The Kinetics of FMS-Related Tyrosine Kinase 3 Ligand (Flt-3L) during Chemoradiotherapy Suggests a Potential Gain from the Earlier Initiation of Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Endpoints and Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Flt-3L Kinetics
3.3. Multiple Factor Analysis (MFA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brandmaier, A.; Formenti, S.C. The Impact of Radiation Therapy on Innate and Adaptive Tumor Immunity. Semin. Radiat. Oncol. 2020, 30, 139–144. [Google Scholar] [CrossRef]
- Cueto, F.J.; Sancho, D. The Flt3L/Flt3 Axis in Dendritic Cell Biology and Cancer Immunotherapy. Cancers 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer 2016, 2, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.-R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.K.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Oba, T.; Makino, K.; Kajihara, R.; Yokoi, T.; Araki, R.; Abe, M.; Minderman, H.; Chang, A.E.; Odunsi, K.; Ito, F. In Situ Delivery of IPSC-Derived Dendritic Cells with Local Radiotherapy Generates Systemic Antitumor Immunity and Potentiates PD-L1 Blockade in Preclinical Poorly Immunogenic Tumor Models. J. Immunother. Cancer 2021, 9, e002432. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, J.; Gu, J.; Xu, B.; Sun, X.; Zhang, S. FMS-Related Tyrosine Kinase 3 Ligand Promotes Radioresistance in Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 2021, 12, 659735. [Google Scholar] [CrossRef]
- Peterlin, P.; Chevallier, P.; Knapper, S.; Collin, M. FLT3 Ligand in Acute Myeloid Leukemia: A Simple Test with Deep Implications. Leuk. Lymphoma 2021, 62, 264–270. [Google Scholar] [CrossRef]
