Simple Summary
Cancer-associated fibroblasts (CAFs) are found in the tumor microenvironment and exhibit several protumorigenic functions. Preclinical studies suggest that CAFs can be reduced, eliminated, or reprogrammed; however, clinical translation has not yet occurred. A better understanding of these cells and their functions will undoubtedly improve cancer treatments. In this review, we summarize current research, highlight major challenges, and discuss future opportunities for improving our knowledge of CAF biology and targeting.
Abstract
Cancer-associated fibroblasts (CAFs) are a heterogenous group of activated fibroblasts and a major component of the tumor stroma. CAFs may be derived from fibroblasts, epithelial cells, endothelial cells, cancer stem cells, adipocytes, pericytes, or stellate cells. These complex origins may underlie their functional diversity, which includes pro-tumorigenic roles in extracellular matrix remodeling, the suppression of anti-tumor immunity, and resistance to cancer therapy. Several methods for targeting CAFs to inhibit tumor progression and enhance anti-tumor immunity have recently been reported. While preclinical studies have shown promise, to date they have been unsuccessful in human clinical trials against melanoma, breast cancer, pancreas cancer, and colorectal cancers. This review summarizes recent and major advances in CAF-targeting therapies, including DNA-based vaccines, anti-CAF CAR-T cells, and modifying and reprogramming CAF functions. The challenges in developing effective anti-CAF treatment are highlighted, which include CAF heterogeneity and plasticity, the lack of specific target markers for CAFs, the limitations in animal models recapitulating the human cancer microenvironment, and the undesirable off-target and systemic side effects. Overcoming these challenges and expanding our understanding of the basic biology of CAFs is necessary for making progress towards safe and effective therapeutic strategies against cancers in human patients.
1. Introduction
Over 1.9 million new cancer cases are expected in the United States in 2022, and solid tumors comprise approximately 90% of these cases [1]. As we continue to expand our knowledge of cancer, we now recognize the tumor microenvironment as a heterogenous, intricate system composed of tumor cells and nonmalignant host components including immune cells, stroma, and vasculature, which shapes the nature of the tumor. In many epithelial tumors, including pancreatic, lung, breast, and colorectal cancers, the stroma can comprise up to 90% of the cancer mass [2]. Within the tumor stroma are both cellular and noncellular components, including collagen, fibroblasts, and mesenchymal stromal cells that provide structure and remodel the tissue. Activated stromal tissue in the pathological context forms desmoplasia or fibrosis, resulting in increased mass and stiffness, which are considered negative prognostic indicators. Fibroblasts constitute one of the most abundant and critical cell types in the tumor stroma and are the major producers of connective tissue extracellular matrix (ECM). Within the tumor microenvironment, various inflammatory cytokines produced by cancer cells, host immune and stromal cells induce the activation of fibroblasts. These activated fibroblasts are termed cancer-associated fibroblasts (CAFs). Through their production of soluble factors, such as cytokines and chemokines, and ECM, CAFs strongly influence surrounding cells. They support tumor progression and metastasis by promoting cancer cell growth, enhancing pro-tumor immune responses, remodeling the ECM, influencing tumor cell drug resistance, and promoting angiogenesis [3,4]. In this review, we focus on CAFs, discuss their biologic tumor-promoting functions and recent advancements in the development of CAF-targeting cancer therapies.
2. Definitions, Origins and Basic Biology of CAFs
2.1. Definition of CAFs
Fibroblasts are found in virtually all organs and normal tissues and contribute to inflammation and fibrosis during wound healing. CAFs are activated fibroblasts with a mesenchymal lineage associated with cancer and contribute to tumor-promoting inflammation and fibrosis. CAFs are defined by a combination of their morphology, association with cancer cells, and lack of lineage markers for epithelial cells, endothelial cells, and hematopoietic cells [4]. CAFs maintain key roles in regulating the biologic function of the tumor stroma and contribute to immune regulation, angiogenesis, and ECM remodeling of the tumor, as well as the generation and maintenance of cancer stem cells, thereby promoting therapeutic resistance.
2.2. Distinction of CAFs from Resting Fibroblasts
CAFs differ in several respects from resting fibroblasts residing in normal tissues. CAFs are generally larger than resting fibroblasts, spindle-shaped, and have indented nuclei and branching cytoplasm. However, the difference between the two is largely a functional distinction. CAFs possess enhanced proliferative, migratory, and secretory properties. CAFs are more metabolically active than untransformed fibroblasts, producing increased extracellular matrix factors such as tenascin, periostin (POSTN), and secreted protein acidic and rich in cysteine (SPARC) [5]. Collagen production by CAFs is abnormal, characterized by increased production and often a more rigid and contractile pattern of collagen deposition [6,7,8]. Several signaling mechanisms are recognized in the transition from resting fibroblast to CAF, including the activation of the Hippo pathway, the loss of p53, and the activation of heat shock factor protein 1 triggered by inflammation and changes in the structure and composition of the ECM [6,9]. CAFs are even found in circulation, akin to circulating tumor cells (CTCs)-circulating CAFs (identified based on the expression of fibroblast activation protein (FAP) and alpha-smooth muscle actin (α-SMA)), and were found in 88% of patients with metastatic breast cancer and in 23% of patients with nonmetastatic disease [9], suggesting a role in metastasis and formation of the pre-metastatic niche.
2.3. Origin of CAFs
CAFs can arise from a myriad of cell precursors, which can also vary between tissues. The origins of all CAFs are not entirely and fully elucidated. Regardless of origin, the transition to CAF is largely irreversible, and yet remains plastic with regard to the CAF phenotype within or across tumor types. CAFs often develop from local resident fibroblast populations but can also differentiate from mesenchymal stromal cells or mesenchymal stem cells (MSCs). MSCs express a similar, less abundant set of surface markers, and possess the ability to differentiate into osteoblasts, chondrocytes, and adipocytes [10,11,12]. Quiescent resident fibroblasts in the liver and pancreas, also known as pancreatic stellate cells, can acquire a CAF phenotype upon activation by tumor growth factor beta (TGFβ) and platelet-derived growth factors (PDGFs) [13,14]. Outside of the fibroblast lineage, CAFs can transdifferentiate from epithelial cells, blood vessels, adipocytes, pericytes, and smooth muscle cells via endothelial to mesenchymal transition (EMT) and endothelial to mesenchymal transition (EndMT). Fibrocytes, circulating mesenchymal cells derived from monocyte precursors, can also become CAFs [15]. Both noninvasive and invasive cancer cells can express the EMT markers β-catenin and vimentin or S100A4, so these are also not unique to CAFs. Importantly, CAFs can secrete proinflammatory cytokines, such as interleukin (IL)-6, which promotes the EMT of cancer cells, forming a positive feedback loop [16,17,18]. The recruitment and activation of CAFs is mediated by hypoxic conditions, oxidative stress, and certain growth factors produced by tumor cells. TGF-β, epidermal growth factor (EGF), fibroblast growth factor type 2 (FGF2), and PDGF are known to act as key regulators of CAF recruitment and activation [19,20]. Additionally, IL-1β from innate immune cells triggers NF-kB activation and production of IL-6 in CAFs via the JAK-STAT pathway, contributing to CAF differentiation [21]. Lysophosphatidic acid produced by cancer cells synergizes with TGF-β to drive the activation and increase the contractility of CAFs [4]. Recent research has also shown that exosomes secreted by murine melanoma, human squamous cell carcinoma, and human breast carcinoma can promote the differentiation of fibroblasts into CAFs, mediated by TGF-β and downstream SMAD signaling pathways [22,23]. Overall, the precise origin and roles of fibroblast populations within the tumor microenvironment remain poorly understood. Further studies using lineage tracing for the cell of origin [24,25] will be essential in deepening our understanding on the origins of CAFs, as well as their evolution during tumorigenesis.
2.4. CAF Phenotypic and Functional Heterogeneity
Tumors are spatially and functionally heterogeneous ecosystems [26], and the variety of sources from which CAFs can arise lend complexity to their phenotype, gene expression, and function. Several biomarkers have been established to detect CAFs, however none are completely exclusive. To date, CAFs are defined as cells that lack the expression of biomarkers for epithelial, endothelial, or hematopoietic cells but express mesenchymal biomarkers such as vimentin, α-SMA, FAP, and platelet-derived growth factor receptor alpha (PDGFR-α), and lack genetic mutations [27,28]. As the phenotypes of CAFs differ between tumor type, CAF studies necessitate the combined application of multiple biomarkers for the detection and identification of these cells. As a result, CAFs are often identified by a combination of α-SMA, tenascin-C, periostin (POSTN), NG2 chondroitin sulfate proteoglycan, PDGFRα/β, and FAP expression. Other mesenchymal markers include vimentin, fibronectin, type I collagen, prolyl 4-hydroxylase, fibroblast surface protein, and fibroblast specific protein-1 (FSP-1)/S100A4 [29]. Biomarkers expressed by both normal fibroblasts and CAFs are summarized in Table 1.

