The Prognostic Impact of Quitting Smoking at or around Diagnosis on the Survival of Patients with Gastrointestinal Cancers: A Systematic Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
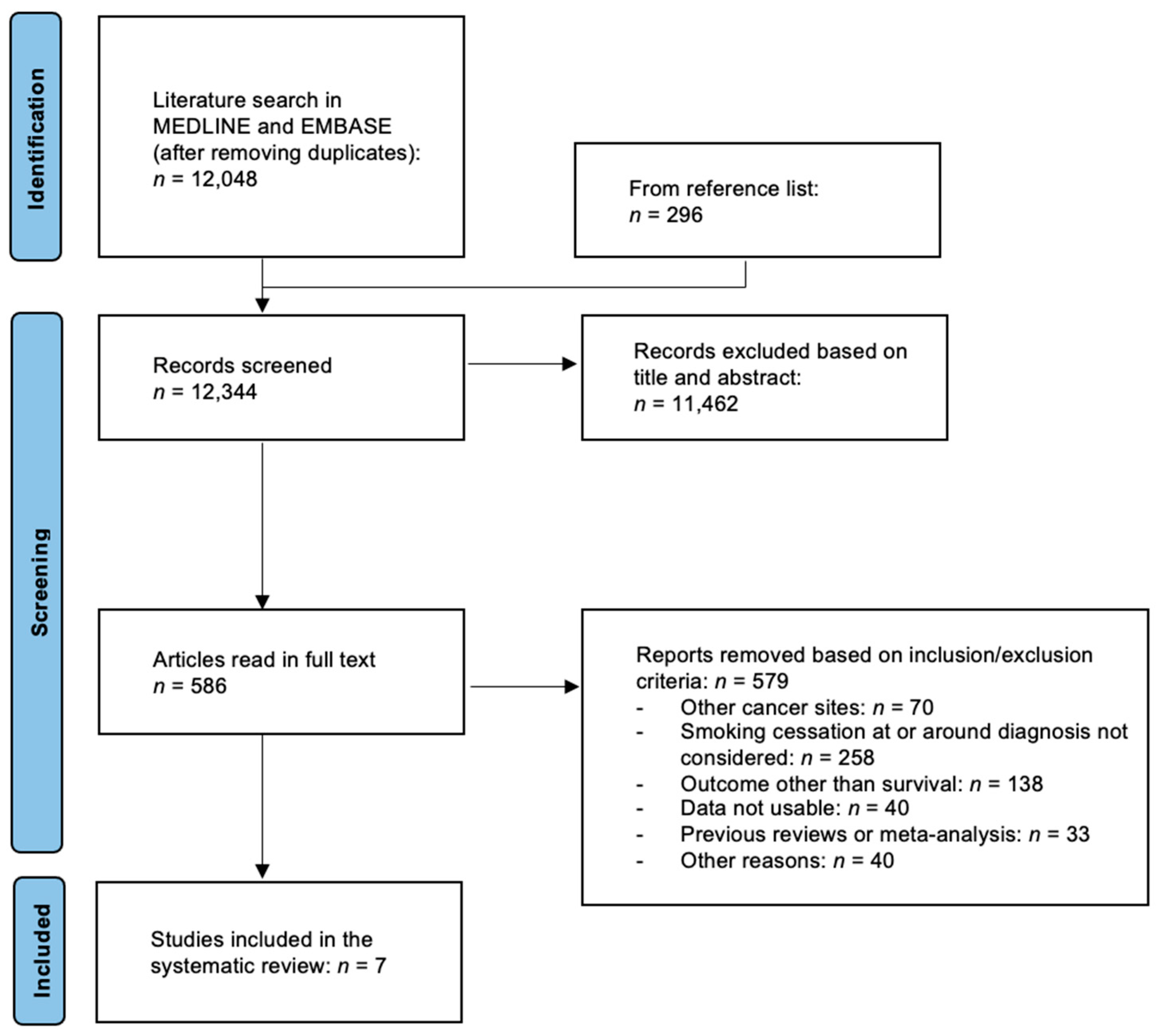
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis and Study Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef] [Green Version]
- Makarova-Rusher, O.V.; Altekruse, S.F.; McNeel, T.S.; Ulahannan, S.; Duffy, A.G.; Graubard, B.I.; Greten, T.F.; McGlynn, K.A. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016, 122, 1757–1765. [Google Scholar] [CrossRef]
- Fan, J.H.; Wang, J.B.; Jiang, Y.; Xiang, W.; Liang, H.; Wei, W.Q.; Qiao, Y.L.; Boffetta, P. Attributable causes of liver cancer mortality and incidence in china. Asian Pac. J. Cancer Prev. 2013, 14, 7251–7256. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulos, D.; Bamia, C.; Lagiou, P.; Fedirko, V.; Trepo, E.; Jenab, M.; Pischon, T.; Nöthlings, U.; Overved, K.; Tjønneland, A.; et al. Hepatocellular carcinoma risk factors and disease burden in a European cohort: A nested case-control study. J. Natl. Cancer Inst. 2011, 103, 1686–1695. [Google Scholar] [CrossRef]
- Caini, S.; Del Riccio, M.; Vettori, V.; Scotti, V.; Martinoli, C.; Raimondi, S.; Cammarata, G.; Palli, D.; Banini, M.; Masala, G.; et al. Quitting Smoking at or Around Diagnosis Improves the Overall Survival of Lung Cancer Patients: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2022, 17, 623–636. [Google Scholar] [CrossRef]
- National Institute for Health Research. PROSPERO International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 28 April 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2016/en (accessed on 14 March 2022).
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- van Houwelingen, H.C.; Arends, L.R.; Stijnen, T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat. Med. 2002, 21, 589–624. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 22 February 2022).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Yang, B.; Jacobs, E.J.; Gapstur, S.M.; Stevens, V.; Campbell, P.T. Active smoking and mortality among colorectal cancer survivors: The Cancer Prevention Study II nutrition cohort. J. Clin. Oncol. 2015, 33, 885–893. [Google Scholar] [CrossRef]
