P-Glycoprotein Activity at Diagnosis Does Not Predict Therapy Outcome and Survival in Canine B-Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.1.1. Biochemistry and Hematology Examinations
2.1.2. Evaluating Adverse Drug Effects
2.1.3. Lymph Node and Bone Marrow Sample Collection and Preparation
2.2. Histopathology and Immunohistochemistry
2.3. Calcein AM Assay
2.4. FACS Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
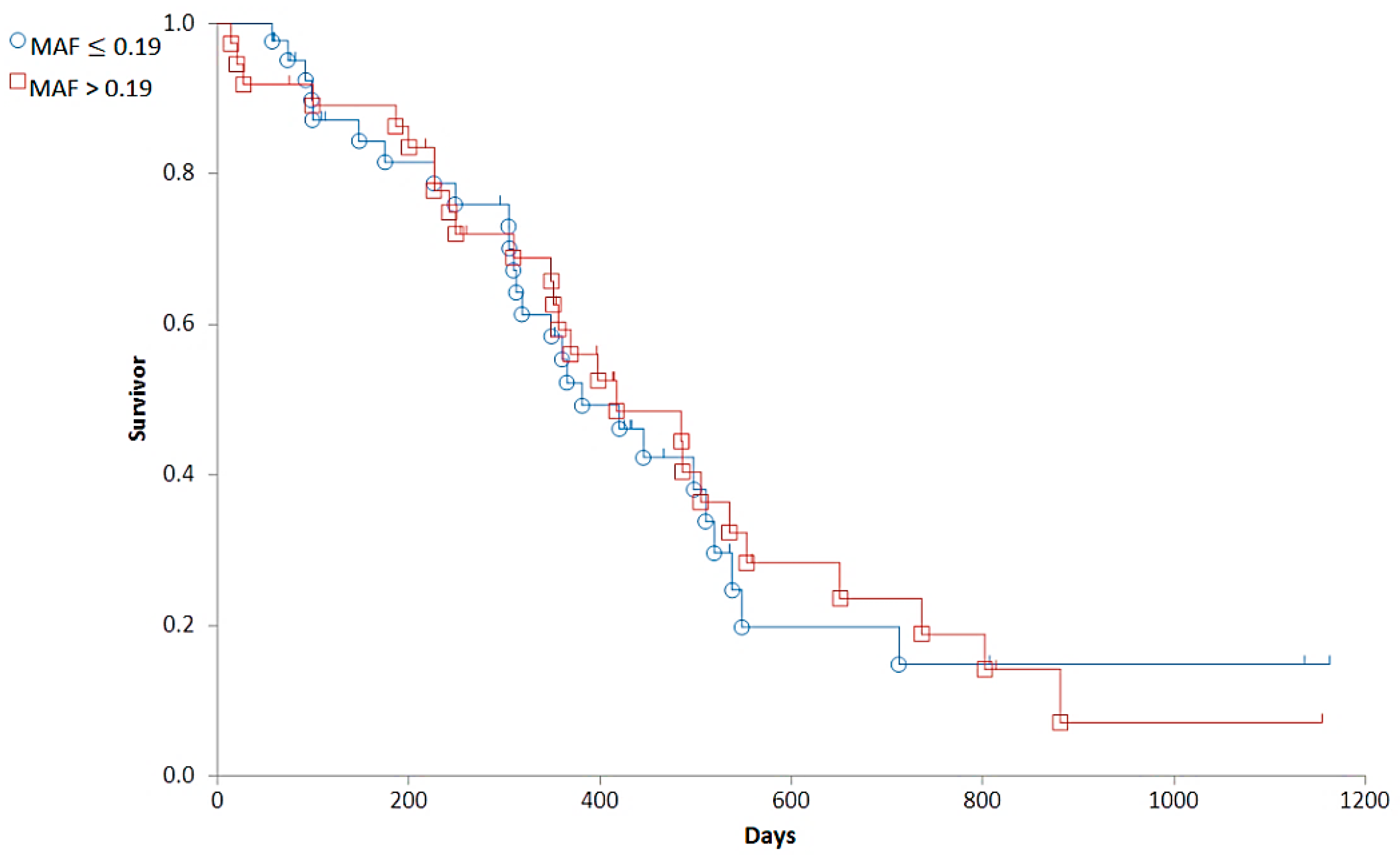
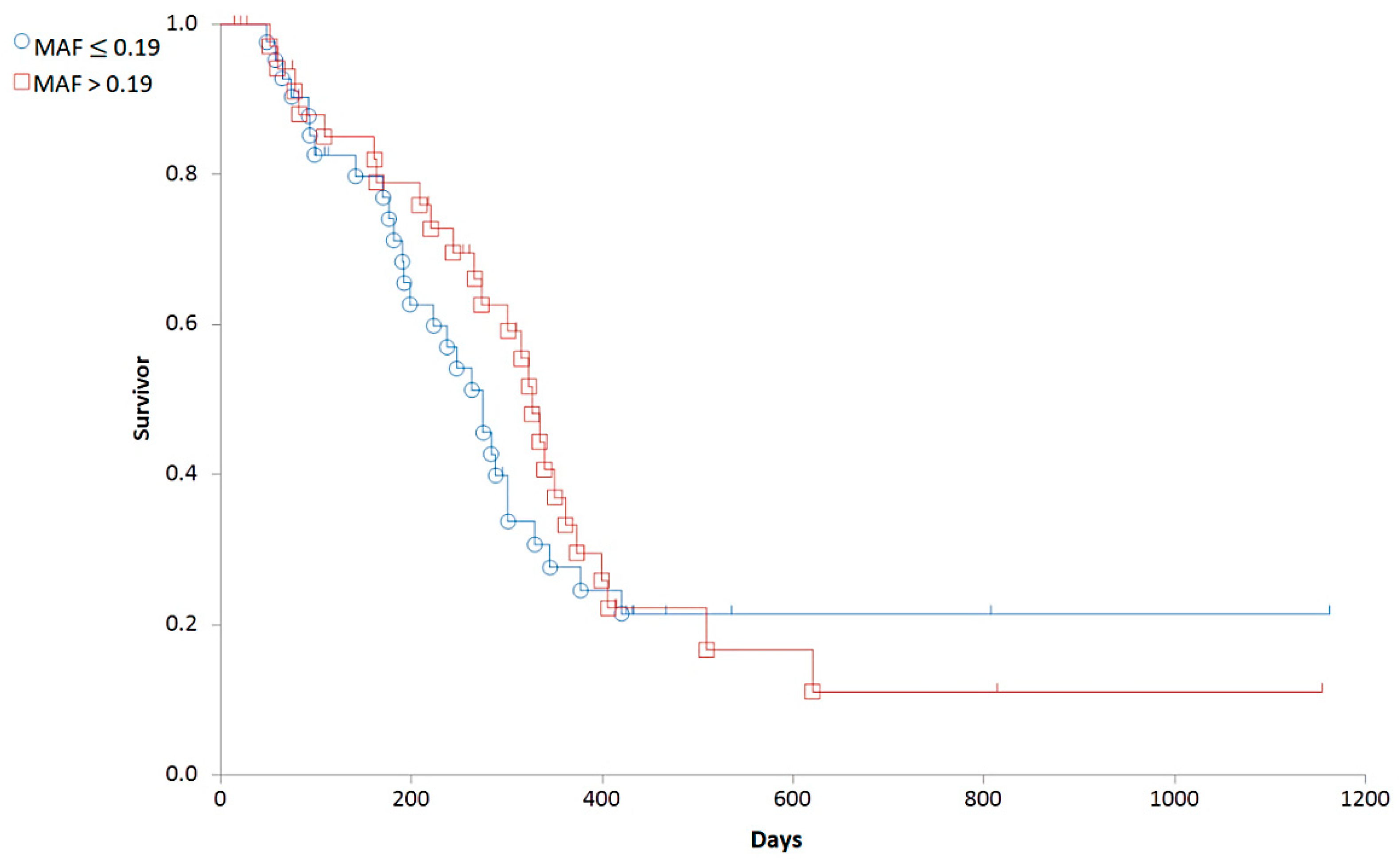
3.2. Correlations between Clinical Stages and Survival Times
3.3. Evaluation of P-gp Activity
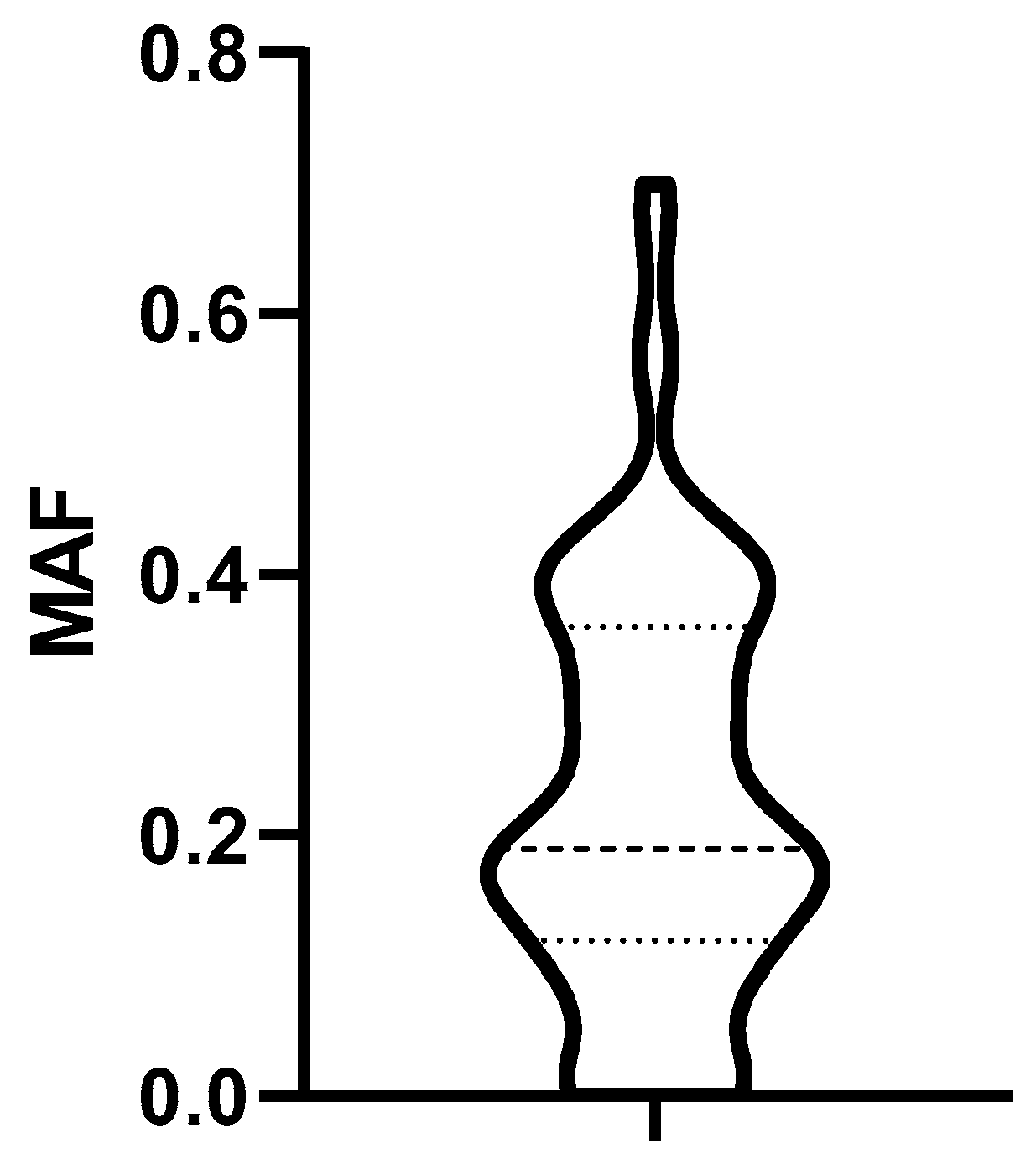
3.3.1. Determination of the Cut off MAF Value
3.3.2. Correlations between MAF and Clinical Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q 2016, 36, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; WB Saunders: Philadelphia, PA, USA, 2019; pp. 688–712. [Google Scholar] [CrossRef]
