Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review
Abstract
:Simple Summary
Abstract
1. Introduction
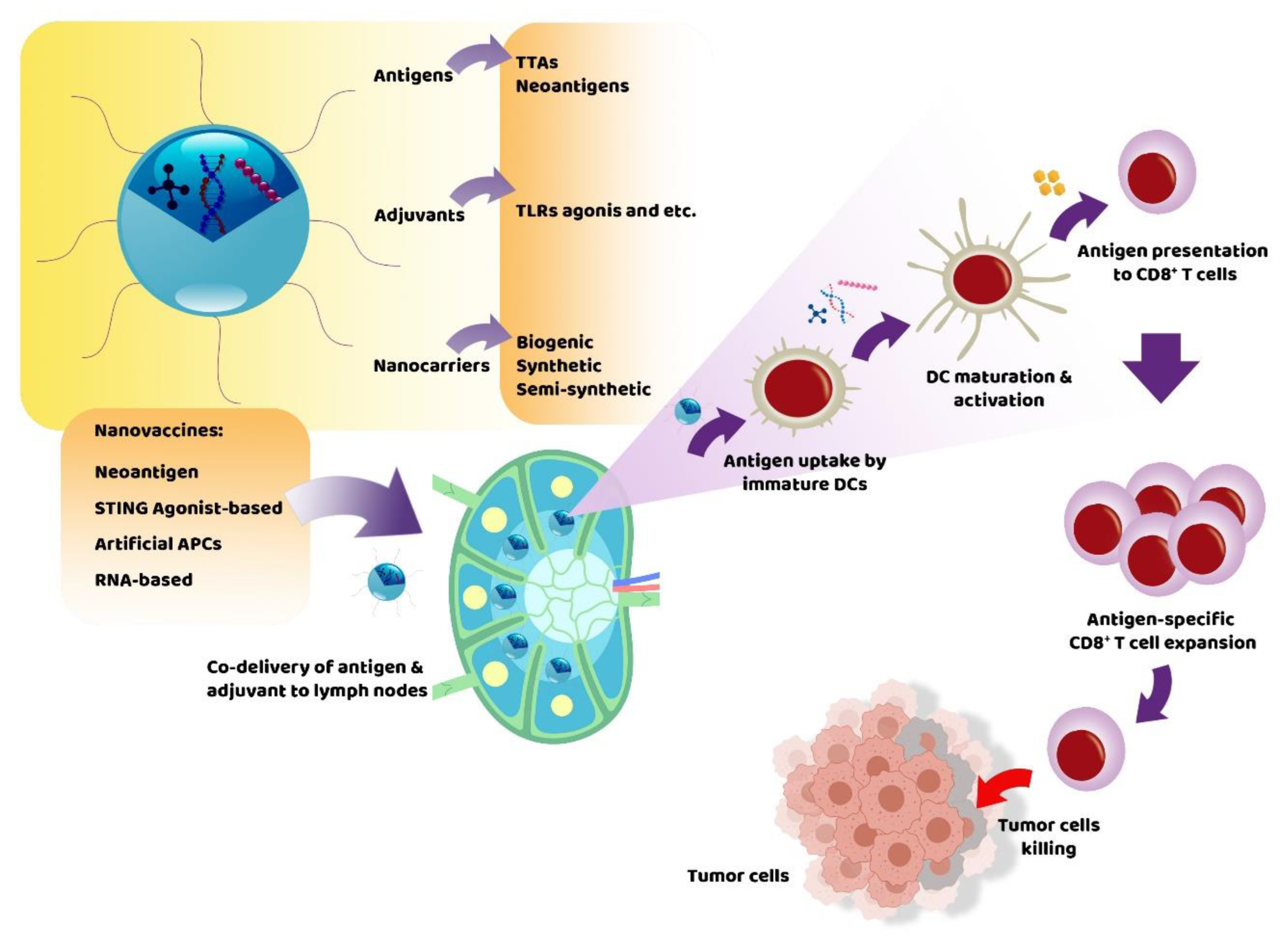
2. What Are Nanovaccines Composed of?
2.1. Antigens
2.2. Immunostimulatory Adjuvants
3. Nanocarriers
3.1. Biogenic Nanocarriers
3.1.1. Outer Membrane Vesicles
3.1.2. Exosomes
3.2. Semi-Biogenic Nanocarriers
3.2.1. Virus-like Particles
3.2.2. Endogenous Protein-Based Nanocarriers
3.2.3. Cell Membrane-Coated Nanocarriers
3.3. Synthetic Nanocarriers
3.3.1. Liposomes
3.3.2. Polymer Nanoparticles
3.3.3. Inorganic Materials
3.4. Self-Adjuvanted Nanocarriers
4. Types of Nanovaccines
4.1. Neoantigen Nanovaccines
4.2. STING Agonist-Based Nanovaccines
4.3. Artificial APCs
4.4. RNA-Based Nanovaccines
5. Nanovaccines in Cancer Therapy
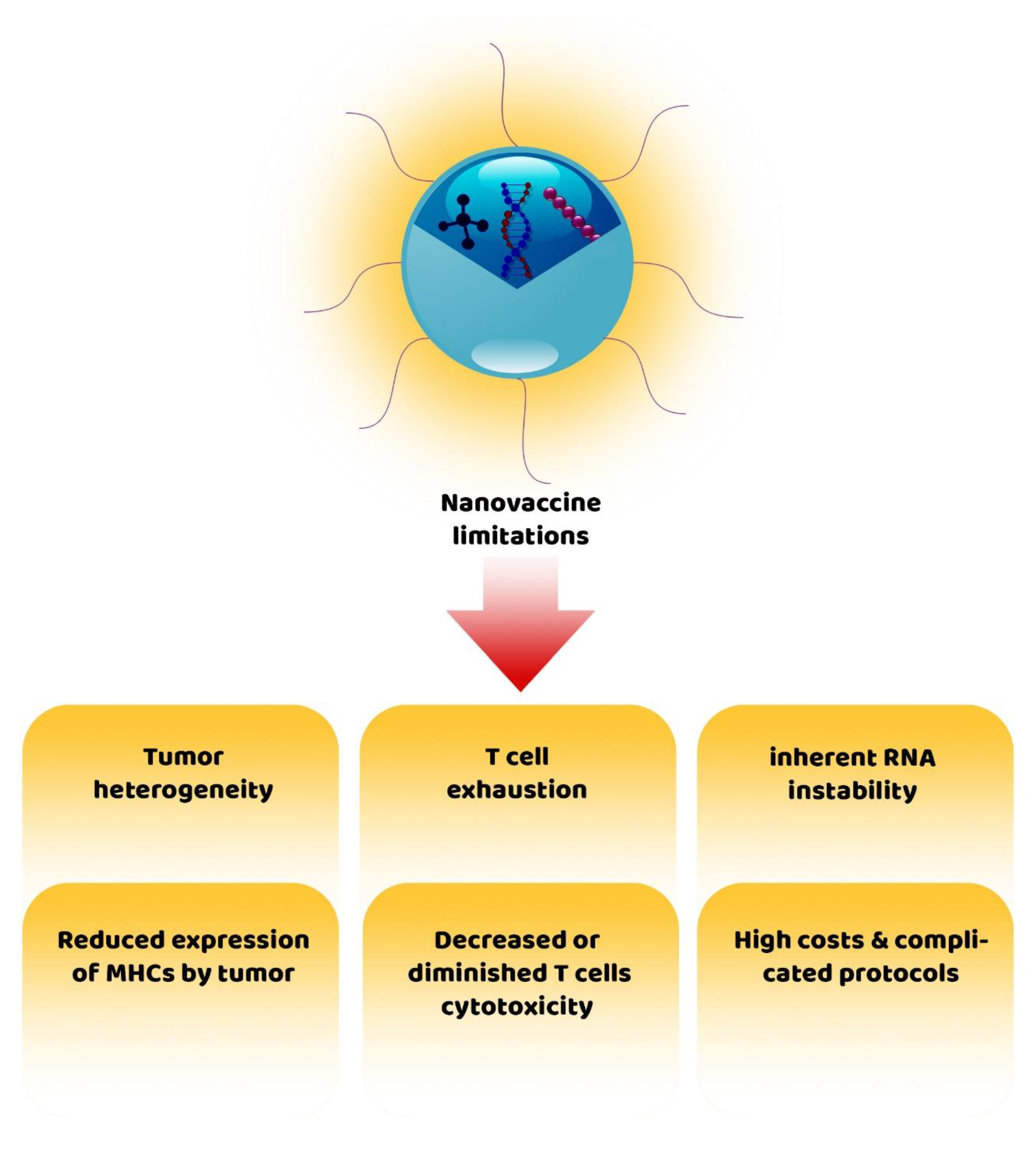
6. Challenges of Nanovaccines for Cancer Therapy
7. Concluding Remarks
Authors Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TME | Tumor microenvironment |
| NPs | Nanoparticles |
| APCs | Antigen-presenting cells |
| COVID-19 | Coronavirus disease of 2019 |
| MHC-I | Major histocompatibility complex I |
| Tregs | Regulatory T cells |
| TSAs | Tumor-specific antigens |
| TAAs | Tumor-associated antigens |
| MAGE-A | Melanoma-associated antigens-A |
| TTK | TTK protein kinase |
| NY-ESO-1 | New York’s esophageal squamous cell carcinoma 1 |
| NRT | Neoantigen reactive T cell |
| STING | Stimulator of interferon genes |
| TLR | Toll-like receptor |
| PLGA | Lactic-co-glycolic acid |
| OMVs | Outer membrane vesicles |
| TCRs | T cell receptors |
| VLPs | Virus-like particles |
| FcRn | Neonatal Fc receptor |
| PLGA | Poly lactic-co-glycolic acid |
| PEG | Polyethylene glycol |
| SNAs | Spherical nucleic acids |
| IFNs | Interferons |
| cGAS | Cytosolic cyclic GMP–AMP synthase |
| CDNs | Cyclic dinucleotides |
| OVA | Ovalbumin |
| BMT | Black mesoporous titania |
| LA | L-arginine |
| PD-L1 | Programmed death-ligand 1 |
| PD-1 | Programmed death-1 |
| PRT | Protamine |
| ICG | Indocyanine green |
| LLC | Lewis’s lung cancer |
| MSNs | Mesoporous silica NPs |
| PDA | Photothermal agent polydopamine |
| PEI | Polyethylenimine |
| GO | Graphene oxide |
| NFKB | Nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB) |
| miRNA | MicroRNA |
| TNFSF4 | TNF superfamily member 4 |
| TILs | Tumor infiltrated lymphocytes |
| TAMs | Tumor-associated macrophages |
| GMP | Good manufacturing practices |
| BDMCs | Bone marrow-derived dendritic cells |
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.A.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, S.; Wang, X.Y.; Zhu, G. Nanovaccines for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1559. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef]
- Jiang, Y.; Krishnan, N.; Zhou, J.; Chekuri, S.; Wei, X.; Kroll, A.V.; Yu, C.L.; Duan, Y.; Gao, W.; Fang, R.H.; et al. Engineered Cell-Membrane-Coated Nanoparticles Directly Present Tumor Antigens to Promote Anticancer Immunity. Adv. Mater. 2020, 32, 2001808. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Biomimetic nanovaccines for COVID-19. Appl. Sci. Technol. Ann. 2020, 1, 176–182. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Ji, W.; Li, L.; Qiu, L.; Zhou, S.; Qian, Z.; Zhang, H. Hybrid Membrane Nanovaccines Combined with Immune Checkpoint Blockade to Enhance Cancer Immunotherapy. Int. J. Nanomed. 2022, 17, 73. [Google Scholar] [CrossRef]