- Kingston, D.; Schmid, M.A.; Onai, N.; Obata-Onai, A.; Baumjohann, D.; Manz, M.G. The Concerted Action of GM-CSF and Flt3-Ligand on in Vivo Dendritic Cell Homeostasis. Blood 2009, 114, 835–843. [Google Scholar] [CrossRef]
- Balog, R.P.; Bacher, R.; Chang, P.; Greenstein, M.; Jammalamadaka, S.; Javitz, H.; Knox, S.J.; Lee, S.; Lin, H.; Shaler, T.; et al. Development of a Biodosimeter for Radiation Triage Using Novel Blood Protein Biomarker Panels in Humans and Non-Human Primates. Int. J. Radiat. Biol. 2020, 96, 22–34. [Google Scholar] [CrossRef]
- Wodnar-Filipowicz, A.; Lyman, S.; Gratwohl, A.; Tichelli, A.; Speck, B.; Nissen, C. Flt3 Ligand Level Reflects Hematopoietic Progenitor Cell Function in Aplastic Anemia and Chemotherapy-Induced Bone Marrow Aplasia. Blood 1996, 88, 4493–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproull, M.; Avondoglio, D.; Kramp, T.; Shankavaram, U.; Camphausen, K. Correlation of Plasma FL Expression with Bone Marrow Irradiation Dose. PLoS ONE 2013, 8, e58558. [Google Scholar] [CrossRef] [PubMed]
- Myerson, R.J.; Garofalo, M.C.; El Naqa, I.; Abrams, R.A.; Apte, A.; Bosch, W.R.; Das, P.; Gunderson, L.L.; Hong, T.S.; Kim, J.J.J.; et al. Elective Clinical Target Volumes for Conformal Therapy in Anorectal Cancer: A Radiation Therapy Oncology Group Consensus Panel Contouring Atlas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuncman, Ł.; Stawiski, K.; Masłowski, M.; Kucharz, J.; Fijuth, J. Dose–Volume Parameters of MRI-Based Active Bone Marrow Predict Hematologic Toxicity of Chemoradiotherapy for Rectal Cancer. Strahlenther. Onkol. 2020, 196, 998–1005. [Google Scholar] [CrossRef]
