Computer-Based Cognitive Training in Children with Primary Brain Tumours: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
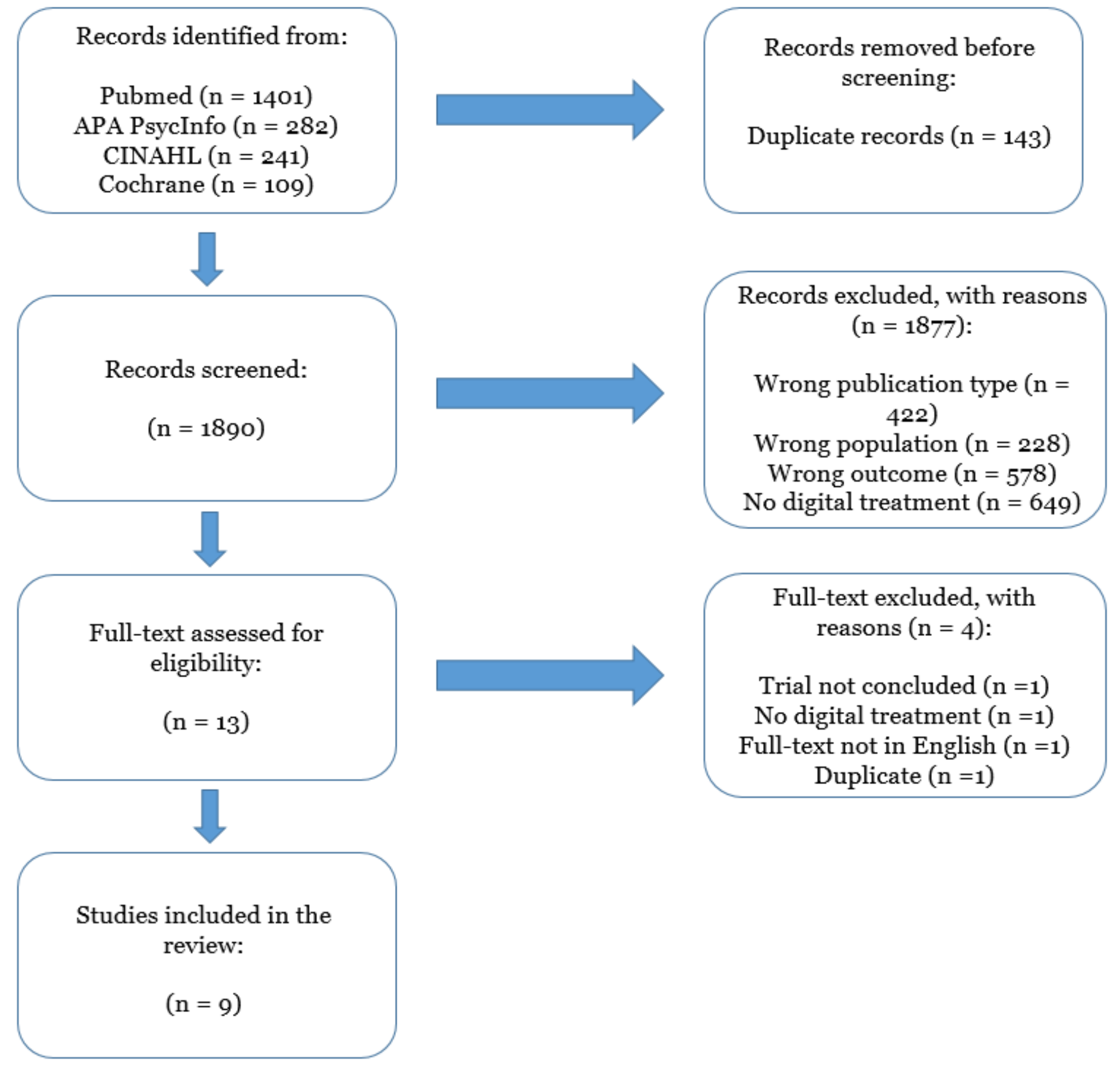
2. Materials and Methods
3. Results
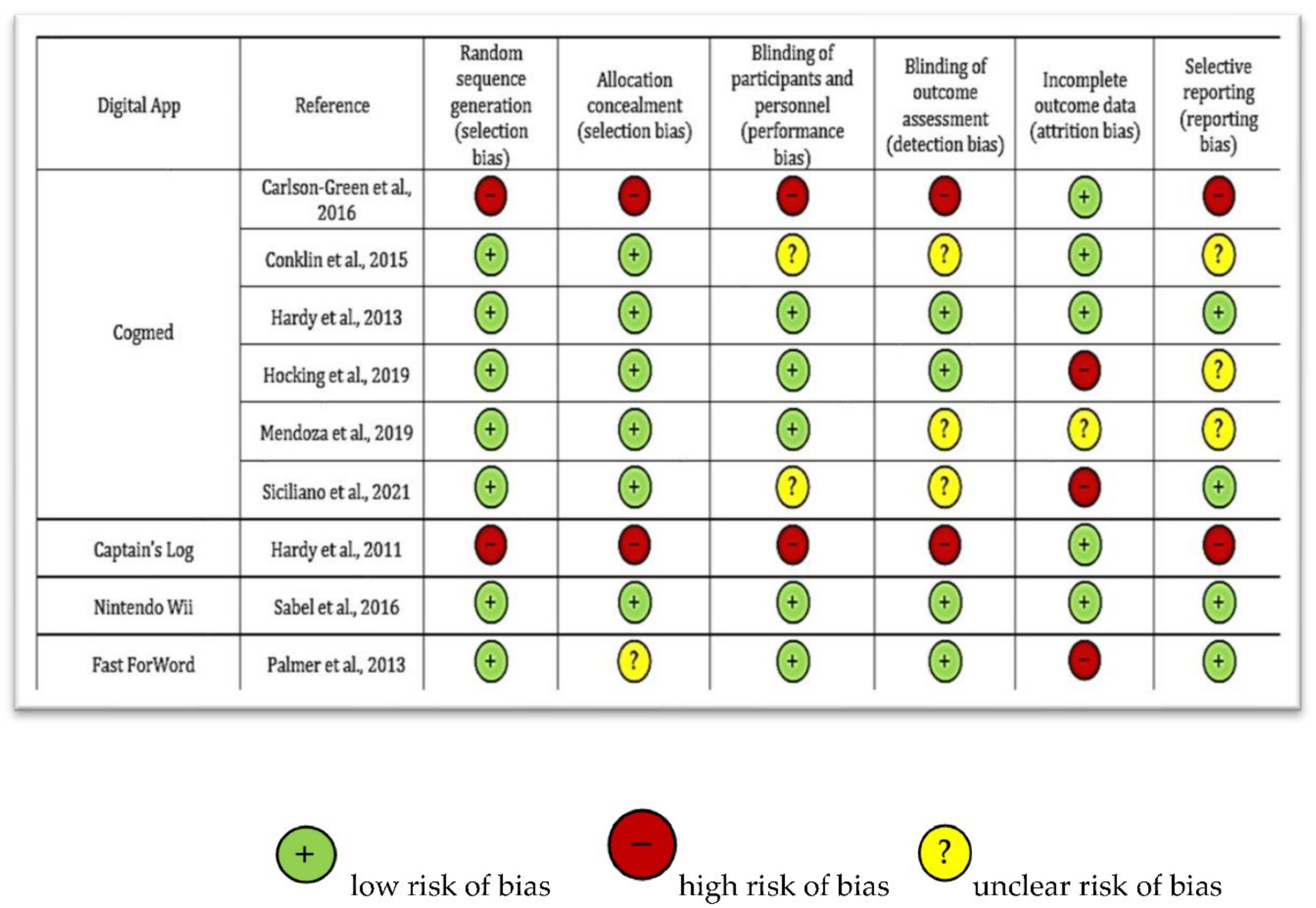
3.1. Quality Assessment of the Studies
3.2. Population
3.3. Cognitive Training Interventions and Outcomes Assessment
3.4. Qualitative Summary of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulhern, R.K.; Butler, R.W. ReviewNeurocognitive sequelae of childhood cancers and their treatment. Pediatr. Rehabil. 2004, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.J.; Krull, K.R.; Wefel, J.S.; Janelsins, M. Cognitive Changes in Cancer Survivors. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.P.; Carroll, C.; White, S.R.; Watson, P.; Spoudeas, H.A.; Hawkins, M.M.; Walker, D.A.; Clare, I.C.H.; Holland, A.J.; Ring, H. Long-term cognitive outcome in adult survivors of an early childhood posterior fossa brain tumour. Int. J. Clin. Oncol. 2020, 25, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Mostow, E.N.; Byrne, J.; Connelly, R.R.; Mulvihill, J.J. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J. Clin. Oncol. 1991, 9, 592–599. [Google Scholar] [CrossRef]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3894–3902. [Google Scholar] [CrossRef]
- Dey, D.; Parihar, V.K.; Szabo, G.G.; Klein, P.M.; Tran, J.; Moayyad, J.; Ahmed, F.; Nguyen, Q.-A.; Murry, A.; Merriott, D.; et al. Neurological Impairments in Mice Subjected to Irradiation and Chemotherapy. Radiat. Res. 2020, 193, 407. [Google Scholar] [CrossRef]