Table 1.
Biomarkers used to identify fibroblasts and cancer-associated fibroblasts.
Historically, activated fibroblasts expressing α-SMA were termed ‘myofibroblasts’, but are now recognized to be only one subset among several within the tumor microenvironment. α-SMA+ CAFs predominantly produce and modulate the ECM, facilitate cell–ECM adhesion, and regulate adaptive immunity [59]. α-SMA+ CAFs are also located more distally to tumor cells. FAP+ CAFs are immunosuppressive with increased ECM alignment and stiffness, and this is hypothesized to be a major factor in the transition from a tumor-resistant to tumor-permissive microenvironment [75]. Stiffness of the tumor stroma influences invasion through tumor cell integrin-dependent mechanotransduction signaling [76], and is correlated with increased metastasis [77,78].
Newer analytic methods such as single-cell RNA sequencing (scRNAseq) and cytometry by time of flight (cyTOF) have begun to help answer questions concerning functional subsets. Functional CAF subsets maintain unique cytokine expression profiles that variably shape the tumor microenvironment. While some CAF subsets do not seem to affect immune cell populations, others in fact, modulate the immune microenvironment, often in pro-tumorigenic ways. The most recent scRNAseq transcriptome data suggest there are between 3 and 7 major subsets of fibroblasts [52,79,80], but some of these groups may have overlapping features, as well as context-dependent and tumor-dependent variability. Nonetheless, there is growing evidence for similar or shared phenotypes across tumor types as discussed in the following sections.
2.5. Functional CAF Subsets in Human Cancers
The analysis of distinct CAF subpopulations at the single cell level has largely been performed in the context of human pancreatic cancer, with several studies also examining these cells in other human tumor types (Table 2). To highlight some of these classifications, in human pancreatic cancer, at least two major CAF phenotypes are defined by their expression of α-SMA and IL-6. A more matrix-secreting, TGF-β–responsive, high-α-SMA, and low-cytokine (e.g., IL-6, IL-11)-expressing myofibroblastic, myCAF population, and inflammatory-type, iCAFs, that exhibit high IL-6 and IL-11 production and low α-SMA expression [51,52,60,81]. Spatial distribution of these two populations also differs—myCAFs are often found adjacent to neoplastic cells whereas iCAFs localize within dense stromal regions distant from neoplastic cells [60]. Interestingly, pancreatic CAFs, formerly quiescent pancreatic stellate cells, are able to transition between the myCAF and iCAF states, although the mechanism by which this occurs is not well understood [60]. A third CAF phenotype, apCAFs, are characterized by major histocompatibility complex (MHC) class II and CD74 expression and are capable of presenting antigens to CD4 T cells, but lack classical costimulatory molecules expressed by professional antigen-presenting cells [52]. MyCAF and iCAF subpopulations have also been identified in human cholangiocarcinoma [82] and bladder cancer [83]. Human triple-negative breast [45,84] and ovarian [85] cancer studies have yielded 3–4 CAF subtypes, designated CAF S1–S4 based on the differential expression of six fibroblast markers (FAP, integrin β1/CD29, α-SMA, S100-A4/FSP1, PDGFRβ, and caveolin-1). Other phenotyping studies in human lung [80], prostate [86], head and neck [87], and colorectal [88,89] cancers similarly classify CAF subpopulations based on high versus low α-SMA expression and/or functional characteristics.

Table 2.
Cancer-associated fibroblast subtypes across different cancers.
2.6. Functional CAF Subsets in Murine Cancers
The three CAF subsets described in human tumors are also found in murine pancreatic cancer models by scRNAseq analysis; ECM-producing myCAFs, inflammatory iCAFs, and a third smaller population of antigen-presenting apCAFs. CAF subsets in spontaneous mouse mammary tumor models (the MMTV-PyVT mouse model) have been categorized into four main groups, vascular CAFs (vCAFs), cycling CAFs (cCAF), matrix CAFs (mCAF), and developmental CAFs (dCAF) [93]. vCAFs are angiogenic, predominantly located near vessels and thought to arise from perivascular cell precursors. cCAFs are considered the proliferating fraction of vCAFs, with similar transcriptional profiles as vCAFs, exhibiting upregulated cell cycle genes (e.g., Nuf2, Mki67, Ccna2, Top2a, and Cep55). mCAFs are descendants of resident fibroblasts, expressing fibulin-1 and PDGFRα, which are often positioned at the invasive front of tumors. dCAFs express development-associated genes and are similar in phenotype and are proximal to cancer cells, suggesting that they may originate from a malignant cell precursor. In 4T1 mouse mammary tumor models, eight subtypes of CAFs divided into in two main populations, pCAF and sCAF, are described based on the selective expression of PDPN or S100A4. The ratio of pCAFs and sCAFs changes with tumor progression and is associated with disease outcome in triple-negative breast cancer patients [95].
CAFs have been functionally categorized in other murine cancers, such as melanoma, as either immune (S1), desmoplastic (S2), or contractile (S3) [90], and in cholangiocarcinoma as either myCAF, iCAF, or mesothelial mesCAF [82]. Overall, the existence of both myofibroblastic and inflammatory CAF populations appears to be the most consistent observation in both human and mouse tumor models. Across cancer types, myCAFs are associated with high ECM production, whereas non-myofibroblastic iCAFs are generally characterized by a secretory, inflammatory phenotype. Lastly, CAFs can also be grouped based on location, e.g., primary tumor, circulation, or metastasis [96,97].
2.7. Challenges in Defining and Detecting CAFs
By far, one of the greatest challenges in defining CAFs is the lack of a pan-specific biomarker. In addition, no standardization nor consensus of biomarkers to identify CAFs currently exist, adding to the difficulty in differentiating CAFs from other mesenchymal cells (e.g., adipocytes or pericytes). This lack of uniform analysis makes interpretation of previous studies and understanding of the full biological implications of these cells difficult. Standardized functional and molecular definitions of fibroblast subtypes also do not yet exist. There is inherent plasticity between CAF subtypes, suggesting these are functional fibroblastic states, as opposed to static fibroblast types, adding to their complexity [98]. CAFs continue to evolve over time and eventually differentiate into subpopulations that promote tumor development in ways that are not only tissue specific but tumor specific. Identifying what triggers this plasticity will also be invaluable in future research, as phenotypic or functional subsets may not function comparably across tumor types. It is increasingly clear that the tumor microenvironment changes throughout cancer progression, and likely so do CAFs. Longitudinal studies, particularly focused on CAF plasticity, are necessary for further insight.
3. Protumorigenic Functions of CAFs
Various components of the tumor microenvironment promote tumor progression and resistance to cancer therapy. For instance, mesenchymal stem cells can secrete vascular endothelial growth factor (VEGF), promoting vessel growth, and prostaglandin E2 (PGE2), impeding the anti-tumor immune response through the suppression of T cell function. Pericytes and adipocytes can produce pro-tumorigenic growth factors and cytokines and even contribute to T cell anergy [99]. Finally, immune cells such as tumor-associated macrophages (TAMs) can promote EMT and inflammation-associated angiogenesis [100]. Here, we focus specifically on the roles of CAFs.
3.1. Tumor-Promoting Secretory Factors
In general, CAFs secrete far more cytokines and chemokines than their resting counterparts. These secreted factors include TGFβ, PDGF, FGF, hepatocyte growth factor (HGF), VEGF, tumor necrosis factor α (TNFα), interferon-γ (IFNγ), CXCL12, IL-6, connective tissue growth factor (CTGFβ), EGF, growth arrest-specific protein 6 (GAS6), galectin-1, secreted frizzled-related protein 1 (SFRP1), sonic hedgehog protein (SHH), and bone morphogenetic protein (BMP), which are tumor-promoting (Figure 1) [6]. As the master regulator of fibrosis and a major secreted factor of CAFs, TGFβ predominantly mediates crosstalk between CAFs and cancer cells. The inhibition of TGFβ signaling using a number of approaches has been shown to significantly inhibit tumor growth and metastasis [101].
