow throughput. It allows the marking of up to 10 biomarkers on a single slide for 10 days (6 h per cycle). Possibility of mechanical tissue damage and formation of artefacts during the coverslip removal procedures. Difficulties with coregistration of images on the the whole slide due to their large number and complicated software service. |
| TSA | It allows spatial-arrangement analysis of multiple targets within a single tissue section. Any primary antibody validated for IHC, regardless of host species, can be used for each target of interest. The autofluorescence can be rectified by a multispectral microscope. Purified fluorophores are commercially accessible. When compared to chromogenic-based methods, multiplex immunofluorescence has a larger linear dynamic range, which makes it easier to study the marker intensity. Costs are comparable to standard chromogenic-based methods. | There is an elevated risk of human-error occurrence, while manual staging is difficult. However, the use of autostainers could help to overcome the problem. There is a risk of “fluorophore bleed-through” or “umbrella effect” due to excessive tyramide deposition. Spectral overlap is a problem when the above seven probes are analysed. |
| IMC | Absence of tissue background signal. Highly quantitative method due to the absence of matrix effects. No need for serial slides to raise the target number or cyclic rounds of labelling–stripping–acquisition of the same tissue section. Up to 40 markers on an individual tissue section at a single-cell level can be analysed. The information on tissue architecture and cellular morphology is preserved. Markers can be analysed in parallel for a single section of tissue with low channel crosstalk. | When compared to fluorescence imaging methods, the subcellular resolution is diminished. Laser-ablated tissue is not reusable for subsequent applications. More expensive than techniques based on fluorophore-conjugated antibodies. Advanced analysis tools are required. Increasing the processing speed is limited in this method. The main limitation is the risk of cross-contamination between laser-ablation spots. The analysed slide is not imaged. Because of the time required to perform ablation, ROI size is limited. Lower sensitivity than fluorescence imaging techniques as it lacks signal amplification or possibility to raise exposure time. |
| MIBI | Absence of tissue background signal. Quantitative information can be obtained from the types of cells and their distribution within the tissue. Markers can be analysed in parallel for a single section of tissue with low channel crosstalk. Image resolution, as well as depth of sample acquisition, can be adjusted. Has the capability of reaching sensitivity as low as parts-per-billion with a dynamic range of 10^5, and preserves very high resolution. It is capable of analysing up to 100 markers on a unique tissue section | More expensive than techniques based on fluorophore-conjugated antibodies. The entire tissue slide is not a digital image; it is only the ROI. |
| CODEX | Can simultaneously reveal up to 60 markers in an individual tissue section. Lack of cross-reactivity (oligonucleotide–oligonucleotide, tissue or cellular DNA). It provides information about biomarkers’ relative number and expression at a spatial level. Relatively cost-effective and quick method. | It lacks a signal-amplification system. Baseline autofluorescence of tissues present. Unified staining protocol demands that each antibody be individually conjugated and validated. The antibodies used in the CODEX system are expensive. |
| DSP | Simultaneous measurement of all markers. Possibility to create up to an 800-plex assay. However, when applying the NGS readout mode, the multiplexing is unlimited. Repeated cycles of high-plex profiling or subsequent DNA sequencing on the same tissue section are available. No autofluorescence is present. | No single-cell expression data. Profiling every cell in a tissue slice at single-cell resolution is costly and tedious. It cannot create an image. |
| InSituPlex | More reproducible than other multiplexing techniques. Lower complexity of the laboratory test, fewer component reagents to prepare, fewer retrieval steps required, automated staining run, and no need for complex prevalidation when compared to multiplex-fluorescence techniques. An assay protocol can be easily implemented in laboratories with the standard fluorescent microscope, because it is compatible with standard IHC workflows and automation instrumentation. It preserves the integrity of the tissue sample. | A small number of publications are available. |
| QuPath | ImageJ | CellProfiler | Icy | |
|---|---|---|---|---|
| Type of imaging | Brightfield and fluorescence | Brightfield and fluorescence | Flow cytometry, brightfield, darkfield, or fluorescence | Brightfield and fluorescence |
| Handle to WSI | Yes | No (needs plugin) | No (needs other programs) | Yes |
| IHC analysis | Yes | Yes | Yes | Yes |
| Bio-format | Yes | Yes (with plugin) | Yes | Yes |
| Other advantages | Built-in cell segmentation and classification software, pixel clarifier, smart annotation tools | Many plugins developed | The user-friendly interface supports 3D images | Supports 3D images, tracking moving cells |
| Disadvantages | Some options require programming skills to use | Some plugins need programming skills to use | Small number of plugins or plugins that overlap in their functionality. | Designed for researchers with software-development skills |
| Ref. | [147] | [148] | [145] | [143] | [127] |
|---|---|---|---|---|---|
| Aim of the study | PD-L1 expression evaluation using digital-image analyses correlated with pathologist interpretation. | Domain adaptation-based deep learning for automated tumour-cell scoring on PD-L1 stained tissue sections. | Automated PD-L1 scoring applying artificial intelligence. | Automated PD-L1 scoring applying open-source software. | QuPath performance testing. |
| Type of cancer | Gastric cancer | Non-small-cell lung cancer | Head and neck squamous cell carcinoma | Non-small-cell lung cancer | Colorectal cancer |
| Method | IHC | IHC | IHC | IHC | IHC |
| Tools | FDA-cleared Aperio Imagescope IHC Membrane Image-Analysis software (ScanScope, Aperio Technologies, Vista, CA, USA) | Deep-learning-based image- analysis software (DASGAN network) | QuPath | QuPath | QuPath |
| Conclusions | No significant difference in interpretation between pathologist and digital analysis | Software replicates the pathologist’s assessment | Comparable results between human-to-human and human-to-AI interpretation. | Similar interpretation between pathologist and digital analysis | There is incipient evidence that software helps in investigating PD-L1 prognostic value in colorectal cancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczkiewicz-Siemion, O.; Sokół, K.; Puton, B.; Borkowska, A.; Szumera-Ciećkiewicz, A. The Role of Pathology-Based Methods in Qualitative and Quantitative Approaches to Cancer Immunotherapy. Cancers 2022, 14, 3833. https://doi.org/10.3390/cancers14153833
Kuczkiewicz-Siemion O, Sokół K, Puton B, Borkowska A, Szumera-Ciećkiewicz A. The Role of Pathology-Based Methods in Qualitative and Quantitative Approaches to Cancer Immunotherapy. Cancers. 2022; 14(15):3833. https://doi.org/10.3390/cancers14153833
Chicago/Turabian StyleKuczkiewicz-Siemion, Olga, Kamil Sokół, Beata Puton, Aneta Borkowska, and Anna Szumera-Ciećkiewicz. 2022. "The Role of Pathology-Based Methods in Qualitative and Quantitative Approaches to Cancer Immunotherapy" Cancers 14, no. 15: 3833. https://doi.org/10.3390/cancers14153833