- Weyer-Jamora, C.; Brie, M.S.; Luks, T.L.; Smith, E.M.; Braunstein, S.E.; Villanueva-Meyer, J.E.; Bracci, P.M.; Chang, S.; Hervey-Jumper, S.L.; Taylor, J.W. Cognitive impact of lower-grade gliomas and strategies for rehabilitation. Neurooncol. Pract. 2021, 8, 117–128. [Google Scholar] [CrossRef]
- Smithson, E.F.; Phillips, R.; Harvey, D.W.; Morrall, M.C.H.J. The use of stimulant medication to improve neurocognitive and learning outcomes in children diagnosed with brain tumours: A systematic review. Eur. J. Cancer 2013, 49, 3029–3040. [Google Scholar] [CrossRef]
- Rossignoli-Palomeque, T.; Perez-Hernandez, E.; González-Marqués, J. Brain Training in Children and Adolescents: Is It Scientifically Valid? Front. Psychol. 2018, 9, 565. [Google Scholar] [CrossRef]
- Bejjanki, V.R.; Zhang, R.; Li, R.; Pouget, A.; Green, C.S.; Lu, Z.L.; Bavelier, D. Action video game play facilitates the development of better perceptual templates. Proc. Natl. Acad. Sci. USA 2014, 111, 16961–16966. [Google Scholar] [CrossRef]
- Kable, J.W.; Caulfield, M.K.; Falcone, M.; McConnell, M.; Bernardo, L.; Parthasarathi, T.; Cooper, N.; Ashare, R.; Audrain-McGovern, J.; Hornik, R.; et al. No Effect of Commercial Cognitive Training on Brain Activity, Choice Behavior, or Cognitive Performance. J. Neurosci. 2017, 37, 7390–7402. [Google Scholar] [CrossRef]
- Bediou, B.; Adams, D.M.; Mayer, R.E.; Tipton, E.; Green, C.S.; Bavelier, D. Meta-analysis of action video game impact on perceptual, attentional, and cognitive skills. Psychol. Bull. 2018, 144, 77–110. [Google Scholar] [CrossRef]
- Sala, G.; Gobet, F. Working memory training in typically developing children: A meta-analysis of the available evidence. Dev. Psychol. 2017, 53, 671–685. [Google Scholar] [CrossRef]