- Kroll, A.V.; Jiang, Y.; Zhou, J.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic nanoparticle vaccines for cancer therapy. Adv. Biosyst. 2019, 3, 1800219. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Guo, J.; Huang, L. Modulation of tumor microenvironment for immunotherapy: Focus on nanomaterial-based strategies. Theranostics 2020, 10, 3099. [Google Scholar] [CrossRef]
- Cai, J.; Wang, H.; Wang, D.; Li, Y. Improving cancer vaccine efficiency by nanomedicine. Adv. Biosyst. 2019, 3, 1800287. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, Y.; Yang, Y.; Miao, Z.; Zhu, J.; Zhong, M.; Feng, C.; Tang, W.; Zhou, J.; Wang, L.; et al. Biomimetic cytomembrane nanovaccines prevent breast cancer development in the long term. Nanoscale 2021, 13, 3594–3601. [Google Scholar] [CrossRef]
- Rabiee, N.; Kiani, M.; Bagherzadeh, M.; Rabiee, M.; Ahmadi, S. Nanoparticle (NP)-Based Delivery Vehicles; Morgan & Claypool Publishers: San Rafael, CA, USA, 2019. [Google Scholar]
- Zhu, G.; Zhang, F.; Ni, Q.; Niu, G.; Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 2017, 11, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Alatrash, G.; Crain, A.K.; Molldrem, J.J. Tumor-associated antigens. In Immune Biology of Allogeneic Hematopoietic Stem Cell Transplantation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–125. [Google Scholar]
- Dalgleish, A.; Pandha, H. Tumor antigens as surrogate markers and targets for therapy and vaccines. Adv. Cancer Res. 2006, 96, 175–190. [Google Scholar]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Fioretti, D.; Iurescia, S.; Fazio, V.M.; Rinaldi, M. DNA vaccines: Developing new strategies against cancer. J. Biomed. Biotechnol. 2010, 2010, 174378. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhang, L. Expression of cancer–testis antigens in esophageal cancer and their progress in immunotherapy. J. Cancer Res. Clin. Oncol. 2019, 145, 281–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Bruggen, P.; Zhang, Y.; Chaux, P.; Stroobant, V.; Panichelli, C.; Schultz, E.S.; Chapiro, J.; Van den Eynde, B.J.; Brasseur, F.; Boon, T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 2002, 188, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.B.; Shearer, M.H.; Jumper, C.A.; Kennedy, R.C. Towards progress on DNA vaccines for cancer. Cell. Mol. Life Sci. 2007, 64, 2391–2403. [Google Scholar] [CrossRef]
- Ross, J.S.; Fletcher, J.A. The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells 1998, 16, 413–428. [Google Scholar] [CrossRef]
- Loeb, K.R.; Loeb, L.A. Significance of multiple mutations in cancer. Carcinogenesis 2000, 21, 379–385. [Google Scholar] [CrossRef]
- Pearlman, A.H.; Hwang, M.S.; Konig, M.F.; Hsiue, E.H.C.; Douglass, J.; DiNapoli, S.R.; Mog, B.J.; Bettegowda, C.; Pardoll, D.M.; Gabelli, S.B.; et al. Targeting public neoantigens for cancer immunotherapy. Nat. Cancer 2021, 2, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Parmiani, G.; De Filippo, A.; Novellino, L.; Castelli, C. Unique human tumor antigens: Immunobiology and use in clinical trials. J. Immunol. 2007, 178, 1975–1979. [Google Scholar] [CrossRef] [Green Version]