- Jang, D.; Choe, S.; Park, J.W.; Jeong, S.Y.; Shin, A. Smoking status before and after colorectal cancer diagnosis and mortality in Korean men: A population-based cohort study. Cancer Med. 2020, 9, 9641–9648. [Google Scholar] [CrossRef] [PubMed]
- Japuntich, S.J.; Kumar, P.; Pendergast, J.F.; Juarez Caballero, G.Y.; Malin, J.L.; Wallace, R.B.; Chrischilles, E.A.; Keating, N.L.; Park, E.R. Smoking Status and Survival Among a National Cohort of Lung and Colorectal Cancer Patients. Nicotine Tob. Res. 2019, 21, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Walter, V.; Jansen, L.; Hoffmeister, M.; Ulrich, A.; Chang-Claude, J.; Brenner, H. Smoking and survival of colorectal cancer patients: Population-based study from Germany. Int. J. Cancer 2015, 137, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Kasza, K.A.; Reid, M.E.; Cummings, K.M.; Marshall, J.R. Smoking at diagnosis and survival in cancer patients. Int. J. Cancer 2013, 132, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Baron, J.; Newcomb, P.A. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: The Seattle Colon Cancer Family Registry. Cancer 2011, 117, 4948–4957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Wang, R.; Gao, Y.T.; Yuan, J.M. Impact of postdiagnosis smoking on long-term survival of cancer patients: The Shanghai cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2404–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.F.; Wei, T.; Liu, X.M.; Liu, C.; Lv, Y. Impact of cigarette smoking on outcome of hepatocellular carcinoma after surgery in patients with hepatitis B. PLoS ONE 2014, 9, e85077. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Wu, W.K.; Chu, K.M.; Wong, H.P.; Lam, E.K.; Tai, E.K.; Koo, M.W.; Cho, C.H. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol. Cancer Res. 2005, 3, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.P.; Yu, L.; Lam, E.K.; Tai, E.K.; Wu, W.K.; Cho, C.H. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol. Sci. 2007, 97, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Vincenzi, B.; Santini, D.; Loupakis, F.; Addeo, R.; Rojas Llimpe, F.L.; Baldi, G.G.; Di Fabio, F.; Del Prete, S.; Pinto, C.; Falcone, A.; et al. Cigarettes smoking habit may reduce benefit from cetuximab-based treatment in advanced colorectal cancer patients. Expert Opin. Biol. Ther. 2009, 9, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Morini, V.; Coluccia, P.; Proietti, S.; D’Anselmi, F.; Pasqualato, A.; Masiello, M.G.; Palombo, A.; De Toma, G.; Bizzarri, M.; et al. Nicotine increases survival in human colon cancer cells treated with chemotherapeutic drugs. Toxicol. In Vitro 2013, 27, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Schaal, C.; Chellappan, S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dino, P.; D’Anna, C.; Sangiorgi, C.; Di Sano, C.; Di Vincenzo, S.; Ferraro, M.; Pace, E. Cigarette smoke extract modulates E-Cadherin, Claudin-1 and miR-21 and promotes cancer invasiveness in human colorectal adenocarcinoma cells. Toxicol. Lett. 2019, 317, 102–109. [Google Scholar] [CrossRef]
- Klemp, I.; Steffenssen, M.; Bakholdt, V.; Thygesen, T.; Sørensen, J.A. Counseling is effective for smoking cessation in head and neck cance patients—A systematic review and meta-analysis. J. Oral Maxillofac. Surg. 2016, 74, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yu, X.; Yu, T.; Xiao, J.; Hunag, Y. Interventions for smoking cessation in people diagnosed with lung cancer. Cochrane Database Syst. Rev. 2019, 6, CD011751. [Google Scholar] [CrossRef] [PubMed]
- Phua, Z.J.; MacInnis, R.J.; Jayasekara, H. Cigarette smoking and risk of second primary cancer: A systematic review and meta-analysis. Cancer Epidemiol. 2022, 78, 102160. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Ú.; Brandon, K.O.; Sutton, S.K.; Brandon, T.H.; Simmons, V.N. Does smoking abstinence predict cancer patients’ quality of life over time? Psychooncology 2019, 28, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic: The MPOWER Package; World Health Organization: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/43818 (accessed on 6 July 2022).
- Girvalaki, C.; Mechili, E.A.; Papadakis, S.; Nikitara, K.; Demin, A.; Trofor, A.; Lila, A.; Harutyunyan, A.; Saliaj, A.; Dimitrievska, D.; et al. Current practices and perceived barriers to tobacco-treatment delivery among healthcare professionals from 15 European countries. The EPACTT Plus project. Tob. Prev. Cessat. 2020, 6, 6. [Google Scholar] [PubMed]
- European Network for Smoking and Tobacco Prevention. 2020 Guidelines for Treating Tobacco Dependence. ISBN 9782930966052. Available online: https://ensp.network/ensp-tdt-guidelines/ (accessed on 6 July 2022).