- Linden, M.; Hawley, C.; Blackwood, B.; Evans, J.; Anderson, V.; O’Rourke, C. Technological aids for the rehabilitation of memory and executive functioning in children and adolescents with acquired brain injury. Cochrane Database Syst. Rev. 2016, 7, CD011020. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Rayyan—Intelligent Systematic Review. Available online: https://www.rayyan.ai/ (accessed on 15 September 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hardy, K.K.; Willard, V.W.; Bonner, M.J. Computerized cognitive training in survivors of childhood cancer: A pilot study. J. Pediatr. Oncol. Nurs. 2011, 28, 27–33. [Google Scholar] [CrossRef]
- Carlson-Green, B.; Puig, J.; Bendel, A. Feasibility and efficacy of an extended trial of home-based working memory training for pediatric brain tumor survivors: A pilot study. Neuro-Oncol. Pract. 2017, 4, 111–120. [Google Scholar] [CrossRef]
- Siciliano, R.E.; Thigpen, J.C.; Desjardins, L.; Cook, J.L.; Steele, E.H.; Gruhn, M.A.; Ichinose, M.; Park, S.; Esbenshade, A.J.; Pastakia, D.; et al. Working memory training in pediatric brain tumor survivors after recent diagnosis: Challenges and initial effects. Appl. Neuropsychol. Child 2022, 11, 412–421. [Google Scholar] [CrossRef]
- Palmer, S.L.; Leigh, L.; Ellison, S.C.; Onar-Thomas, A.; Wu, S.; Qaddoumi, I.; Armstrong, G.T.; Wright, K.; Wetmore, C.; Broniscer, A.; et al. Feasibility and Efficacy of a Computer-Based Intervention Aimed at Preventing Reading Decoding Deficits Among Children Undergoing Active Treatment for Medulloblastoma: Results of a Randomized Trial. J. Pediatr. Psychol. 2014, 39, 450–458. [Google Scholar] [CrossRef]