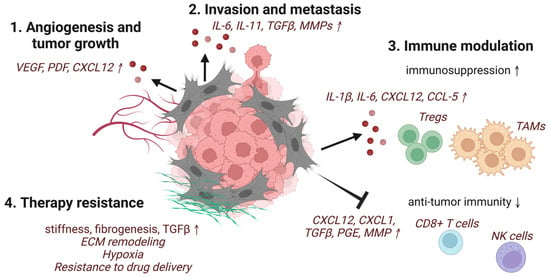
Figure 1.
Schematic representation of selected pro-tumorigenic functions of CAFs. CAFs induce (1) angiogenesis and tumor growth, (2) invasion and metastasis of cancer cells, (3) modulation of the immune system, including recruitment and activation of immune suppressors and inhibition of anti-tumor effector cells, and (4) therapy-resistance through ECM production and remodeling (created with BioRender.com accessed 19 June 2022).
CAFs have also been demonstrated to induce EMT and promote the growth and migration of cancer cells via IL-6 [102,103]. Elevated levels of CAF-derived IL-6 induces the activation of the JAK/STAT3 signaling pathway, leading to tumor cell proliferation mediated by the activation of cyclin D1, among other cell cycle mediators. Tumor survival is enhanced by the activation of downstream BCL2-like protein 1 (BCL-xL). STAT3 also induces the expression of angiogenic factor VEGF. During tumor neovascularization, degradation of the basement membrane and ECM occurs, with a contribution from matrix metalloproteinases (MMPs) [104], to allow for endothelial cells to migrate and generate new vessels. This process, in turn, enhances cancer invasion and metastasis. The hyperactivation of STAT3 in anti-tumor immune cells exerts a negative regulatory effect that also contributes to an immunosuppressive tumor microenvironment [105]. Other signaling pathways governing the tumor-promoting ability of CAFs include PDGF-PDGFR, which acts through paracrine signaling on cancer cells to drive tumor growth [29].
3.2. Resistance to Chemotherapies and Radiation
Classic chemotherapy targets rapidly proliferating cells, but does not eliminate all CAFs, nor those cancer cells that become drug resistant. CAFs can also contribute to the development of resistant cancer phenotypes following cycles of chemotherapies. Several in vitro experiments demonstrate that DNA damage induced by chemotherapies prompted increased cancer cell invasion and survival through stromal-derived paracrine signaling via cytokines and exosomes [6]. For example, this occurs via glial-derived neurotrophic factor (GDNF) production in prostate cancer [106], IL-6 in lymphoma [107], and exosome secretion in colorectal cancer [108]. Chemotherapy-induced genotoxic stress can also trigger a DNA damage secretory program resulting in the release of numerous inflammatory (IL-6/8), angiogenic (VEGF, CXCL1), mitogenic (amphiregulin), and pro-EMT (HGF) factors [98].
Several chemotherapy drugs have been reported to induce CAF-like phenotypes in resting fibroblasts and promote stemness in breast [109] and colorectal cancers [108]. This is thought to occur following an exposure of cancer cells to a hypoxic environment, which activates hypoxia-inducible factor (HIF-1α) and sonic hedgehog-GLI signaling [109,110]. CAF-mediated TGF-β signaling synergizes with HIF-1α signaling and enhances the expression of GLI2 in cancer cells, inducing stemness. This results in a resistance to chemotherapy. In fact, a high expression of the HIF-1α/TGF-β is associated with an increased risk of colorectal cancer recurrence in patients undergoing chemotherapy [110]. Similarly, studies have found that CAFs contribute to drug resistance and reduce the efficacy of anti-EGFR cetuximab [111], gemcitabine [112], and the tyrosine kinase inhibitor gefitinib [113].
As with the examples of chemotherapies described above, radiation therapy impedes cancer cell growth through DNA damage. Radiation affects not only cancer cells but also the tissue microenvironment surrounding cancer cells, which includes immune cells, endothelial cells, vasculature, and fibroblasts. Fibroblasts are highly resistant to radiation, even at high doses. Irradiated fibroblasts can overcome apoptotic signals and become senescent but have also been demonstrated to convert to a more activated CAF phenotype [114,115,116]. In one study, radiation exposure activated CAFs and upregulated their expression of CXCL12, which directly acted on pancreatic cancer cells via CXCR4, promoting EMT and invasion in vitro and in vivo [117]. The enhanced expression of CXCL12, HGF, MMPs, and TGF-β in irradiated fibroblasts was found to increase invasion and EMT in cancer cells as indicated by the increased expression of vimentin, snail and beta-catenin, and decreased E-cadherin expression. [114,115,116].
The CAF-directed resistance to radiotherapy and post-radiation recurrence of cancers is reported to be associated with activation of the autophagy pathway. It is likely this response is at least in part related to CAF-secreted IGF1/2, CXCL12, and β-hydroxybutyrate, leading to increased reactive oxygen species (ROS), enhancing protein phosphatase 2A (PP2A) activity, repressing mTOR activation, and ultimately resulting in autophagy in cancer cells after irradiation [118,119]. The IGF2 neutralizing antibody and autophagy inhibitor 3-MA consistently reduced the CAF-promoted tumor relapse in tumor-bearing mice after radiotherapy [118]. Combining CAF-targeted therapies and chemotherapy or radiation could yield a more powerful and robust anti-tumor response.
3.3. Immunomodulatory Role of CAFs
Advances in immune checkpoint inhibitors, such as PD-1 and CTLA-4, have brought much attention to the immune cell–tumor crosstalk; however, less is known about the contribution of stromal components to the immune milieu. Recent studies suggest CAFs mediate the tumor immune landscape via the secretion of various cytokines, growth factors, chemokines, exosomes, and other effector molecules, ultimately shaping an immunosuppressive tumor microenvironment, enabling cancer cells to evade immune surveillance, and limiting immunotherapy strategies.
In general, CAFs shape the tumor microenvironment by the production of proinflammatory cytokines, including IL-1β and IL-6 [21,120], and express the ligands CXCL12 [30], CXCL1 [121], and G-CSF [122] that can drive downstream immunosuppressive signaling pathways. For instance, CXCL12 regulates interactions between tumor cells and surrounding cells in the tumor microenvironment, promoting tumor survival, proliferation, angiogenesis, and metastasis. It also promotes the recruitment of immunosuppressive cells and their precursors, notably bone marrow mesenchymal stem cells and monocytes that differentiate into TAMs. Inflammatory CAFs, or iCAFs, highly express CXCL12, which binds to CXCR4 [52]. Blocking the CXCL12-CXCR4 interaction can induce cancer regression in pre-clinical models [30,123]. CAFs also interact with T cells, NK cells, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), mast cells, tumor-associated neutrophils (TANs) and TAMs in the tumor microenvironment, generally resulting in an immunopermissive environment.
CAFs prevent CD8+ cytotoxic T cell activity and recruitment within tumors, in part through TGF-β [124,125,126] and CXCL12 [127]. Both TGF-β c and CXCL12 are known to contribute to cytotoxic T cell exclusion by attenuation of the anti–PD-L1 response [30]. While limiting anti-tumor cytotoxic T cells, CAFs can also increase intratumoral Treg recruitment and scRNAseq revealed the upregulation of PD-1 and CTLA4 in Tregs. CAFs appear to attract, help accumulate, and promote the survival of FOXP3+ Tregs in human triple-negative breast cancer [84]. FoxP3+ Tregs are known to restrain host-antitumor-immunity, and thereby lend an unfavorable prognosis in a number of cancers. Although the precise mechanism of crosstalk between Tregs and CAFs remains unclear, high numbers of both cell types are found in stromal regions and are associated with low survival in cancers such as lung adenocarcinoma [128].
NK cells are well-known innate effector cells; however, their function can be impaired by CAFs through the inhibition of NK receptor activation, cytotoxic activity, and cytokine production [129,130]. Netrin G1 (NetG1) expression on CAFs can suppress the cytotoxic function of NK cells and support the survival of cancer cells in nutrient-deprived environments and is thus linked to a poor prognosis in cancers such as pancreatic ductal adenocarcinoma [131]. Tumor-infiltrating DCs are also critical to the anti-tumor immune response, and their functionality can similarly be impaired by CAFs. By activating the IL-6-mediated STAT3 pathway, CAFs in hepatocellular carcinoma transdifferentiated DCs into regulatory DCs (rDCs) that produced inhibitory cytokines and enzymes such as indoleamine 2,3-dioxygenase (IDO) [132]. VEGF produced by CAFs is also involved in the abnormal differentiation and impaired antigen presenting function of DCs via inhibition of NF-κB activation [133].