- Petrizzo, A.; Tagliamonte, M.; Mauriello, A.; Costa, V.; Aprile, M.; Esposito, R.; Caporale, A.; Luciano, A.; Arra, C.; Tornesello, M.L.; et al. Unique true predicted neoantigens (TPNAs) correlates with anti-tumor immune control in HCC patients. J. Transl. Med. 2018, 16, 286. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, P.; Banchereau, J.; Bhardwaj, N.; Cockett, M.; Disis, M.L.; Dranoff, G.; Gilboa, E.; Hammond, S.A.; Hershberg, R.; Korman, A.J.; et al. The Human Vaccines Project: A roadmap for cancer vaccine development. Sci. Transl. Med. 2016, 8, ps9–ps334. [Google Scholar] [CrossRef]
- Li, L.; Goedegebuure, S.P.; Gillanders, W. Cancer vaccines: Shared tumor antigens return to the spotlight. Signal Transduct. Target. Ther. 2020, 5, 251. [Google Scholar] [CrossRef]
- Adjuvants and Vaccines. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 2 May 2022).
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; McArdle, S.E. Cancer vaccines: Adjuvant potency, importance of age, lifestyle, and treatments. Front. Immunol. 2021, 11, 3850. [Google Scholar] [CrossRef]
- Schijns, V.E. Mechanisms of vaccine adjuvant activity: Initiation and regulation of immune responses by vaccine adjuvants. Vaccine 2003, 21, 829–831. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer vaccines, adjuvants, and delivery systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Bishop, C.J.; Kozielski, K.L.; Green, J.J. Exploring the role of polymer structure on intracellular nucleic acid delivery via polymeric nanoparticles. J. Control. Release 2015, 219, 488–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovsky, N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV vaccines and the role of TLR agonists in immune response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef] [PubMed]
- Schetters, S.T.; Jong, W.S.; Horrevorts, S.K.; Kruijssen, L.J.; Engels, S.; Stolk, D.; Daleke-Schermerhorn, M.H.; Garcia-Vallejo, J.; Houben, D.; Unger, W.W.; et al. Outer membrane vesicles engineered to express membrane-bound antigen program dendritic cells for cross-presentation to CD8+ T cells. Acta Biomater. 2019, 91, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef]
- Manna, I.; Quattrone, A.; De Benedittis, S.; Vescio, B.; Iaccino, E.; Quattrone, A. Exosomal miRNA as peripheral biomarkers in Parkinson’s disease and progressive supranuclear palsy: A pilot study. Parkinsonism Relat. Disord. 2021, 93, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Maisano, D.; Mimmi, S.; Dattilo, V.; Marino, F.; Gentile, M.; Vecchio, E.; Fiume, G.; Nisticò, N.; Aloisio, A.; de Santo, M.P.; et al. A novel phage display based platform for exosome diversity characterization. Nanoscale 2022, 14, 2998–3003. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Roy, P.; Noad, R. Virus-like particles as a vaccine delivery system: Myths and facts. Hum. Vaccines 2008, 4, 5–12. [Google Scholar] [CrossRef]
- Deschuyteneer, M.; Elouahabi, A.; Plainchamp, D.; Plisnier, M.; Soete, D.; Corazza, Y.; Lockman, L.; Giannini, S.; Deschamps, M. Molecular and structural characterization of the L1 virus-like particles that are used as vaccine antigens in Cervarix™, the AS04-adjuvanted HPV-16 and-18 cervical cancer vaccine. Hum. Vaccines 2010, 6, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baslé, E.; Joubert, N.; Pucheault, M. Protein chemical modification on endogenous amino acids. Chem. Biol. 2010, 17, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Z.; Liu, T.; Li, X.; Hu, X.; Wei, X.; Zhang, X.; Tan, W. Smart human-serum-albumin–As2O3 nanodrug with self-amplified folate receptor-targeting ability for chronic myeloid leukemia treatment. Angew. Chem. Int. Ed. 2017, 56, 10845–10849. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Chen, Q.; Yi, X.; Wang, G.; Chen, J.; Ning, P.; Yang, K.; Liu, Z. Radionuclide I-131 labeled albumin-paclitaxel nanoparticles for synergistic combined chemo-radioisotope therapy of cancer. Theranostics 2017, 7, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lynn, G.M.; Jacobson, O.; Chen, K.; Liu, Y.; Zhang, H.; Ma, Y.; Zhang, F.; Tian, R.; Ni, Q.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1954. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Qu, X.; Zhu, W.; Xiang, M.; Yang, J.; Zhang, K.; Wei, Y.; Chen, S. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat. Commun. 2014, 5, 3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, B.T.; Zhang, L. Cell membrane-camouflaged nanoparticles for drug delivery. J. Control. Release 2015, 220, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, R.H.; Hu, C.M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef] [PubMed]
- Taneichi, M.; Ishida, H.; Kajino, K.; Ogasawara, K.; Tanaka, Y.; Kasai, M.; Mori, M.; Nishida, M.; Yamamura, H.; Mizuguchi, J.; et al. Antigen chemically coupled to the surface of liposomes are cross-presented to CD8+ T cells and induce potent antitumor immunity. J. Immunol. 2006, 177, 2324–2330. [Google Scholar] [CrossRef] [Green Version]
- Ignatius, R.; Mahnke, K.; Rivera, M.; Hong, K.; Isdell, F.; Steinman, R.M.; Pope, M.; Stamatatos, L. Presentation of proteins encapsulated in sterically stabilized liposomes by dendritic cells initiates CD8+ T-cell responses in vivo. Blood J. Am. Soc. Hematol. 2000, 96, 3505–3513. [Google Scholar]
- Vangasseri, D.P.; Cui, Z.; Chen, W.; Hokey, D.A.; Falo, L.D., Jr.; Huang, L. Immunostimulation of dendritic cells by cationic liposomes. Mol. Membr. Biol. 2006, 23, 385–395. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Huang, L. Mechanism of adjuvant activity of cationic liposome: Phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol. Immunol. 2007, 44, 3672–3681. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.A.; Forrest, M.L.; Alani, A.W.; Kwon, G.S.; Robinson, J.R. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J. Pharm. Sci. 2010, 99, 2018–2031. [Google Scholar] [CrossRef]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747. [Google Scholar] [CrossRef] [Green Version]
- Rietscher, R.; Schröder, M.; Janke, J.; Czaplewska, J.; Gottschaldt, M.; Scherließ, R.; Hanefeld, A.; Schubert, U.S.; Schneider, M.; Knolle, P.A.; et al. Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity. Eur. J. Pharm. Biopharm. 2016, 102, 20–31. [Google Scholar] [CrossRef]
- Song, C.; Noh, Y.-W.; Lim, Y.T. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int. J. Nanomed. 2016, 11, 3753. [Google Scholar]