| Author, Year | Country | Sex (% Men) | Age (Years) | Cancer Site | Smoking Status | Years of Diagnosis | Tumour Stage | Treatments | Follow-Up Duration (Years) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Smokers at Diagnosis (a) | Continued Smokers | Quitters | |||||||||
| Jang, 2020 (b) | South Korea | 100.0% | <60: 38.6%, ≥60: 61.4% | colon (100%) | 15,564 (71.4%) | 2420 (11.1%) | 3808 (17.5%) | 2002–2016 | NA | surgery alone (67.7%), surgery with RT or CHT (28.6%), RT or CHT alone (3.7%) | median 6.3 |
| rectum (100%) | 10,415 (68.1%) | 1756 (11.5%) | 3116 (20.4%) | ||||||||
| Japuntich, 2019 | USA | 45.1% | <60: 35.5%, ≥60: 64.5% | colon-rectum (100%) | 2634 (84.7%) | 289 (9.3%) | 187 (6.0%) | 2003–2005 | I, II (47.7%), III (39.8%), IV (12.5%) | surgery (57.9%), CHT (33.0%), RT (9.1%) | max 7.0 |
| Walter, 2015 | Germany | 59.3% | median 69 (range 30–96) | colon (59.2%), rectum (40.8%) | 2690 (86.2%) | 276 (8.9%) | 153 (4.9%) | 2003–2010 | I (22.2%), II (30.8%), III (32.7%), IV (14.3%) | surgery (100%), other treatments (NA) | median 4.9 |
| Zhang, 2014 | China | 83.8% | mean 48.9 | liver HBV+ (100%) | 193 (63.9%) | 22 (7.3%) | 87 (28.8%) | 2008–2011 | NA | surgery + HBV treatment (100%) | median 2.2 |
| Tao, 2013 (c) | China | NA | NA | stomach (100%) | 227 (62.7%) | 135 (% in either category NA) | 1986–2010 | NA | surgery, CHT, RT (% NA) | mean 5.3 | |
| NA | NA | colon-rectum (100%) | 134 (54.0%) | 114 (% in either category NA) | 1986–2010 | NA | surgery, CHT, RT (% NA) | ||||
| Warren, 2013 | USA | 56.6% | mean 60.2 | colon-rectum (100%) | 291 (81.1%) | 47 (13.1%) | 21 (5.8%) | 1982–1998 | local (27.3%), regional (36.2%), distant (36.5%) | NA | min 12.0, max 27.7 |
| Phipps, 2011 | USA | 53.7% | range 18–74 | colon (64.3%), rectum (34.4%), NA (1.3%) | 1.851 (81.9%) | 152 (6.7%) | 258 (11.4%) | 1998–2007 | I (32.1%), II (15.2%), III (12.5%), IV (20.1%), NA (20.1%) | NA | max 12.0 |
| Author, Year | Quitters | Continued Smokers |
|---|---|---|
| Jang, 2020 | Stopped smoking within 1 year after diagnosis. | Continued to smoke in the first year after diagnosis. |
| Japuntich, 2019 | Quit less than 1 year before diagnosis. | Active smokers at diagnosis. |
| Walter, 2015 | Quit less than 1 year before diagnosis. | Active smokers at diagnosis. |
| Zhang, 2014 | Quit smoking within 1 year of diagnosis. | Continued smokers after surgery, for at least 1 year or until death. |
| Tao, 2013 | Never smoked cigarettes after diagnosis. | Continued to smoke until death or the latest follow-up interview. |
| Warren, 2013 | Quit less than one year before diagnosis. | Active smokers at diagnosis. |
| Phipps, 2011 | Quit smoking at the post-diagnosis interview (median 6.9 months after diagnosis). | Continued to smoke at the post-diagnosis interview. |
| Author, Year | Smoking Status | HR | 95% CI | Variables Used for Statistical Adjustment |
|---|---|---|---|---|
| Colorectal Cancer, Overall Survival | ||||
| Jang, 2020 | continued smokers | 1.00 (ref) | age, comorbidities, alcohol intake, BMI, physical activity levels | |
| quit smoking (colon) | 1.06 | 0.90–1.25 | ||
| quit smoking (rectal) | 1.10 | 0.92–1.32 | ||
| Japuntich, 2019 | continued smokers | 1.00 (ref) | age, sex, tumour stage, comorbidities, alcohol intake, BMI, other | |
| quit smoking | 0.87 | 0.64–1.18 | ||
| Walter, 2015 | never + former smokers | 1.00 (ref) | age, sex, tumour stage, comorbidities, alcohol intake, BMI, other | |
| continued smokers | 1.10 | 0.86–1.41 | ||