MDSCs can be also be recruited to the tumor microenvironment by CAFs via CCL2 [134], thereby suppressing CD8 T cell proliferation and IFN-γ production [135]. Mast cells, which can be both tumor-suppressing and tumor-promoting, can be recruited by CAFs via CXCL12 in a CXCR4-dependent manner [136]. In vitro, mast cells and CAFs can act together to induce the malignant transformation of benign epithelial cells [137]. Furthermore, N2, or protumorigenic polarization of neutrophils within the tumor can be induced through CAF-derived cardiotrophin-like cytokine factor 1 (CLCF1), which upregulates CXCL6 and TGF-β on tumor cells [138]. Neutrophils may also be directly recruited by CAFs through the secretion of CXCL12 or the expression of CXCR2, thus becoming TANs [139,140]. CAFs regulate the activation, survival, and function of TANs through the IL-6/STAT3-PDL1 signaling axis [139].
Like other cells in the tumor microenvironment, TAMs and CAFs have synergistic effects and are often detected in similar areas of tumor tissue. Their combined presence is a negative prognostic predictive indicator in human cancers [141]. Likewise, CAFs are involved in monocyte recruitment, macrophage differentiation, and polarization toward tumor-promoting, or an M2 phenotype [142,143], through the secretion of macrophage colony-stimulating factor 1 (M-CSF1), IL-6, CCL2 [144], and IL-8 [145]. M2 macrophages are reciprocally able to stimulate CAF activation through IL-6 and CXCL12 [142]. While research accumulates on the interactions of CAFs and immune cells in the tumor microenvironment, many ongoing questions remain unanswered. Undoubtedly, understanding CAF and immune cell interactions will provide the basis for novel strategies for targeted immunotherapies.
4. Targeting CAFs: Anti-Cancer Therapies
Significant advances have been made in CAF-targeted therapies in recent years. Predominantly, these methods aim to (1) directly or indirectly deplete CAFs, (2) reduce or eliminate the tumor-promoting and immunosuppressive functions of CAFs, or (3) normalize or reprogram CAFs to a more quiescent state. Those strategies are summarized here.
4.1. Chemotherapy Targeting CAFs
As discussed previously, FAP is expressed on subsets of CAFs in various tumors. FAP is a membrane-bound serine postprolyl peptidase that differs from other dipeptidyl prolyl peptidases in that it also has endopeptidase activity [146]. A competitive inhibitor of prolyl peptidase, Val-boroPro (Talabostat) is an oral drug that showed some tumor growth control by degrading ECM in mice [147]. However, in human clinical trials for metastatic colorectal cancers, no therapeutic efficacy was observed [148]. Sibrotuzumab is a humanized anti-FAP monoclonal antibody (clone F19) that inhibits the dipeptidyl peptidase activity of FAP [149]. Unlike F19, Sibrotuzumab did not demonstrate inhibitory activity and failed to suppress the growth of pancreatic cancers in patients, despite documented evidence of accumulation of the antibody in the tumor using a radiolabeled version of the antibody (iodine-131-labeled Sibrotuzumab) imaged by single photon emission computed tomography (SPECT) [150].
Taking advantage of the unique enzymatic activity of FAP, anti-CAF prodrugs or protoxins contain cytotoxic agents coupled with a dipeptide containing a FAP cleavage site [146,151]. These prodrugs remain inactive when systemically delivered and are proteolytically activated upon cleavage by FAP, which is expressed on CAFs in the tumor. Intratumoral injection of these prodrugs produced tumor lysis and growth inhibition in human breast and prostate cancer xenografts [146,151,152]. Another class of drugs are the immunotoxins that use an antibody to specifically deliver a toxin to the target cells. Anti-FAP-PE39 immunotoxin suppressed mammary tumor growth and increased the recruitment of tumor-infiltrating lymphocytes [153]. A monoclonal antibody conjugated with a tubulin binding drug maytrasinoid and a bispecific antibody simultaneously targeting FAP on CAFs and death receptor 5 on tumor cells has shown potent anti-tumor effects [153,154]. In another strategy, nanoparticles such as FAP-targeted liposomes have been explored as carriers to specifically deliver therapeutic drugs (e.g., doxorubicin, anti-Tenascin C) to CAFs [155,156] or to remodel the tumor microenvironment [157,158]. Despite the success of preclinical strategies, including substantially attenuating the growth of tumor xenografts in various cancer models with minimal to no toxicity [152,159,160,161], clinical translation is still in its early stages.
4.2. Immunotherapy
Various strategies to enhance immunity against FAP-expressing cells (i.e., CAFs) and to suppress cancer growth have been explored. Vaccination against FAP using dendritic cells transfected with FAP mRNA led to the suppressed growth of implanted and intravenously injected tumors [162]. The efficacy was enhanced when a co-vaccination against FAP and a tumor cell-associated antigen was used. These DC vaccines, synergistically combined with an anti-fibrotic agent, showed promising activation of both innate and adaptive immunity. Enhanced NK cell activity, anti-tumoral humoral immunity, and a cytotoxic CD8+ T cell response was observed in three different tumor models [162]. Similarly, adenoviral anti-FAP vaccines are able to selectively deplete CAFs by stimulating a CD8+ T cell response, leading to the inhibition of tumor growth and metastasis in several murine cancer models [163,164,165,166]. In a landmark study using a transgenic mouse expressing the diphtheria toxin receptor under the FAP promoter, depleting FAP+ CAFs by diphtheria toxin administration improved anti-cancer vaccination efficacy [167]. An orally administered anti-FAP DNA vaccine notably suppressed neoangiogenesis, tumor growth, and the metastasis of orthotopically injected breast carcinoma cells [163]. Adding doxorubicin substantially increased the intratumoral uptake of the drug and prolonged lifespans of vaccinated mice [168].
Adoptive chimeric antigen receptor (CAR)-T cell therapy can also be used to directly target CAFs [159,166,169]. FAP-specific CAR-T cells deplete most FAP+ cells, including CAFs, and restrict tumor stroma generation, resulting in the improved uptake and anti-tumor effects of chemotherapeutic drugs. Unfortunately, several studies have observed severe side effects using this approach, such as significant bone marrow toxicity and cachexia [170,171]. More selective and yet unknown targets may improve the precision of CAF-based therapies, which remains an active field of research [50].
Finally, near-infrared photoimmunotherapy (NIR-PIT) is an innovative approach to CAF depletion that has been used to directly and specifically deplete FAP-expressing cells in the tumor microenvironment. Tumor growth was inhibited using a co-culture xenograft model of human esophageal squamous cell carcinoma without adverse effects [172]. Anti-FAP+ CAF therapy combined with 5-fluorouracil (5-FU) could overcome chemoresistance compared with 5-FU alone [173].
4.3. Functional Modification/Reprogramming
Strategies that aim to revert activated CAFs to quiescence include the use of all-trans-retinoic acid (ATRA) [174,175,176], minnelide (which de-regulates the TGF-β signaling pathway) [177,178], and calcipotriol, a vitamin D receptor ligand [179,180]. The angiotensin receptor II antagonist losartan has been shown to decrease TGF-β-mediated activation of CAFs, reducing desmoplasia and increasing drug delivery and efficacy of immunotherapy [181,182,183]. Losartan, in combination with other traditional chemotherapies to treat pancreatic cancer, is currently under investigation in clinical trials [184]. Recent strategies seek to block immunosuppressive ligands of major CAF signaling pathways such as IL-6 [185,186], LIF [187], and TGF-β [124,126] in order to suppress or kill cancer cells.
The CXCL12/CXCR4 axis is important in cancer progression and immunosuppression. CXCL12 produced by CAFs recruits CXCR4-expressing endothelial progenitor cells and immune suppressive Tregs, which contributes to angiogenesis and tumor growth [45,127]. The abrogation of CXCR4 signaling in CAFs using the CXCR4 inhibitor plerixafor significantly reduced fibrosis, leading to vasculature normalization, increased cytotoxic T cell infiltration, decreased immunosuppressive cell populations, and increased checkpoint inhibitor efficacy [123]. Other strategies that inhibit CAF functions include TGF-β blockade [188], NFkB inhibitors to overcome chemotherapy resistance [189], and Smoothened (SMO) hedgehog pathway inhibitors (IPI-926) [190].