- Mendoza, L.K.; Ashford, J.M.; Willard, V.W.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; Wang, F.; Zhang, H.; et al. Social Functioning of Childhood Cancer Survivors after Computerized Cognitive Training: A Randomized Controlled Trial. Children 2019, 6, E105. [Google Scholar] [CrossRef]
- Hocking, M.C.; Paltin, I.; Quast, L.F.; Barakat, L.P. Acceptability and Feasibility in a Pilot Randomized Clinical Trial of Computerized Working Memory Training and Parental Problem-Solving Training with Pediatric Brain Tumor Survivors. J. Pediatr. Psychol. 2019, 44, 669–678. [Google Scholar] [CrossRef]
- Hardy, K.K.; Willard, V.W.; Allen, T.M.; Bonner, M.J. Working memory training in survivors of pediatric cancer: A randomized pilot study. Psychooncology 2013, 22, 1856–1865. [Google Scholar] [CrossRef]
- Sabel, M.; Sjölund, A.; Broeren, J.; Arvidsson, D.; Saury, J.-M.; Gillenstrand, J.; Emanuelson, I.; Blomgren, K.; Lannering, B. Effects of physically active video gaming on cognition and activities of daily living in childhood brain tumor survivors: A randomized pilot study. Neuro-Oncol. Pract. 2017, 4, 98–110. [Google Scholar] [CrossRef]
- Cogmed. Available online: https://www.cogmed.com (accessed on 12 January 2022).
- Captain’s Log. Available online: https://www.braintrain.com/captains-log-for-educators/ (accessed on 12 January 2022).
- Fast Forword. Available online: https://www.scilearn.com/program/ (accessed on 12 January 2022).
- Nintendo Wii. Available online: https://www.nintendo.com (accessed on 12 January 2022).