| quit smoking | 0.97 | 0.70–1.33 | ||
| Tao, 2013 (a) | continued smokers | 1.00 (ref) | age, pack-years, treatment received, other | |
| quit smoking | 0.29 | 0.14–0.59 | ||
| Warren, 2013 (a) (b) | continued smokers | 1.00 (ref) | age, tumour stage, pack-years, BMI, other | |
| quit smoking | 1.19 | 0.44–3.33 | ||
| Phipps, 2011 | never smokers | 1.00 (ref) | age, sex, other | |
| continued smokers | 1.50 | 1.14–1.97 | ||
| quit smoking | 1.52 | 1.21–1.90 | ||
| Colorectal cancer, disease-specific survival | ||||
| Walter, 2015 | never + former smokers | 1.00 (ref) | age, sex, tumour stage, comorbidities, alcohol intake, BMI, other | |
| continued smokers | 1.10 | 0.83–1.45 | ||
| quit smoking | 0.87 | 0.60–1.25 | ||
| Warren, 2013 (a) (b) | continued smokers | 1.00 (ref) | age, tumour stage, pack-years, BMI, other | |
| quit smoking | 0.85 | 0.25–2.94 | ||
| Phipps, 2011 | never smokers | 1.00 (ref) | age, sex, other | |
| continued smokers | 1.47 | 1.07–2.03 | ||
| quit smoking | 1.32 | 1.00–1.74 | ||
| Colorectal cancer, recurrence-free survival | ||||
| Walter, 2015 | never + former smokers | 1.00 (ref) | age, sex, tumour stage, comorbidities, alcohol intake, BMI, other | |
| continued smokers | 1.18 | 0.93–1.51 | ||
| quit smoking | 1.00 | 0.73–1.36 | ||
| Colorectal cancer, disease-free survival | ||||
| Walter, 2015 | never + former smokers | 1.00 (ref) | age, sex, tumour stage, comorbidities, alcohol intake, BMI, other | |
| continued smokers | 1.18 | 0.95–1.48 | ||
| quit smoking | 1.04 | 0.78–1.39 | ||
| Stomach cancer, overall survival | ||||
| Tao, 2013 (a) | continued smokers | 1.00 (ref) | age, pack-years, treatment received, other | |
| quit smoking | 0.74 | 0.37–1.43 | ||
| Liver cancer (HBV-positive patients), recurrence-free survival | ||||
| Zhang, 2014 | continued smokers | 1.00 (ref) | none (extracted from KM curve) | |
| quit smoking | 0.41 | 0.25–0.65 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caini, S.; Del Riccio, M.; Vettori, V.; Raimondi, S.; Assedi, M.; Vignati, S.; Bonaccorsi, G.; Cattaruzza, M.S.; Bellerba, F.; Vagnoni, G.; et al. The Prognostic Impact of Quitting Smoking at or around Diagnosis on the Survival of Patients with Gastrointestinal Cancers: A Systematic Literature Review. Cancers 2022, 14, 3857. https://doi.org/10.3390/cancers14163857
Caini S, Del Riccio M, Vettori V, Raimondi S, Assedi M, Vignati S, Bonaccorsi G, Cattaruzza MS, Bellerba F, Vagnoni G, et al. The Prognostic Impact of Quitting Smoking at or around Diagnosis on the Survival of Patients with Gastrointestinal Cancers: A Systematic Literature Review. Cancers. 2022; 14(16):3857. https://doi.org/10.3390/cancers14163857
Chicago/Turabian StyleCaini, Saverio, Marco Del Riccio, Virginia Vettori, Sara Raimondi, Melania Assedi, Silvano Vignati, Guglielmo Bonaccorsi, Maria Sofia Cattaruzza, Federica Bellerba, Giulia Vagnoni, and et al. 2022. "The Prognostic Impact of Quitting Smoking at or around Diagnosis on the Survival of Patients with Gastrointestinal Cancers: A Systematic Literature Review" Cancers 14, no. 16: 3857. https://doi.org/10.3390/cancers14163857
APA StyleCaini, S., Del Riccio, M., Vettori, V., Raimondi, S., Assedi, M., Vignati, S., Bonaccorsi, G., Cattaruzza, M. S., Bellerba, F., Vagnoni, G., Duroni, G., & Gandini, S. (2022). The Prognostic Impact of Quitting Smoking at or around Diagnosis on the Survival of Patients with Gastrointestinal Cancers: A Systematic Literature Review. Cancers, 14(16), 3857. https://doi.org/10.3390/cancers14163857