- Li, J.; Ren, H.; Zhang, Y. Metal-based nano-vaccines for cancer immunotherapy. Coord. Chem. Rev. 2022, 455, 214345. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, I.H.; Kang, S.; Kim, D.; Choi, M.; Saw, P.E.; Shin, E.C.; Jon, S. Gold nanoparticles displaying tumor-associated self-antigens as a potential vaccine for cancer immunotherapy. Adv. Healthc. Mater. 2014, 3, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Radovic-Moreno, A.F.; Chernyak, N.; Mader, C.C.; Nallagatla, S.; Kang, R.S.; Hao, L.; Walker, D.A.; Halo, T.L.; Merkel, T.J.; Rische, C.H.; et al. Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. USA 2015, 112, 3892–3897. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Ma, B.; Ma, Y.; Cao, P.; Leng, X.; Huang, P.; Ji, T.; Lu, X.; Liu, L. Drug-Loaded Liposomal Spherical Nucleic Acid as an Effective Cancer Nanovaccine. Nucleic Acids 2021. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 2016, 79, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Stieneker, F.; Kreuter, J.; Löwer, J. High Antibody Titres in Mice with Polymethylmethacrylate Nanoparticles as Adjuvant for HIV Vaccines. AIDS 1991, 5, 431–435. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Jiao, J.; Hu, H.M. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat. Nanotechnol. 2011, 6, 645–650. [Google Scholar] [CrossRef]
- Abdulnasser Harfoush, S.; Hannig, M.; Le, D.D.; Heck, S.; Leitner, M.; Omlor, A.J.; Tavernaro, I.; Kraegeloh, A.; Kautenburger, R.; Kickelbick, G.; et al. High-dose intranasal application of titanium dioxide nanoparticles induces the systemic uptakes and allergic airway inflammation in asthmatic mice. Respir. Res. 2020, 21, 168. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of nanoparticles in medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpisheh, V.; Afjadi, J.F.; Afjadi, M.N.; Haeri, M.S.; Sough, T.S.A.; Asl, S.H.; Edalati, M.; Atyabi, F.; Masjedi, A.; Hajizadeh, F.; et al. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int. J. Biol. Macromol. 2021, 167, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Qian, L.; Ke, Y.; Feng, X.; Chen, X.; Liu, F.; Yu, L.; Zhang, L.; Tao, Y.; Xu, R.; et al. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J. Nanobiotechnol. 2022, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.W.; Marshall, E.A.; Bell, J.C.; Lam, W.L. cGAS–STING and cancer: Dichotomous roles in tumor immunity and development. Trends Immunol. 2018, 39, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, S.K.; Barbie, D.A. Tumor cGAMP awakens the natural killers. Immunity 2018, 49, 585–587. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, J.; Zheng, X.; Liu, Z.; Zhang, X.; Li, Y.; Wilhelm, J.; Cao, J.; Huang, G.; Zhang, J.; et al. Intratumoral administration of STING-activating nanovaccine enhances T cell immunotherapy. J. ImmunoTher. Cancer 2022, 10, e003960. [Google Scholar] [CrossRef]
- Hamilos, D.L. Antigen presenting cells. Immunol. Res. 1989, 8, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Bergh, O.; Thorsby, E. Antigen-presenting properties of human vascular endothelial cells. J. Exp. Med. 1980, 152, 249s–255s. [Google Scholar] [PubMed]
- Rodríguez-Pinto, D. B cells as antigen presenting cells. Cell. Immunol. 2005, 238, 67–75. [Google Scholar] [CrossRef]
- Geppert, T.; Lipsky, P. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: Differential ability to function as antigen-presenting cells despite comparable Ia expression. J. Immunol. 1985, 135, 3750–3762. [Google Scholar]
- Bal, V.; McIndoe, A.; Denton, G.; Hudson, D.; Lombardi, G.; Lamb, J.; Lechler, R. Antigen presentation by keratinocytes induces tolerance in human T cells. Eur. J. Immunol. 1990, 20, 1893–1897. [Google Scholar] [CrossRef]