- Pages, J. Multiple Factor Analysis: Main Features and Application to Sensory Data. Rev. Colomb. Estad. 2004, 27, 1–26. [Google Scholar]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple Factor Analysis: Principal Component Analysis for Multitable and Multiblock Data Sets: Multiple Factor Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes from the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef]
- Corrò, C.; Dutoit, V.; Koessler, T. Emerging Trends for Radio-Immunotherapy in Rectal Cancer. Cancers 2021, 13, 1374. [Google Scholar] [CrossRef]
- Liu, C.; Wang, P.; Sun, Y.; Dou, X.; Hu, X.; Zou, W.; Sun, Y.; Hu, Q.; Yue, J. Neoadjuvant Chemoradiotherapy Changes the Landscape of Soluble Immune Checkpoint Molecules in Patients with Locally Advanced Rectal Cancer. Front. Oncol. 2022, 12, 756811. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markey, K.A.; Kuns, R.D.; Browne, D.J.; Gartlan, K.H.; Robb, R.J.; Martins, J.P.; Henden, A.S.; Minnie, S.A.; Cheong, M.; Koyama, M.; et al. Flt-3L Expansion of Recipient CD8α+ Dendritic Cells Deletes Alloreactive Donor T Cells and Represents an Alternative to Posttransplant Cyclophosphamide for the Prevention of GVHD. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1604–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.C. The Growing Landscape of FLT3 Inhibition in AML. Hematology 2019, 2019, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=flt-3l+radiotherapy&cntry=&state=&city=&dist= (accessed on 8 August 2022).
- Mell, L.K.; Kochanski, J.D.; Roeske, J.C.; Haslam, J.J.; Mehta, N.; Yamada, S.D.; Hurteau, J.A.; Collins, Y.C.; Lengyel, E.; Mundt, A.J. Dosimetric Predictors of Acute Hematologic Toxicity in Cervical Cancer Patients Treated with Concurrent Cisplatin and Intensity-Modulated Pelvic Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1356–1365. [Google Scholar] [CrossRef]