5. Future Perspectives
CAFs play an integral role in the promotion of tumor growth. However, the origin and functional roles of unique CAF subsets are yet to be fully understood, as well as their niche within various tumor types. Determining the spatial and temporal dynamics of CAFs and their cell-to-cell interactions in the tumor microenvironment will add critical information to our knowledge on these fascinating cells. While much of CAF biology has been modeled in vitro, it has been repeatedly demonstrated that CAFs in culture do not fully recapitulate the heterogeneity of CAFs in vivo [87,191,192]. Increasing the strategic use of animal models, including humanized and genetically engineered mouse models, is critical to further understanding the origin, plasticity, and phenotypes of CAFs over time. To this end, the future of the field will undoubtedly include new and emerging technologies such as fate mapping and scRNAseq to assess changes in both stromal cells and immune cells through tumor progression, digital or multiplex spatial profiling of proteins or RNA in tissue to assess spatial changes in the tumor microenvironment, spatial transcriptomics, digital pathology and three-dimensional tissue clearing, and 3D culture systems. Intravital microscopy will provide live visualization of cell-to-cell interactions in vivo. These technologies will bring breakthroughs, such as the identification of even more CAF subpopulations, their cellular interactions, and further insights into CAF heterogeneity and plasticity.
In addition to these analytic methods, a consensus on CAF biomarkers will need to be reached, so that similar phenotypes may be compared across tumor types and preclinical models used in different laboratories. Improving therapeutic delivery methods, such as targeted CAF therapy, rather than stromal-directed therapy, is now becoming more common. FAP shows promise as a CAF marker for CAR-targeted therapy. Recently emerged FAP imaging using gallium-68 labeled small-molecule FAP inhibitor (FAPI) as a tracer for positron emission tomography (PET) suggests superiority in detecting FAP+ cell-containing cancers in patients compared with [fluorine-18]fluoro-deoxy-glucose PET in various cancers [193]. FAPI PET could be used to predetermine the candidacy for anti-FAP CAF-targeted therapies as well as to evaluate the therapy efficacy.
6. Conclusions
This review summarizes key advances in CAF-directed therapies and highlights new techniques for the molecular targeting of CAFs. Although a dominant cell type in the tumor microenvironment, CAFs are difficult to precisely target for therapy because of their heterogeneity. These challenges must be overcome to have a meaningful impact from benchtop to clinical intervention. The origins of CAFs across cancer types remains elusive, as does the complete picture of subtypes and functional heterogeneity. Minimizing off target and systemic effects is an ongoing challenge. Finally, the combination of CAF immunotherapies with existing therapies may be valuable and remains an active area of investigation. These methods potentially add further insights to our knowledge of CAF biology but can also help improve precision cancer therapeutics and patient outcomes.
Author Contributions
R.A.G. and N.S. conceptualized and wrote the article. R.A.G. generated the tables and figure. R.A.G., N.S. and P.L.C. revised the article, tables, and figure. All authors have read and agreed to the published version of the manuscript.
Funding
This article was prepared with funding from the Intramural Research Programs of the National Cancer Institute at the National Institutes of Health, ZIA BC010657.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, E.; Wu, N.C.; Cadavid, J.L.; McGuigan, A.P. The life cycle of cancer-associated fibroblasts within the tumour stroma and its importance in disease outcome. Br. J. Cancer 2020, 122, 931–942. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Belhabib, I.; Zaghdoudi, S.; Lac, C.; Bousquet, C.; Jean, C. Extracellular matrices and cancer-associated fibroblasts: Targets for cancer diagnosis and therapy? Cancers 2021, 13, 3466. [Google Scholar] [CrossRef]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Pankova, D.; Chen, Y.; Terajima, M.; Schliekelman, M.J.; Baird, B.N.; Fahrenholtz, M.; Sun, L.; Gill, B.J.; Vadakkan, T.J.; Kim, M.P.; et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res. 2016, 14, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal stem or stromal cells: Toward a better understanding of their biology? Transfus. Med. Hemother. 2010, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Paunescu, V.; Bojin, F.M.; Tatu, C.A.; Gavriliuc, O.I.; Rosca, A.; Gruia, A.T.; Tanasie, G.; Bunu, C.; Crisnic, D.; Gherghiceanu, M.; et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differences. J. Cell Mol. Med. 2011, 15, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Helms, E.; Onate, M.K.; Sherman, M.H. Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 2020, 10, 648–656. [Google Scholar] [CrossRef]
- Norton, J.; Foster, D.; Chinta, M.; Titan, A.; Longaker, M. Pancreatic cancer associated fibroblasts (CAF): Under-explored target for pancreatic cancer treatment. Cancers 2020, 12, 1347. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Long, K.B.; Tooker, G.; Tooker, E.; Luque, S.L.; Lee, J.W.; Pan, X.; Beatty, G.L. IL6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 2017, 16, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Wang, G.; Wang, T.; Fu, B.; Zhang, Y.; Huang, L.; Deng, Y.; Chen, G.; Wu, X.; Chen, J.; et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in hepatocellular carcinoma. Int. J. Biol. Sci. 2020, 16, 2542–2558. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, B.; Hu, Q.; Qin, Y.; Xu, W.; Liu, W.; Yu, X.; Xu, J. The impact of cancer-associated fibroblasts on major hallmarks of pancreatic cancer. Theranostics 2018, 8, 5072–5087. [Google Scholar] [CrossRef] [PubMed]
- Lohr, M.; Schmidt, C.; Ringel, J.; Kluth, M.; Muller, P.; Nizze, H.; Jesnowski, R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001, 61, 550–555. [Google Scholar] [PubMed]
- Aoyagi, Y.; Oda, T.; Kinoshita, T.; Nakahashi, C.; Hasebe, T.; Ohkohchi, N.; Ochiai, A. Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br. J. Cancer 2004, 91, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFbeta signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Jeong, H.E.; Seo, H.; Cho, S.; Kim, K.; Na, D.; Chung, S.; Park, J.; Choi, N.; Kang, J.Y. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018, 76, 146–153. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef]
- Togo, S.; Polanska, U.M.; Horimoto, Y.; Orimo, A. Carcinoma-associated fibroblasts are a promising therapeutic target. Cancers 2013, 5, 149–169. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef]
- Arnold, J.N.; Magiera, L.; Kraman, M.; Fearon, D.T. Tumoral immune suppression by macrophages expressing fibroblast activation protein-alpha and heme oxygenase-1. Cancer Immunol. Res. 2014, 2, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Wang, L.S.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Farahani, R.M.; Xaymardan, M. Platelet-derived growth factor receptor alpha as a marker of mesenchymal stem cells in development and stem cell biology. Stem Cells Int. 2015, 2015, 362753. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Kitano, H.; Kageyama, S.; Hewitt, S.M.; Hayashi, R.; Doki, Y.; Ozaki, Y.; Fujino, S.; Takikita, M.; Kubo, H.; Fukuoka, J. Podoplanin expression in cancerous stroma induces lymphangiogenesis and predicts lymphatic spread and patient survival. Arch. Pathol. Lab. Med. 2010, 134, 1520–1527. [Google Scholar] [CrossRef]
- Astarita, J.L.; Acton, S.E.; Turley, S.J. Podoplanin: Emerging functions in development, the immune system, and cancer. Front. Immunol. 2012, 3, 283. [Google Scholar] [CrossRef]
- Kerrigan, A.M.; Navarro-Nuñez, L.; Pyz, E.; Finney, B.A.; Willment, J.A.; Watson, S.P.; Brown, G.D. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J. Thromb. Haemost. 2012, 10, 484–486. [Google Scholar] [CrossRef]
- Shindo, K.; Aishima, S.; Ohuchida, K.; Fujiwara, K.; Fujino, M.; Mizuuchi, Y.; Hattori, M.; Mizumoto, K.; Tanaka, M.; Oda, Y. Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas. Mol. Cancer 2013, 12, 168. [Google Scholar] [CrossRef]