- Cheever, M.A.; Higano, C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Fadel, T.R.; Sharp, F.A.; Vudattu, N.; Ragheb, R.; Garyu, J.; Kim, D.; Hong, E.; Li, N.; Haller, G.L.; Pfefferle, L.D.; et al. A carbon nanotube–polymer composite for T-cell therapy. Nat. Nanotechnol. 2014, 9, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, F.; Wang, R.; Cen, T.; Liu, S.; Zhao, Z.; Li, R.; Xu, L.; Zhang, G.; Xu, Z.; et al. An armed oncolytic virus enhances the efficacy of tumor-infiltrating lymphocyte therapy by converting tumors to artificial antigen presenting cells in situ. Mol. Ther. 2022. [Google Scholar] [CrossRef]
- Lin, Y.X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA nanotechnology-mediated cancer immunotherapy. Theranostics 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Sun, X. Recent advances in mRNA vaccine delivery. Nano Res. 2018, 11, 5338–5354. [Google Scholar] [CrossRef]
- Lundqvist, A.; Noffz, G.; Pavlenko, M.; Sæbøe-Larssen, S.; Fong, T.; Maitland, N.; Pisa, P. Nonviral and viral gene transfer into different subsets of human dendritic cells yield comparable efficiency of transfection. J. Immunother. 2002, 25, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Uchida, S.; Ishii, T.; Itaka, K.; Kataoka, K. Messenger RNA-based therapeutics for the treatment of apoptosis-associated diseases. Sci. Rep. 2015, 5, 15810. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Xiong, Q.; Lee, G.Y.; Ding, J.; Li, W.; Shi, J. Biomedical applications of mRNA nanomedicine. Nano Res. 2018, 11, 5281–5309. [Google Scholar] [CrossRef]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hongxia, Z.; Xinru, Y.; Xiaojuan, W.; Zining, W.; Feifei, X.; Jun, W.; Xiaojun, X. Abstract LB-205: A lipoplex-based mRNA nanovaccine for cancer immunotherapy. Cancer Res. 2019, 79 (Suppl. S13), LB-205. [Google Scholar] [CrossRef]
- Xiao, B.; Li, D.; Xu, H.; Zhou, X.; Xu, X.; Qian, Y.; Yu, F.; Hu, H.; Zhou, Z.; Liu, X.; et al. An MRI-trackable therapeutic nanovaccine preventing cancer liver metastasis. Biomaterials 2021, 274, 120893. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lv, J.; Zhuang, Q.; Yang, Z.; Cao, Z.; Xu, L.; Pei, P.; Wang, C.; Wu, H.; Dong, Z.; et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hou, Z.; Liu, S.; Liang, S.; Ding, B.; Zhao, Y.; Chang, M.; Han, G.; Kheraif, A.A.A.; Lin, J. A Multifunctional Nanovaccine Based on L-Arginine-Loaded Black Mesoporous Titania: Ultrasound-Triggered Synergistic Cancer Sonodynamic Therapy/Gas Therapy/Immunotherapy with Remarkably Enhanced Efficacy. Small 2021, 17, 2005728. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Zhao, J.; Pan, J.; Liu, C.; Guo, X.; Zhou, S. Personalized Nanovaccine Coated with Calcinetin-Expressed Cancer Cell Membrane Antigen for Cancer Immunotherapy. Nano Lett. 2021, 21, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Xu, Q.; Zhang, Z.; Wei, Z.; Yong, T.; Bie, N.; Zhang, X.; Li, X.; Li, J.; Gan, L.; et al. Biomimetic sonodynamic therapy-nanovaccine integration platform potentiates Anti-PD-1 therapy in hypoxic tumors. Nano Today 2021, 38, 101195. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, L.; Cui, X.; Wu, X.; Zhang, Y.; Guan, X.; Ma, J.; Zhang, W. Cooperating minimalist nanovaccine with PD-1 blockade for effective and feasible cancer immunotherapy. J. Adv. Res. 2022, 35, 49–60. [Google Scholar] [CrossRef]