- Sproull, M.; Kramp, T.; Tandle, A.; Shankavaram, U.; Camphausen, K. Multivariate Analysis of Radiation Responsive Proteins to Predict Radiation Exposure in Total-Body Irradiation and Partial-Body Irradiation Models. Radiat. Res. 2017, 187, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.J.; Oh, J.H.; Apte, A.; Son, C.H.; Deasy, J.O.; Goodman, K.A. Clinical and Dosimetric Predictors of Acute Hematologic Toxicity in Rectal Cancer Patients Undergoing Chemoradiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 113, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Huchet, A.; Belkacémi, Y.; Frick, J.; Prat, M.; Muresan-Kloos, I.; Altan, D.; Chapel, A.; Gorin, N.C.; Gourmelon, P.; Bertho, J.M. Plasma Flt-3 Ligand Concentration Correlated with Radiation-Induced Bone Marrow Damage during Local Fractionated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 508–515. [Google Scholar] [CrossRef]
- Prat, M.; Demarquay, C.; Frick, J.; Dudoignon, N.; Thierry, D.; Bertho, J.M. Use of Flt3 Ligand to Evaluate Residual Hematopoiesis after Heterogeneous Irradiation in Mice. Radiat. Res. 2006, 166, 504–511. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.; Chamseddine, I.; Lee, W.H.; Kim, H.R.; Lee, I.J.; Hong, M.H.; Cho, B.C.; Lee, C.G.; Cho, S.; et al. Lymphocyte Dynamics during and after Chemo-Radiation Correlate to Dose and Outcome in Stage III NSCLC Patients Undergoing Maintenance Immunotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 168, 1–7. [Google Scholar] [CrossRef]