- Augsten, M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front. Oncol. 2014, 4, 62. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Popova, S.N.; Brown, E.R.; Barsyte-Lovejoy, D.; Navab, R.; Shih, W.; Li, M.; Lu, M.; Jurisica, I.; Penn, L.Z.; et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11754–11759. [Google Scholar] [CrossRef] [PubMed]
- Blandin, A.F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 integrins as therapeutic targets to disrupt hallmarks of cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef] [PubMed]
- Navab, R.; Strumpf, D.; To, C.; Pasko, E.; Kim, K.S.; Park, C.J.; Hai, J.; Liu, J.; Jonkman, J.; Barczyk, M.; et al. Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene 2016, 35, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin. Cancer Biol. 2020, 62, 166–181. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463–479. [Google Scholar] [CrossRef]
- Simpkins, S.A.; Hanby, A.M.; Holliday, D.L.; Speirs, V. Clinical and functional significance of loss of caveolin-1 expression in breast cancer-associated fibroblasts. J. Pathol. 2012, 227, 490–498. [Google Scholar] [CrossRef]
- Chen, D.; Che, G. Value of caveolin-1 in cancer progression and prognosis: Emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (review). Oncol. Lett. 2014, 8, 1409–1421. [Google Scholar] [CrossRef]
- Shimizu, K.; Kirita, K.; Aokage, K.; Kojima, M.; Hishida, T.; Kuwata, T.; Fujii, S.; Ochiai, A.; Funai, K.; Yoshida, J.; et al. Clinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 321–328. [Google Scholar] [CrossRef]
- Scatena, C.; Fanelli, G.; Fanelli, G.N.; Menicagli, M.; Aretini, P.; Ortenzi, V.; Civitelli, S.P.; Innocenti, L.; Sotgia, F.; Lisanti, M.P.; et al. New insights in the expression of stromal caveolin 1 in breast cancer spread to axillary lymph nodes. Sci. Rep. 2021, 11, 2755. [Google Scholar] [CrossRef]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018, 172, 841–856.e16. [Google Scholar] [CrossRef]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Kisselbach, L.; Merges, M.; Bossie, A.; Boyd, A. CD90 Expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 2009, 59, 31–44. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Schliekelman, M.J.; Creighton, C.J.; Baird, B.N.; Chen, Y.; Banerjee, P.; Bota-Rabassedas, N.; Ahn, Y.H.; Roybal, J.D.; Chen, F.; Zhang, Y.; et al. Thy-1(+) cancer-associated fibroblasts adversely impact lung cancer prognosis. Sci. Rep. 2017, 7, 6478. [Google Scholar] [CrossRef] [PubMed]
- Sauzay, C.; Voutetakis, K.; Chatziioannou, A.; Chevet, E.; Avril, T. CD90/Thy-1, a cancer-associated cell surface signaling molecule. Front. Cell Dev. Biol. 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Hsia, L.T.; Ashley, N.; Ouaret, D.; Wang, L.M.; Wilding, J.; Bodmer, W.F. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl. Acad. Sci. USA 2016, 113, E2162–E2171. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2015, 28, 831–833. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef]
- Österreicher, C.H.; Penz-Österreicher, M.; Grivennikov, S.I.; Guma, M.; Koltsova, E.K.; Datz, C.; Sasik, R.; Hardiman, G.; Karin, M.; Brenner, D.A. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. USA 2011, 108, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Liu, X.; Kammertoens, T.; Blankenstein, T.; Qin, Z. Fibroblast-specific protein 1/S100A4-positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res. 2013, 73, 2770–2781. [Google Scholar] [CrossRef]
- De Wever, O.; Nguyen, Q.D.; Van Hoorde, L.; Bracke, M.; Bruyneel, E.; Gespach, C.; Mareel, M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004, 18, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Lowy, C.M.; Oskarsson, T. Tenascin C in metastasis: A view from the invasive front. Cell Adhes. Migr. 2015, 9, 112–124. [Google Scholar] [CrossRef]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adhes. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Planche, A.; Bacac, M.; Provero, P.; Fusco, C.; Delorenzi, M.; Stehle, J.C.; Stamenkovic, I. Identification of prognostic molecular features in the reactive stroma of human breast and prostate cancer. PLoS ONE 2011, 6, e18640. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Xu, X.E.; Wang, X.Q.; Cui, S.J.; Xu, L.L.; Jiang, Y.H.; Zhang, Y.; Yan, H.B.; Zhang, Q.; Qiao, J.; et al. Identification of colonic fibroblast secretomes reveals secretory factors regulating colon cancer cell proliferation. J. Proteom. 2014, 110, 155–171. [Google Scholar] [CrossRef]
- Qin, X.; Yan, M.; Zhang, J.; Wang, X.; Shen, Z.; Lv, Z.; Li, Z.; Wei, W.; Chen, W. TGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasis. Sci. Rep. 2016, 6, 20587. [Google Scholar] [CrossRef]
- Yu, B.; Wu, K.; Wang, X.; Zhang, J.; Wang, L.; Jiang, Y.; Zhu, X.; Chen, W.; Yan, M. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Jia, D.; Liu, Z.; Deng, N.; Tan, T.Z.; Huang, R.Y.; Taylor-Harding, B.; Cheon, D.J.; Lawrenson, K.; Wiedemeyer, W.R.; Walts, A.E.; et al. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016, 382, 203–214. [Google Scholar] [CrossRef]
- Nishishita, R.; Morohashi, S.; Seino, H.; Wu, Y.; Yoshizawa, T.; Haga, T.; Saito, K.; Hakamada, K.; Fukuda, S.; Kijima, H. Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol. Lett. 2018, 15, 6195–6202. [Google Scholar] [CrossRef]
- Vázquez-Villa, F.; García-Ocaña, M.; Galván, J.A.; García-Martínez, J.; García-Pravia, C.; Menéndez-Rodríguez, P.; González-del Rey, C.; Barneo-Serra, L.; de Los Toyos, J.R. COL11A1/(pro)collagen 11A1 expression is a remarkable biomarker of human invasive carcinoma-associated stromal cells and carcinoma progression. Tumour. Biol. 2015, 36, 2213–2222. [Google Scholar] [CrossRef]
- Gascard, P.; Tlsty, T.D. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016, 30, 1002–1019. [Google Scholar] [CrossRef]
- Barrett, R.; Pure, E. Cancer-associated fibroblasts: Key determinants of tumor immunity and immunotherapy. Curr. Opin. Immunol. 2020, 64, 80–87. [Google Scholar] [CrossRef]
- Bayer, S.V.; Grither, W.R.; Brenot, A.; Hwang, P.Y.; Barcus, C.E.; Ernst, M.; Pence, P.; Walter, C.; Pathak, A.; Longmore, G.D. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. Elife 2019, 8, e45508. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Grither, W.R.; Van Hove, S.; Biswas, H.; Ponik, S.M.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. Mechanical signals regulate and activate SNAIL1 protein to control the fibrogenic response of cancer-associated fibroblasts. J. Cell Sci. 2016, 129, 1989–2002. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Muller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwe, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef]
- Avery, D.; Govindaraju, P.; Jacob, M.; Todd, L.; Monslow, J.; Pure, E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018, 67, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Affo, S.; Nair, A.; Brundu, F.; Ravichandra, A.; Bhattacharjee, S.; Matsuda, M.; Chin, L.; Filliol, A.; Wen, W.; Song, X.; et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 2021, 39, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef]
- Givel, A.M.; Kieffer, Y.; Scholer-Dahirel, A.; Sirven, P.; Cardon, M.; Pelon, F.; Magagna, I.; Gentric, G.; Costa, A.; Bonneau, C.; et al. miR200-regulated CXCL12beta promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat. Commun. 2018, 9, 1056. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, G.; Yang, Y.; Wang, F.; Xiao, Y.T.; Zhang, N.; Bian, X.; Zhu, Y.; Yu, Y.; Liu, F.; et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 2021, 23, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lovrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Valdés-Mora, F.; Salomon, R.; Gloss, B.S.; Law, A.M.K.; Venhuizen, J.; Castillo, L.; Murphy, K.J.; Magenau, A.; Papanicolaou, M.; Rodriguez de la Fuente, L.; et al. Single-cell transcriptomics reveals involution mimicry during the specification of the basal breast cancer subtype. Cell Rep. 2021, 35, 108945. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.; Levi-Galibov, O.; David, E.; Bornstein, C.; Giladi, A.; Dadiani, M.; Mayo, A.; Halperin, C.; Pevsner-Fischer, M.; Lavon, H.; et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nat. Cancer. 2020, 1, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Tan, Z.W.; Zhu, P.; Tan, N.S. Cancer-associated fibroblasts in tumor microenvironment-Accomplices in tumor malignancy. Cell Immunol. 2019, 343, 103729. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef]