- Petermann, F.; Petermann, U. Wechsler Intelligence Scale for Children, 4th ed.; Manual 1: Grundlagen, Testauswertung und Interpretation: Übersetzung und Adaptation der WISC-IV® von David Wechsler. 2., ergänzte Auflage. (Pearson Assessment & Information GmbH, ed.); Pearson: London, UK, 2014. [Google Scholar]
- Zelazo, P.D.; Bauer, P.J.; Fox, N.A. National Institutes of Health Toolbox Cognition Battery (NIH Toolbox CB): Validation for Children between 3 and 15 Years; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Conners, C.K.; Sitarenios, G.; Parker, J.D.; Epstein, J.N. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J. Abnorm. Child Psychol. 1998, 26, 257–268. [Google Scholar] [CrossRef]
- Klingberg, T.; Forssberg, H.; Westerberg, H. Training of Working Memory in Children With ADHD. J. Clin. Exp. Neuropsychol. 2002, 24, 781–791. [Google Scholar] [CrossRef]
- Klingberg, T.; Fernell, E.; Olesen, P.J.; Johnson, M.; Gustafsson, P.; Dahlström, K.; Gillberg, C.G.; Forssberg, H.; Westerberg, H. Computerized Training of Working Memory in Children With ADHD-A Randomized, Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 177–186. [Google Scholar] [CrossRef]
- Holmes, J.; Gathercole, S.E.; Dunning, D.L. Adaptive training leads to sustained enhancement of poor working memory in children. Dev. Sci. 2009, 12, F9–F15. [Google Scholar] [CrossRef]
- Reddick, W.E.; White, H.A.; Glass, J.O.; Wheeler, G.C.; Thompson, S.J.; Gajjar, A.; Leigh, L.; Mulhern, R.K. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer 2003, 97, 2512–2519. [Google Scholar] [CrossRef]
- Fraley, C.E.; Thigpen, J.C.; Pearson, M.M.; Kuttesch, J.F., Jr.; Desjardins, L.; Hoskinson, K.R.; Alvarado-Gonzalez, A.; Esbenshade, A.J.; Pastakia, D.; Friedman, D.L.; et al. Predictors of cognitive function in pediatric brain tumor patients: Pre-surgery through 24-month follow-up. Appl. Neuropsychol. Child 2021, 10, 340–347. [Google Scholar] [CrossRef]
- Shultz, E.L.; Lehmann, V.; Rausch, J.R.; Keim, M.C.; Winning, A.M.; Olshefski, R.S.; Vannatta, K.A.; Compas, B.E.; Gerhardt, C.A. Family estimates of risk for neurocognitive late effects following pediatric cancer: From diagnosis through the first three years of survivorship: Shultz et al. Pediatr. Blood Cancer 2017, 64, e26462. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Oldrati, V.; Oprandi, M.C.; Ferrari, E.; Poggi, G.; Borgatti, R.; Urgesi, C.; Bardoni, A. Remote Technology-Based Training Programs for Children with Acquired Brain Injury: A Systematic Review and a Meta-Analytic Exploration. Behav. Neurol. 2019, 2019, 1346987. [Google Scholar] [CrossRef] [PubMed]
- Resch, C.; Rosema, S.; Hurks, P.; de Kloet, A.; van Heugten, C. Searching for effective components of cognitive rehabilitation for children and adolescents with acquired brain injury: A systematic review. Brain Inj. 2018, 32, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Odone, A.; Buttigieg, S.; Ricciardi, W.; Azzopardi-Muscat, N.; Staines, A. Public health digitalization in Europe. Eur. J. Public Health 2021, 29 (Suppl. S3), e1. [Google Scholar] [CrossRef]
- European Commission. Guidance Document Medical Devices—Scope, Field of Application, Definition—Qualification and Classification of Stand Alone Software—MEDDEV 2.1/6. Updated July 2016. Available online: http://ec.europa.eu/DocsRoom/documents/17921/attachments/1/translations (accessed on 10 June 2022).