- Cho, Y.; Park, S.; Byun, H.K.; Lee, C.G.; Cho, J.; Hong, M.H.; Kim, H.R.; Cho, B.C.; Kim, S.; Park, J.; et al. Impact of Treatment-Related Lymphopenia on Immunotherapy for Advanced Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1065–1073. [Google Scholar] [CrossRef]
- Kim, K.H.; Pyo, H.; Lee, H.; Oh, D.; Noh, J.M.; Ahn, Y.C.; Yoon, H.I.; Moon, H.; Lee, J.; Park, S.; et al. Dynamics of Circulating Immune Cells During Chemoradiotherapy in Patients with Non-Small Cell Lung Cancer Support Earlier Administration of Anti-PD-1/PD-L1 Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, S.K.; Lee, K.H.; Frost, N.; Breder, V.; Kowalski, D.M.; Pollock, T.; Levchenko, E.; Reguart, N.; Martinez-Marti, A.; Houghton, B.; et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients with Unresectable, Locally Advanced, Stage III Non–Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021, 7, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.; Hecht, M.; Schmidt, M.; Haderlein, M.; Jost, T.; Büttner-Herold, M.; Weber, K.; Denz, A.; Grützmann, R.; Hartmann, A.; et al. Influence of Gender on Radiosensitivity during Radiochemotherapy of Advanced Rectal Cancer. Cancers 2021, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.-D.; Ghadimi, M.; Minsky, B.D.; Rödel, C.; Fokas, E. Association of Sex With Toxic Effects, Treatment Adherence, and Oncologic Outcomes in the CAO/ARO/AIO-94 and CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trials of Rectal Cancer. JAMA Oncol. 2020, 6, 294–296. [Google Scholar] [CrossRef]

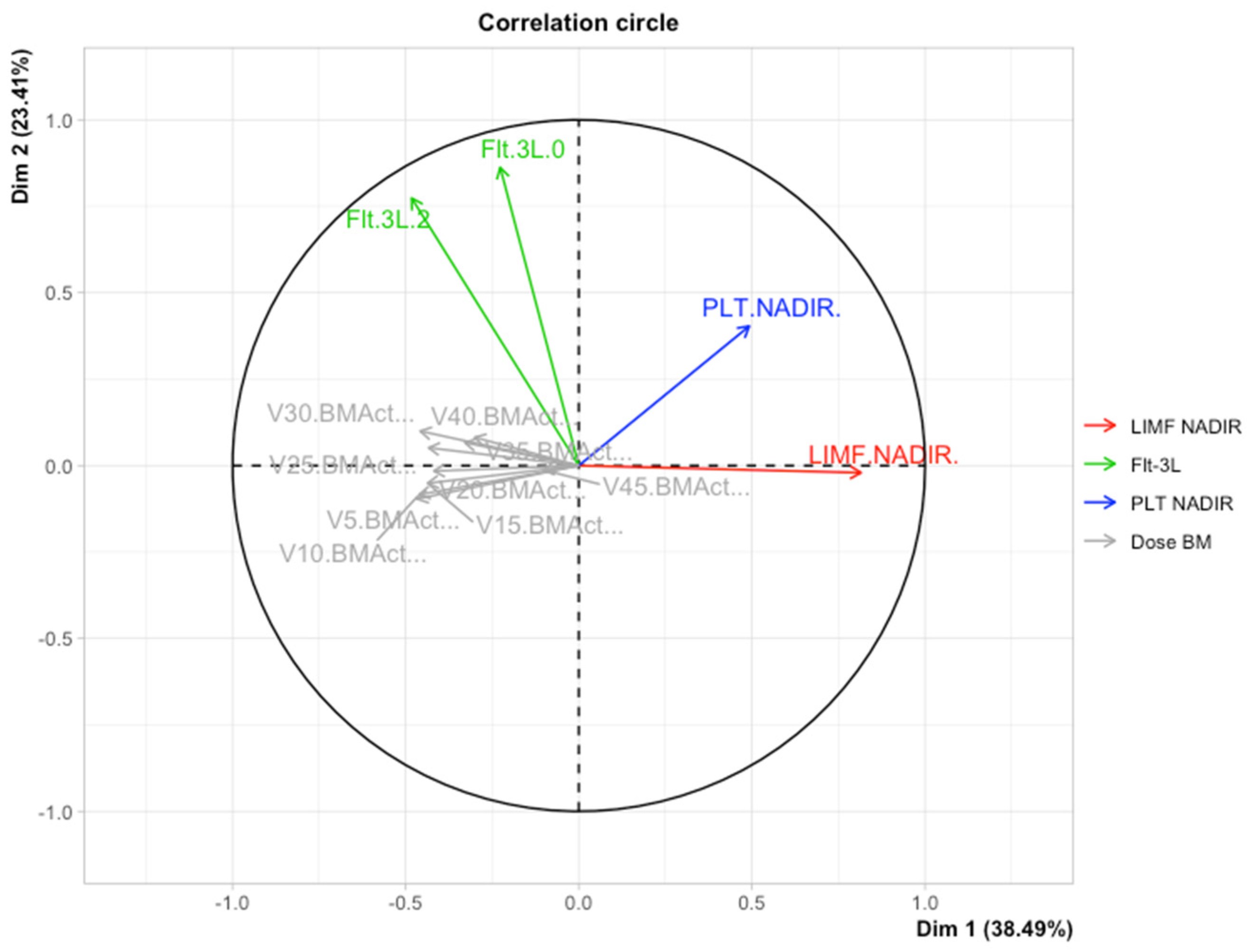
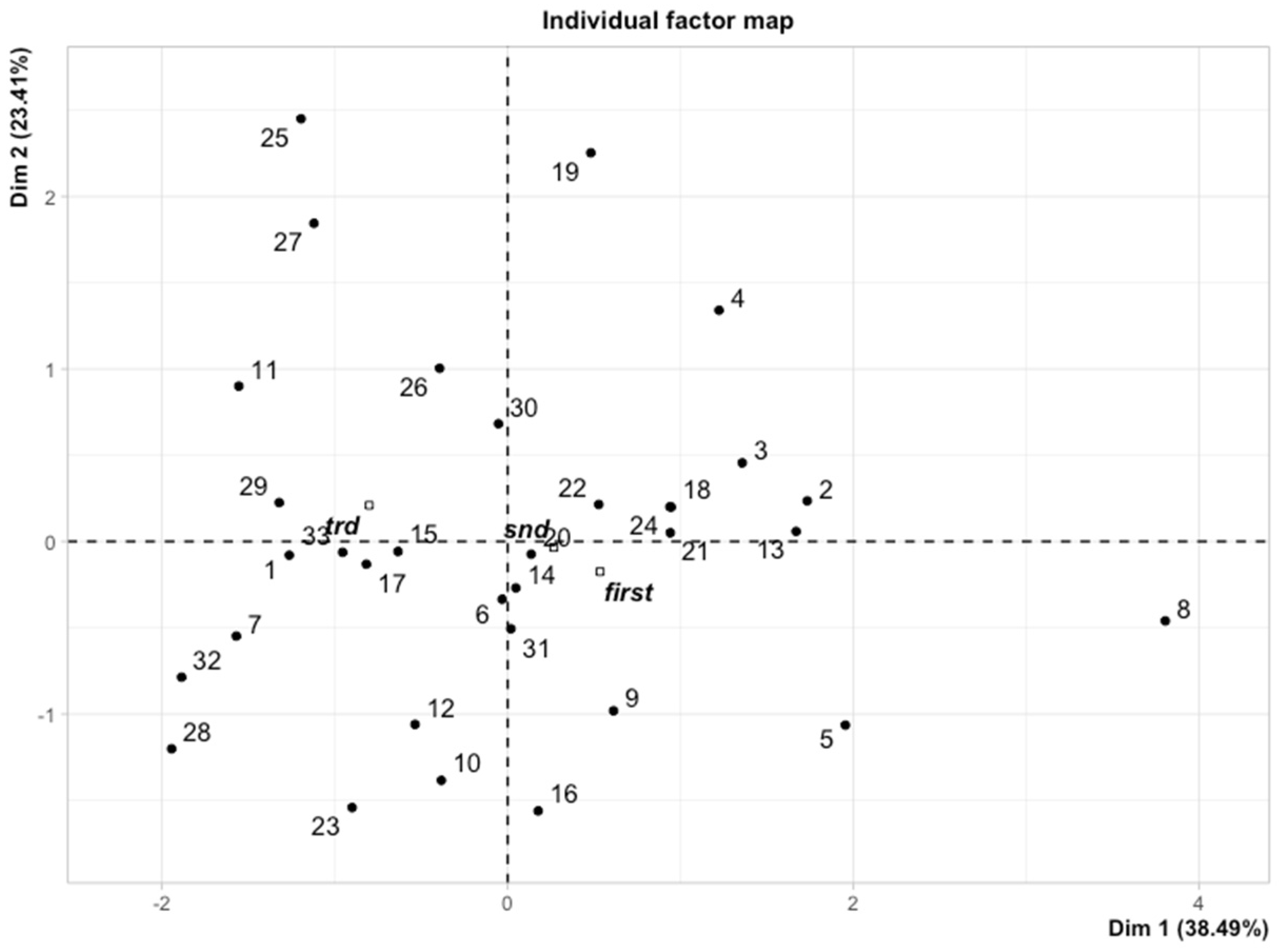
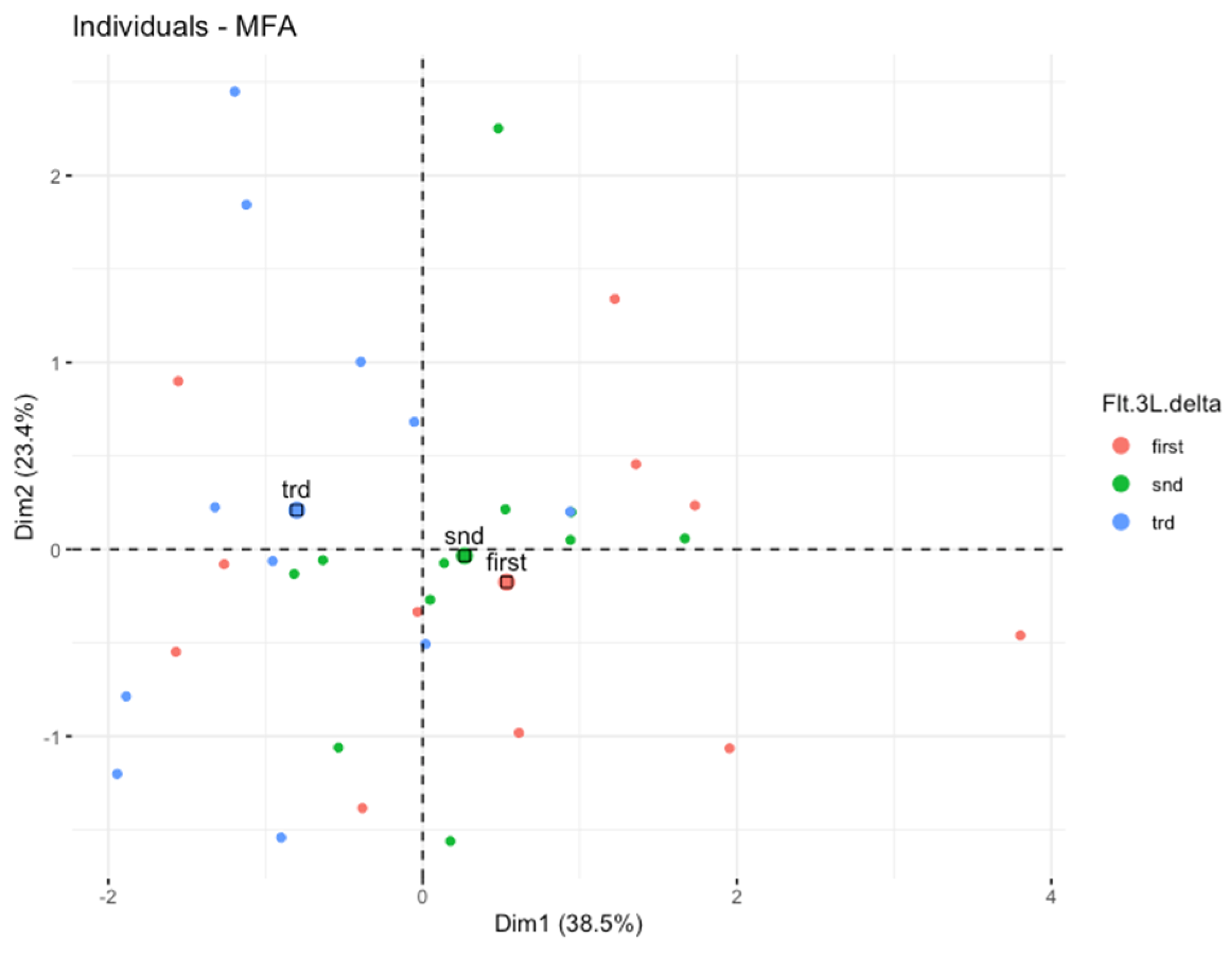

| Data | Flt-3L 2 and Flt-3L 0 | Flt-3L 4 and Flt-3L 0 | Flt-3L 5/6 and Flt-3L 0 | Flt-3L 4 and Flt-3L 2 | Flt-3L 5/6 and Flt-3L 2 | Flt-3L 5/6 and Flt-3L 4 |