- Zhang, T.; Xiong, H.; Ma, X.; Gao, Y.; Xue, P.; Kang, Y.; Sun, Z.J.; Xu, Z. Supramolecular Tadalafil Nanovaccine for Cancer Immunotherapy by Alleviating Myeloid-Derived Suppressor Cells and Heightening Immunogenicity. Small Methods 2021, 5, 2100115. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, S.; Shin, H.; Kim, T.; Yu, B.; Kim, J.; Yoo, D.; Jon, S. Sequential and timely combination of a cancer nanovaccine with immune checkpoint blockade effectively inhibits tumor growth and relapse. Angew. Chem. 2020, 132, 14736–14746. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, Y.; Teng, X.; Wang, Y.; Huo, S.; Qing, G.; Ni, Q.; Li, X.; Wang, J.; Ye, X.; et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2020, 15, 1053–1064. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, B.; Wang, D.; Sun, F.; Song, R.; Shao, Q.; Wang, H.; Yu, H.; Li, Y. Engineering polymeric prodrug nanoplatform for vaccination immunotherapy of cancer. Nano Lett. 2020, 20, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; He, T.; Liu, P.; Yi, Z.; Zhu, S.; Liang, X.; Kang, E.; Gong, C.; Liu, X. Self-Adjuvanted Molecular Activator (SeaMac) Nanovaccines Promote Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2002080. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, W.; Chen, G.; Zhang, M.; Tang, X.; Wang, H.; Zhao, P.; Xu, Q.; Wu, Z.; Lin, X.; et al. Recombinant cancer nanovaccine for targeting tumor-associated macrophage and remodeling tumor microenvironment. Nano Today 2021, 40, 101244. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, T.; Zheng, Y.; Yang, S.L.; Yu, Z.; Duo, Y.; Zhang, Y.; Adah, D.; Shi, L.; Sun, Z.; et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale 2020, 12, 19939–19952. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, L.; Guo, Q.; Zuo, Y.; Wang, N.; Wang, H.; Kong, D.; Zhu, D.; Zhang, L. Robust Nanovaccine Based on Polydopamine-Coated Mesoporous Silica Nanoparticles for Effective Photothermal-Immunotherapy against Melanoma. Adv. Funct. Mater. 2021, 31, 2010637. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In situ transforming RNA nanovaccines from polyethylenimine functionalized graphene oxide hydrogel for durable cancer immunotherapy. Nano Lett. 2021, 21, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, H.; Hu, Y.; Wu, J.; Zhang, S.; Feng, Y.; Lin, L.; Tian, H.; Chen, X. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy. Bioact. Mater. 2022, 7, 167–180. [Google Scholar] [CrossRef]
- Ma, L.; Diao, L.; Peng, Z.; Jia, Y.; Xie, H.; Li, B.; Ma, J.; Zhang, M.; Cheng, L.; Ding, D.; et al. Immunotherapy and Prevention of Cancer by Nanovaccines Loaded with Whole-Cell Components of Tumor Tissues or Cells. Adv. Mater. 2021, 33, 2104849. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Liu, Z.; Le, Z.; Nie, T.; Qiao, D.; Su, Y.; Mai, H.; Chen, Y.; Liu, L. Therapeutic nanovaccines sensitize EBV-associated tumors to checkpoint blockade therapy. Biomaterials 2020, 255, 120158. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Song, H.; Shi, X.; Zhang, Y.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. PolyTLR7/8a-conjugated, antigen-trapping gold nanorods elicit anticancer immunity against abscopal tumors by photothermal therapy-induced in situ vaccination. Biomaterials 2021, 275, 120921. [Google Scholar] [CrossRef] [PubMed]