| Author | Year | No. of Participants (Cases and Controls) | Gender (M, F%) and Mean Age | Mean Age at Diagnosis (Years) | Type of Neoplastic Disease | Intervention | Cognitive Abilities Trained | Attrition | Results (Summary of Findings) |
|---|---|---|---|---|---|---|---|---|---|
| Carlos-Green | 2016 | N = 21 (21 intervention) No control group. | NR; age range 8 to 18 years. | 6 (1–14) | Medulloblastoma (N = 11), Germinoma (N = 4), Ependymoma (N = 4), and other tumour types (N = 2). | Name: Cogmed. Participants in this study were asked to complete 35 training sessions over 8 to 12 weeks and were contacted by telephone to check progress and enhance motivation. At the end of the training, participants and their parents also completed questionnaires assessing their satisfaction with the program. Participants were assessed through a follow-up testing 6 months after the training completion. | Attention;Working memory; Executive functions. | 2/21 (9.5%) participants did not complete all the training sessions. | The efficacy of Cogmed was examined 6 months after completing the intervention. Improvements: Working memory (verbal and visual-spatial tasks), academic math test, executive functioning (emotional and behavioural control, ability to transition/shift between activities, planning and organizational skills, ability to monitor their behaviour). |
| Conklin | 2015 | N = 68. Intervention (N = 34); Control group waiting list (N = 34). | Cases: 18 M (52.9%) 16 F (48.1%), age 12.2 ± 2.5; Controls: 18 M (52.9%) 16 F (48.1%), age 11.8 ± 2.4. | Cases: 5.2 ± 2.9; Controls: 4.6 ± 2.7. | Cases: 23 ALL, 11 BT (8 Medulloblastoma, 2 Glioma, 1 Ependymoma); Controls: 24 ALL, 10 BT (7 Medulloblastoma, 3 Ependymoma). | Name: Cogmed. Participants were randomly assigned to the intervention. The intervention group was asked to complete 25 at-home training sessions over 5 to 9 weeks. Training progress was monitored over the Internet and coaching telephone calls were used to provide feedback and help maintain motivation. All participants had a final cognitive assessment after 6 months. | Attention; Working memory; Executive functions. | Cogmed group: 4 participants (11.8%) incomplete trainings. 30 follow-up assessments. Controls: 2 dropouts (5.9%). 32 follow-up assessments. | Improvements in the intervention group: Spatial span backward short-term (p = 0.002); WISC-IV spatial span forward (p = 0.012); CPT-II omissions (p = 0.036), WM (WISC-IV digit span backward, p = 0.017; WISC-IV working memory index, p = 0.022), and processing speed (CPT-II reaction time, p = 0.020). Improvements also regarding attention and executive functions compared with the control group participants (CPRS-3 inattention, p = 0.009; CPRS-3 executive function, p = 0.002). |
| Hardy | 2011 | N = 9 (9 intervention) No control group. | Cases: 5 M (56%) 4 F (44%), age 13.3 ± 2.4. | NR | Cases: 3 ALL, 6 BT (1 Primitive neuroectodermal tumour, 3 Medulloblastoma, 2 Ependymoma). | Name: Captain’s Log. Participants were asked to complete a 50 min/week training session for 12 weeks. 3 months after the completion, participants returned to the clinic for follow-up testing. | Problem solving; Working memory; Attention. | 1 participant (11.1%) did not complete all follow-up visits. | Working memory scores increased from baseline to the follow-up assessment [F(2,15.11) = 3.16; p = 0.07]. Digit span forward had a significant increase over time [F(2,15.09) = 6.79; p < 0.01)]. Attention problems [F(2,15.10) = 6.98; p < 0.01], significantly decreased across 3 time points. Digit span backward [F(2,15.27) = 0.10; NS] and number sequencing [F(2,15.38) = 0.40; NS] did not improve significantly post-intervention. |