|---|---|---|---|---|---|---|
| Z | −4.91 | −4.08 | −4.58 | −0.49 | −1.37 | −0.94 |
| Bilateral asymptomatic significance | <0.01 | <0.01 | <0.01 | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuncman, Ł.; Orzechowska, M.; Stawiski, K.; Masłowski, M.; Ciążyńska, M.; Gottwald, L.; Milecki, T.; Fijuth, J. The Kinetics of FMS-Related Tyrosine Kinase 3 Ligand (Flt-3L) during Chemoradiotherapy Suggests a Potential Gain from the Earlier Initiation of Immunotherapy. Cancers 2022, 14, 3844. https://doi.org/10.3390/cancers14163844
Kuncman Ł, Orzechowska M, Stawiski K, Masłowski M, Ciążyńska M, Gottwald L, Milecki T, Fijuth J. The Kinetics of FMS-Related Tyrosine Kinase 3 Ligand (Flt-3L) during Chemoradiotherapy Suggests a Potential Gain from the Earlier Initiation of Immunotherapy. Cancers. 2022; 14(16):3844. https://doi.org/10.3390/cancers14163844
Chicago/Turabian StyleKuncman, Łukasz, Magdalena Orzechowska, Konrad Stawiski, Michał Masłowski, Magdalena Ciążyńska, Leszek Gottwald, Tomasz Milecki, and Jacek Fijuth. 2022. "The Kinetics of FMS-Related Tyrosine Kinase 3 Ligand (Flt-3L) during Chemoradiotherapy Suggests a Potential Gain from the Earlier Initiation of Immunotherapy" Cancers 14, no. 16: 3844. https://doi.org/10.3390/cancers14163844
APA StyleKuncman, Ł., Orzechowska, M., Stawiski, K., Masłowski, M., Ciążyńska, M., Gottwald, L., Milecki, T., & Fijuth, J. (2022). The Kinetics of FMS-Related Tyrosine Kinase 3 Ligand (Flt-3L) during Chemoradiotherapy Suggests a Potential Gain from the Earlier Initiation of Immunotherapy. Cancers, 14(16), 3844. https://doi.org/10.3390/cancers14163844