- Kyi, C.; Postow, M.A. Immune checkpoint inhibitor combinations in solid tumors: Opportunities and challenges. Immunotherapy 2016, 8, 821–837. [Google Scholar] [CrossRef] [Green Version]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Haque, A.; Alasmary, M.Y.; Abdel-Wahab, B.A.; Akhter, S. Emerging advances in synthetic cancer nano-vaccines: Opportunities and challenges. Expert Rev. Vaccines 2020, 19, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 2018, 49, 178–193.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nanovaccine | Type of Study/Cancer | Mechanism of Action | Outcomes | Ref |
|---|---|---|---|---|
| OMPN (OVA, MnO2, and polydopamine) | In vitro/Animal model/orthotopic melanoma |
|
| [105] |
| F-PEI/OVA (OVA, fluoropolymer) | In vitro/Animal model/orthotopic melanoma and breast cancer |
|
| [106] |
| BMT@LA (L-arginine, black mesoporous titania) | Animal model/bilateral U14 tumor model |
|
| [107] |
| banNV (Adpgk neoantigen, R848, CpG) | In vitro/Animal model/ MC38 colorectal cancer cell |
|
| [108] |
| PLGA nanovaccine (calcinetin, R837) | In vitro/Animal model Luc-4T1 cells |
|
| [109] |
| cMn-MOF@CM Mn-MOF, CpG, OVA | In vitro/Animal model Melanoma B16 |
|
| [110] |
| (Antigenic peptide, CpG oligodeoxynucleotides and cationic polymer NP) | In vitro/Animal model Breast carcinoma 4T1 cells |
|
| [111] |
| PCO (PRT/CpG/OVA) | In vitro/Animal model BDMCs, B16 melanoma cells |
|
| [112] |
| Nanoprodrug (FIT NPs, tadalafil ICG photosensitizer) | In vitro/Animal model CT-26 cells/ Colon cancer |
|
| [113] |
| OVAPEP-SLNP@CpG (Small lipid nanoparticle, CpG, OVA) | In vitro/Animal model Prophylactic and therapeutic E.G7 tumor models |
|
| [114] |
| NTV (p[OEGMA4-DMAEMA22]-p[MA] 30 with conjugation of NDP or PDP with an acid-sensitive acetal bond, OVA241–27) | In vitro/Animal model Human papillomavirus-E6/E7 and B16F10-OVA and tumor mice models |
|
| [115] |
| Neoantigen-loaded Nanovaccine (Acid-activatable polymeric conjugate of the DMXAA and neoantigen) | In vitro/Animal model B16-OVA melanoma and 4T1 breast tumor |
|
| [116] |
| SeaMac (Polymer NPs, neoantigen) | In vitro/Animal model colon carcinoma 26 (CT26) and B16-F10 tumor models |
|
| [117] |
| LrTL (Trichosanthin, legumain, liposome) | In vitro/Animal model Lewis’s lung cancer (LLC), B16-F10, intracranial LLC xenograft, and CT-26 colon cancer |
|
| [118] |
| hEX@BP (Black phosphorus quantum dots and exosomes) | In vitro/Animal model LLC cells |
|
| [119] |
| MSNs-ABC@PDA-OVA (Mesoporous silica NPs, OVA, photothermal agent polydopamine, and antigen release promoter ammonium bicarbonate) | In vitro/Animal model Melanoma |
|
| [120] |
| PEI-functionalized GO transformable hydrogel (Polyethylenimine, graphene oxide and R848-laden) | In vitro/Animal model B16-OVA cells |
|
| [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Lan, H.; Jin, K.; Gong, D.; Qian, J. Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers 2022, 14, 3842. https://doi.org/10.3390/cancers14163842
Fang X, Lan H, Jin K, Gong D, Qian J. Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers. 2022; 14(16):3842. https://doi.org/10.3390/cancers14163842
Chicago/Turabian StyleFang, Xingliang, Huanrong Lan, Ketao Jin, Daojun Gong, and Jun Qian. 2022. "Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review" Cancers 14, no. 16: 3842. https://doi.org/10.3390/cancers14163842
APA StyleFang, X., Lan, H., Jin, K., Gong, D., & Qian, J. (2022). Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers, 14(16), 3842. https://doi.org/10.3390/cancers14163842