| Hardy | 2013 | N = 20. Intervention (N = 13); Active control group (N = 7; training: Not-adaptive Cogmed). | Cases: 8 M (61.5%) 5 F (38.5%), age 12.7 ± 2.77; Controls: 4 M (57.1%) 3 F (42.9%), age 10.7 ± 1.89. | Cases: 4.9 ± 3.54; Controls: 5.7 ± 2.88. | Cases: 7 ALL, 6 BT (2 Medulloblastoma/PNET, 3 Ependymoma, 1 other tumour type); Controls: 4 ALL, 3 BT (2 Medulloblastoma/PNET, 1 other tumour type) | Name: Cogmed. Participants were randomly assigned to the success-adapted computer intervention or not-adaptive active control condition. Participants were asked to complete 25 at-home training sessions (3 to 5 sessions a week) over 5 to 8 weeks. Participants were assisted by a treatment coach to motivate them and solve problems. Follow-up assessment after 3 months. | Attention; Working memory; Executive functions. | Cases: 2 incomplete trainings (15.4%); Controls: 1 incomplete training (14.3%). | Symbolic working memory task from the WRAML2—the cases increased significantly [F = 4.57, p = 0.05] compared with the controls during the intervention period, while this effect was no longer significant at the 3-month follow-up [F = 3.65, p = 0.08]. Cases experienced a greater improvement in parent-reported learning problems on the Conner-3 [F = 4.65, p = 0.05]. Moreover, 45% of cases exhibited improvement consistent with the RCI, even after the 3-month follow-up. |
| Hocking | 2019 | N = 27. Standard intervention (N = 14); Active control group (N = 13; training: Cogmed + Parent intervention). | 14 M (51.9%) 13 F (48.1%), mean age 11.07 (7–16). | Cases: 4.96 ± 3.48. | 7 Astrocytoma, 6 Medulloblastoma, 6 Ependymoma, 1 low-grade glioma, 7 other BTs. | Name: Cogmed. Participants in both groups were assigned to 25 computer sessions over 5 to 6 weeks (30–45 min for each session). Participants in the combined intervention were also exposed to a “Parent intervention”: Phone sessions for parents in the combined group included six sessions (duration: 30–45 min) regarding manualised problem-solving skills training (PSST). | Attention; Working memory; Executive functions. | 5 participants (18.5%) lost to follow-up in both standard and combined group. In the next 3 months, standard group lost 3 further participants. | Completers: Better performance in baseline auditory attention abilities (digit span forward) than non-completers and they also showed a reduction of working memory difficulties in completers than non-completers. |
| Mendoza | 2019 | N = 68. Intervention (N = 34); Control group waiting list (N = 34). | Cases: 18 M (53%) 16 F (47%), age 12.21 ± 2.47; Controls: 18 M (53%) 16 F (47%), age 11.82 ± 2.42. | Cases: 5.15 ± 2.92; Controls: 4.62 ± 2.68. | Cases: 23 ALL, 11 BT (8 Medulloblastoma, 2 Glioma, 1 Ependymoma); Controls: 24 ALL, 10 BT (7 Medulloblastoma, 3 Ependymoma). | Name: Cogmed. Participants were randomly assigned to computerised training or waitlist control groups. Participants in the Cogmed intervention group were asked to complete 25 at-home training sessions over 5 to 9 weeks. The exercises increased or decreased in difficulty and complexity based on performance. Progress and participants’ motivation were monitored by coaching phone calls. | Attention; Working memory; Executive functions. | Cogmed group: 4 incomplete trainings (11.8%). 30 follow-up assessments. Controls: 2 drop-outs (5.9%). 32 follow-up assessments. | From baseline to post-intervention assessment, the intervention group showed greater improvement than the control group on: Attention and working memory (WISC-IV spatial span forward, digit span backward, working memory index, p < 0.05; WISC-IV spatial span backward p < 0.001). Improvements also in executive functioning and attention. |