- Vickman, R.E.; Faget, D.V.; Beachy, P.; Beebe, D.; Bhowmick, N.A.; Cukierman, E.; Deng, W.M.; Granneman, J.G.; Hildesheim, J.; Kalluri, R.; et al. Deconstructing tumor heterogeneity: The stromal perspective. Oncotarget 2020, 11, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Okamoto, O.K. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015, 2015, 868475. [Google Scholar] [CrossRef]
- Kim, O.H.; Kang, G.H.; Noh, H.; Cha, J.Y.; Lee, H.J.; Yoon, J.H.; Mamura, M.; Nam, J.S.; Lee, D.H.; Kim, Y.A.; et al. Proangiogenic TIE2(+)/CD31 (+) macrophages are the predominant population of tumor-associated macrophages infiltrating metastatic lymph nodes. Mol. Cells 2013, 36, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X. Transforming growth factor-beta signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef] [PubMed]
- Goulet, C.R.; Champagne, A.; Bernard, G.; Vandal, D.; Chabaud, S.; Pouliot, F.; Bolduc, S. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling. BMC Cancer 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, F.; Cui, J.Y.; Chen, L.; Chen, Y.T.; Liu, B.W. CAFs enhance paclitaxel resistance by inducing EMT through the IL6/JAK2/STAT3 pathway. Oncol. Rep. 2018, 39, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Huber, R.M.; Lucas, J.M.; Gomez-Sarosi, L.A.; Coleman, I.; Zhao, S.; Coleman, R.; Nelson, P.S. DNA damage induces GDNF secretion in the tumor microenvironment with paracrine effects promoting prostate cancer treatment resistance. Oncotarget 2015, 6, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Hemann, M.T. DNA damage-mediated induction of a chemoresistant niche. Cell 2010, 143, 355–366. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Mu, L.; Huang, K.; Li, X.; Tao, D.; Wu, Y.; Qin, J. Fibroblast-derived exosomes contribute to chemoresistance through priming cancer stem cells in colorectal cancer. PLoS ONE 2015, 10, e0125625. [Google Scholar] [CrossRef] [PubMed]
- Peiris-Pages, M.; Sotgia, F.; Lisanti, M.P. Chemotherapy induces the cancer-associated fibroblast phenotype, activating paracrine Hedgehog-GLI signalling in breast cancer cells. Oncotarget 2015, 6, 10728–10745. [Google Scholar] [CrossRef]
- Tang, Y.A.; Chen, Y.F.; Bao, Y.; Mahara, S.; Yatim, S.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef]
- Garvey, C.M.; Lau, R.; Sanchez, A.; Sun, R.X.; Fong, E.J.; Doche, M.E.; Chen, O.; Jusuf, A.; Lenz, H.J.; Larson, B.; et al. Anti-EGFR Therapy induces egf secretion by cancer-associated fibroblasts to confer colorectal cancer chemoresistance. Cancers 2020, 12, 1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Head, R.; Liu, J.; Ruzinova, M.B.; Lim, K.H. Tumor-stroma IL1β-IRAK4 feedforward circuitry drives tumor fibrosis, chemoresistance, and poor prognosis in pancreatic cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef]
- Ishibashi, M.; Neri, S.; Hashimoto, H.; Miyashita, T.; Yoshida, T.; Nakamura, Y.; Udagawa, H.; Kirita, K.; Matsumoto, S.; Umemura, S.; et al. CD200-positive cancer associated fibroblasts augment the sensitivity of epidermal growth factor receptor mutation-positive lung adenocarcinomas to EGFR tyrosine kinase inhibitors. Sci. Rep. 2017, 7, 46662. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Tan, Y.; Wei, Q.; Yu, W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal 2019, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Ansems, M.; Span, P.N. The tumor microenvironment and radiotherapy response; a central role for cancer-associated fibroblasts. Clin. Transl. Radiat. Oncol. 2020, 22, 90–97. [Google Scholar] [CrossRef]
- Ragunathan, K.; Upfold, N.L.E.; Oksenych, V. Interaction between fibroblasts and immune cells following DNA damage induced by ionizing radiation. Int. J. Mol. Sci. 2020, 21, 8635. [Google Scholar] [CrossRef]
- Li, D.; Qu, C.; Ning, Z.; Wang, H.; Zang, K.; Zhuang, L.; Chen, L.; Wang, P.; Meng, Z. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am. J. Cancer Res. 2016, 6, 2192–2206. [Google Scholar]
- Wang, Y.; Gan, G.; Wang, B.; Wu, J.; Cao, Y.; Zhu, D.; Xu, Y.; Wang, X.; Han, H.; Li, X.; et al. Cancer-associated fibroblasts promote irradiated cancer cell recovery through autophagy. EBioMedicine 2017, 17, 45–56. [Google Scholar] [CrossRef]
- Yang, N.; Lode, K.; Berzaghi, R.; Islam, A.; Martinez-Zubiaurre, I.; Hellevik, T. Irradiated tumor fibroblasts avoid immune recognition and retain immunosuppressive functions over natural killer cells. Front. Immunol. 2020, 11, 602530. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 2018, 49, 178–193.e7. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Owens, P.; Gorska, A.E.; Chytil, A.; Ye, F.; Shi, C.; Weaver, V.M.; Kalluri, R.; Moses, H.L.; Novitskiy, S.V. Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells. Cancer Immunol. Res. 2017, 5, 718–729. [Google Scholar] [CrossRef]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ishii, G.; Hiraoka, N.; Hirayama, S.; Yamauchi, C.; Aokage, K.; Hishida, T.; Yoshida, J.; Nagai, K.; Ochiai, A. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013, 104, 409–415. [Google Scholar] [CrossRef]
- Balsamo, M.; Scordamaglia, F.; Pietra, G.; Manzini, C.; Cantoni, C.; Boitano, M.; Queirolo, P.; Vermi, W.; Facchetti, F.; Moretta, A.; et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 20847–20852. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yi, S.; Liu, W.; Jia, C.; Wang, G.; Hua, X.; Tai, Y.; Zhang, Q.; Chen, G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med. Oncol. 2013, 30, 663. [Google Scholar] [CrossRef]
- Francescone, R.; Barbosa Vendramini-Costa, D.; Franco-Barraza, J.; Wagner, J.; Muir, A.; Lau, A.N.; Gabitova, L.; Pazina, T.; Gupta, S.; Luong, T.; et al. Netrin G1 promotes pancreatic tumorigenesis through cancer-associated fibroblast-driven nutritional support and immunosuppression. Cancer Discov. 2021, 11, 446–479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Deng, Y.N.; Yi, H.M.; Wang, G.Y.; Fu, B.S.; Chen, W.J.; Liu, W.; Tai, Y.; Peng, Y.W.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Hodi, F.S. The intersection between tumor angiogenesis and immune suppression. Clin. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017, 32, 654–668.e5. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hwang, R.F.; Logsdon, C.D.; Ullrich, S.E. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 2013, 73, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Lister, N.L.; Hashimoto, K.; Teng, L.; Flandes-Iparraguirre, M.; Eder, A.; Sanchez-Herrero, A.; Niranjan, B. Tissue engineered human prostate microtissues reveal key role of mast cell-derived tryptase in potentiating cancer-associated fibroblast (CAF)-induced morphometric transition in vitro. Biomaterials 2019, 197, 72–85. [Google Scholar] [CrossRef]
- Song, M.; He, J.; Pan, Q.Z.; Yang, J.; Zhao, J.; Zhang, Y.J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-associated fibroblast-mediated cellular crosstalk supports hepatocellular carcinoma progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 422. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Herrera, M.; Herrera, A.; Domínguez, G.; Silva, J.; García, V.; García, J.M.; Gómez, I.; Soldevilla, B.; Muñoz, C.; Provencio, M.; et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013, 104, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-de-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Brannon, A.L.; Del Vecchio, A.; Garcia, P.E.; Penny, M.K.; Kane, K.T.; Vinta, A.; Buckanovich, R.J.; di Magliano, M.P. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia 2016, 18, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Ameen, Z.; Collins, A.; Wojcik, S.; Mair, M.; Young, G.S.; Fuchs, J.R.; Eubank, T.D.; Frankel, W.L.; Bekaii-Saab, T.; et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013, 73, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10, 273. [Google Scholar] [CrossRef]
- LeBeau, A.M.; Brennen, W.N.; Aggarwal, S.; Denmeade, S.R. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 2009, 8, 1378–1386. [Google Scholar] [CrossRef]
- Adams, S.; Miller, G.T.; Jesson, M.I.; Watanabe, T.; Jones, B.; Wallner, B.P. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res. 2004, 64, 5471–5480. [Google Scholar] [CrossRef]