| Palmer | 2013 | N = 81. Intervention (N = 43); Control group waiting list (N = 38). | Cases: 24 M (55.8%) 19 F (44.2%), age NR; Controls: 26 M (68.4%) 12 F (31.6%), age NR. | Cases: 9.38 ± 3.12; Controls: 9.27 ± 3.18. | Cases: 43 Medulloblastoma; Controls: 38 Medulloblastoma. | Name: Fast ForWord. In addition to the standard-of-care, patients were asked to complete the Fast ForWord computer-based training program 48 min/day, 5 days/week, for 6 weeks—30 sessions, with a total training time of 1440 min. Participants were assisted by a teacher and their performance was monitored. 5-year follow-up period. | Working memory; Attention; Auditory processing and sequencing; Reading ability. | Cases: 3 incomplete trainings (6.9%); 2 incomplete assessments (4.7%). | Patients with high-risk disease (p = 0.0042) and younger age at diagnosis (p < 0.0001) had more declines in reading during the follow-up. Older patients at diagnosis date had less decline in reading (p = 0.0008) and decoding (p = 0.0367). |
| Sabel | 2016 | N = 13. Intervention (N = 7); Control group waiting list (N = 6). | Cases: 3 M (43%) 4 F (57%), age 11.9 ± 3.6; Controls: 3 M (50%) 3 F (50%), age 13.2 ± 1.9. | NR | Cases: 1 Anaplastic Astrocytoma, 2 Germinoma, 1 Medulloblastoma, 2 Pilocytic Astrocytoma, 1 Supratentorial Primitive Neuroectodermal tumour; Controls: 1 Choroid Plexus Carcinoma, 1 Germinoma, 2 Medulloblastoma, 1 Pilocytic Astrocytoma, 1 Supratentorial Primitive Neuroectodermal tumour. | Name: Nintendo Wii, Wii-Fit. Patients were randomly assigned to the intervention. Participants were asked to complete a minimum of 30 min/day, at least 5 days/week, over 10 to 12 weeks. Activity levels were measured via a multisensory activity monitor for 1 week at baseline, every second week during the intervention period, and for 1 week after the waiting list period. | Body coordination; Hand-eye coordination; Fine motor control. | No attrition found. | The intervention group exhibited improvement in: Motor (p = 0.012) and process (p = 0.002) skills after active video gaming. There were no significant changes in cognitive tests, although positive trends in selective (p = 0.078) and sustained attention (p = 0.090). |
| Siciliano | 2021 | N = 41. Intervention (N = 20); Active control group (N = 21; training: Not-adaptive Cogmed). | Cases: 13 M (65%) 7 F (35%), age 12.31 ± 2.57; Controls: 13 M (57%) 7 F (43%), age 11.67 ± 2.81. | NR | NR | Name: Cogmed. Participants were randomly assigned to adaptive or not-adaptive versions. Participants were asked to complete 25 sessions (30–45 min) for 5 days a week, over a 5-week period. Coaches supported participants one to two times per week. Follow-up: 10 to 20 weeks post-intervention, and the final one 6 months after the previous assessment. | Attention; Working memory; Executive functions. | 15/41 participants (36.6%) did not complete T2 assessment. The T3 and T4 assessment completion did not vary by group. | WMI and NTCB scores significantly improved immediately post-intervention compared with baseline scores. No significant differences between adaptive and not-adaptive conditions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciancalepore, F.; Tariciotti, L.; Remoli, G.; Menegatti, D.; Carai, A.; Petruzzellis, G.; Miller, K.P.; Delli Priscoli, F.; Giuseppi, A.; Premuselli, R.; et al. Computer-Based Cognitive Training in Children with Primary Brain Tumours: A Systematic Review. Cancers 2022, 14, 3879. https://doi.org/10.3390/cancers14163879
Sciancalepore F, Tariciotti L, Remoli G, Menegatti D, Carai A, Petruzzellis G, Miller KP, Delli Priscoli F, Giuseppi A, Premuselli R, et al. Computer-Based Cognitive Training in Children with Primary Brain Tumours: A Systematic Review. Cancers. 2022; 14(16):3879. https://doi.org/10.3390/cancers14163879
Chicago/Turabian StyleSciancalepore, Francesco, Leonardo Tariciotti, Giulia Remoli, Danilo Menegatti, Andrea Carai, Giuseppe Petruzzellis, Kiersten P. Miller, Francesco Delli Priscoli, Alessandro Giuseppi, Roberto Premuselli, and et al. 2022. "Computer-Based Cognitive Training in Children with Primary Brain Tumours: A Systematic Review" Cancers 14, no. 16: 3879. https://doi.org/10.3390/cancers14163879