- Narra, K.; Mullins, S.R.; Lee, H.O.; Strzemkowski-Brun, B.; Magalong, K.; Christiansen, V.J.; McKee, P.A.; Egleston, B.; Cohen, S.J.; Weiner, L.M.; et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol. Ther. 2007, 6, 1691–1699. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; al-Batran, S.E.; Hartmann, F.; Hartung, G.; Jager, D.; Renner, C.; Tanswell, P.; Kunz, U.; Amelsberg, A.; Kuthan, H.; et al. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 2003, 26, 44–48. [Google Scholar] [CrossRef]
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.-T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer1. Clin. Cancer Res. 2003, 9, 1639–1647. [Google Scholar]
- Huang, S.; Fang, R.; Xu, J.; Qiu, S.; Zhang, H.; Du, J.; Cai, S. Evaluation of the tumor targeting of a FAPα-based doxorubicin prodrug. J. Drug Target 2011, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein–expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xiao, L.; Joo, K.I.; Liu, Y.; Zhang, C.; Liu, S.; Conti, P.S.; Li, Z.; Wang, P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int. J. Cancer 2016, 138, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hu, B.; Li, S.; Zhang, C.; Liu, Y.; Wang, P. A multi-antigen vaccine in combination with an immunotoxin targeting tumor-associated fibroblast for treating murine melanoma. Mol. Ther. Oncolytics 2016, 3, 16007. [Google Scholar] [CrossRef] [PubMed]
- Tansi, F.L.; Rüger, R.; Böhm, C.; Steiniger, F.; Kontermann, R.E.; Teichgraeber, U.K.; Fahr, A.; Hilger, I. Activatable bispecific liposomes bearing fibroblast activation protein directed single chain fragment/Trastuzumab deliver encapsulated cargo into the nuclei of tumor cells and the tumor microenvironment simultaneously. Acta Biomater. 2017, 54, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Rabenhold, M.; Steiniger, F.; Fahr, A.; Kontermann, R.E.; Rüger, R. Bispecific single-chain diabody-immunoliposomes targeting endoglin (CD105) and fibroblast activation protein (FAP) simultaneously. J. Control. Release 2015, 201, 56–67. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.; Sun, J.; Song, Q.; He, B.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine 2016, 12, 131–141. [Google Scholar] [CrossRef]
- Truffi, M.; Mazzucchelli, S.; Bonizzi, A.; Sorrentino, L.; Allevi, R.; Vanna, R.; Morasso, C.; Corsi, F. Nano-strategies to target breast cancer-associated fibroblasts: Rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci. 2019, 20, 1263. [Google Scholar] [CrossRef]
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 2008, 14, 4584–4592. [Google Scholar] [CrossRef]
- Cheng, J.D.; Dunbrack, R.L., Jr.; Valianou, M.; Rogatko, A.; Alpaugh, R.K.; Weiner, L.M. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model1. Cancer Res. 2002, 62, 4767–4772. [Google Scholar]
- Brünker, P.; Wartha, K.; Friess, T.; Grau-Richards, S.; Waldhauer, I.; Koller, C.F.; Weiser, B.; Majety, M.; Runza, V.; Niu, H.; et al. RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers FAP-dependent, avidity-driven DR5 hyperclustering and tumor cell apoptosis. Mol. Cancer Ther. 2016, 15, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Fassnacht, M.; Nair, S.; Boczkowski, D.; Gilboa, E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005, 65, 11156–11163. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Kruger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef]
- Zhang, Y.; Ertl, H.C. Depletion of FAP+ cells reduces immunosuppressive cells and improves metabolism and functions CD8+T cells within tumors. Oncotarget 2016, 7, 23282–23299. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, F.F.; Geng, F.; Liu, C.L.; Xu, P.; Lu, Z.Z.; Yu, B.; Wu, H.; Wu, J.X.; Zhang, H.H.; et al. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4T1 murine breast cancer model. Cancer Immunol. Immunother. 2016, 65, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R.; Lei-Rossmann, J.; Muntzer, A.; Scott, E.M.; Hagel, J.; Campo, L.; Bryant, R.J.; Verrill, C.; Lambert, A.; et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018, 78, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830. [Google Scholar] [CrossRef]
- Geng, F.; Bao, X.; Dong, L.; Guo, Q.-Q.; Guo, J.; Xie, Y.; Zhou, Y.; Yu, B.; Wu, H.; Wu, J.-X.; et al. Doxorubicin pretreatment enhances FAPα/survivin co-targeting DNA vaccine anti-tumor activity primarily through decreasing peripheral MDSCs in the 4T1 murine breast cancer model. Oncoimmunology 2020, 9, 1747350. [Google Scholar] [CrossRef]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Roberts, E.W.; Deonarine, A.; Jones, J.O.; Denton, A.E.; Feig, C.; Lyons, S.K.; Espeli, M.; Kraman, M.; McKenna, B.; Wells, R.J.; et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013, 210, 1137–1151. [Google Scholar] [CrossRef]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.C.; Restifo, N.P.; Rosenberg, S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013, 210, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Noma, K.; Ohara, T.; Kashima, H.; Sato, H.; Kato, T.; Urano, S.; Katsube, R.; Hashimoto, Y.; Tazawa, H.; et al. Photoimmunotherapy for cancer-associated fibroblasts targeting fibroblast activation protein in human esophageal squamous cell carcinoma. Cancer Biol. Ther. 2019, 20, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Katsube, R.; Noma, K.; Ohara, T.; Nishiwaki, N.; Kobayashi, T.; Komoto, S.; Sato, H.; Kashima, H.; Kato, T.; Kikuchi, S.; et al. Fibroblast activation protein targeted near infrared photoimmunotherapy (NIR PIT) overcomes therapeutic resistance in human esophageal cancer. Sci. Rep. 2021, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Ene-Obong, A.; Clear, A.J.; Watt, J.; Wang, J.; Fatah, R.; Riches, J.C.; Marshall, J.F.; Chin-Aleong, J.; Chelala, C.; Gribben, J.G.; et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013, 145, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Froeling, F.E.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011, 141, 1486–1497. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Xu, Y.; Zhao, X.; Zhang, Y.; Yang, X.; Wang, Y.; Zhao, R.; Anderson, G.J.; Zhao, Y.; et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018, 9, 3390. [Google Scholar] [CrossRef]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef]
- Dauer, P.; Zhao, X.; Gupta, V.K.; Sharma, N.; Kesh, K.; Gnamlin, P.; Dudeja, V.; Vickers, S.M.; Banerjee, S.; Saluja, A. Inactivation of cancer-associated-fibroblasts disrupts oncogenic signaling in pancreatic cancer cells and promotes its regression. Cancer Res. 2018, 78, 1321–1333. [Google Scholar] [CrossRef]
- Ding, N.; Yu, R.T.; Subramaniam, N.; Sherman, M.H.; Wilson, C.; Rao, R.; Leblanc, M.; Coulter, S.; He, M.; Scott, C.; et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Chen, I.X.; Tong, R.; Ng, M.R.; Martin, J.D.; Naxerova, K.; Wu, M.W.; Huang, P.; Boucher, Y.; Kohane, D.S.; et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 10674–10680. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2561. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncol. 2019, 5, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G.; et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018, 67, 320–332. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, W.; Collins, M.A.; Bednar, F.; Rakshit, S.; Zetter, B.R.; Stanger, B.Z.; Chung, I.; Rhim, A.D.; di Magliano, M.P. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013, 73, 6359–6374. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019, 569, 131–135. [Google Scholar] [CrossRef]
- Grauel, A.L.; Nguyen, B.; Ruddy, D.; Laszewski, T.; Schwartz, S.; Chang, J.; Chen, J.; Piquet, M.; Pelletier, M.; Yan, Z.; et al. TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat. Commun. 2020, 11, 6315. [Google Scholar] [CrossRef]
- Godwin, P.; Baird, A.M.; Heavey, S.; Barr, M.P.; O’Byrne, K.J.; Gately, K. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Front. Oncol. 2013, 3, 120. [Google Scholar] [CrossRef]
- Steele, N.G.; Biffi, G.; Kemp, S.B.; Zhang, Y.; Drouillard, D.; Syu, L.; Hao, Y.; Oni, T.E.; Brosnan, E.; Elyada, E.; et al. Inhibition of hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2023–2037. [Google Scholar] [CrossRef]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., 2nd; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Waise, S.; Parker, R.; Rose-Zerilli, M.J.J.; Layfield, D.M.; Wood, O.; West, J.; Ottensmeier, C.H.; Thomas, G.J.; Hanley, C.J. An optimised tissue disaggregation and data processing pipeline for characterising fibroblast phenotypes using single-cell RNA sequencing. Sci. Rep. 2019, 9, 9580. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).