- Richards, K.L.; Suter, S.E. Man’s best friend: What can pet dogs teach us about non-Hodgkin’s lymphoma? Immunol. Rev. 2015, 263, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Frantz, A.M.; Modiano, J.F. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: Recent progress and applications. Vet. Immunol. Immunopathol. 2014, 159, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Gelain, M.E.; Comazzi, S. The dog as a possible animal model for human non-Hodgkin lymphoma: A review. Hematol. Oncol. 2013, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; San Myint, M.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2011, 48, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Vezzali, E.; Parodi, A.L.; Marcato, P.S.; Bettini, G. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet. Comp. Oncol. 2010, 8, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Ginn, P.E. Immunohistochemical detection of P-glycoprotein in formalin-fixed and paraffin-embedded normal and neoplastic canine tissues. Vet. Pathol. 1996, 33, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E. Mechanisms of Drug Resistance in Veterinary Oncology- A Review with an Emphasis on Canine Lymphoma. Vet. Sci. 2015, 12, 150–184. [Google Scholar] [CrossRef]
- Lage, H. An overview of cancer multidrug resistance: A still unsolved problem. Cell. Mol. Life Sci. 2008, 65, 3145–3167. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.P.; Cordon-Cardo, C. On the origins of clinical drug resistance. Semin. Cancer Biol. 1991, 2, 227–233. [Google Scholar] [PubMed]
- Virkel, G.; Ballent, M.; Lanusse, C.; Lifschitz, A. Role of ABC Transporters in Veterinary Medicine: Pharmaco- Toxicological Implications. Curr. Med. Chem. 2019, 26, 1251–1269. [Google Scholar] [CrossRef]
- Szakács, G.; Paterson, J.; Ludwig, J.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Allen, J.D.; Brinkhuis, R.F.; van Deemter, L.; Wijnholds, J.; Schinkel, A.H. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000, 60, 5761–5766. [Google Scholar] [PubMed]
- Nooter, K.; Herweijer, H. Multidrug resistance (mdr) genes in human cancer. Br. J. Cancer 1991, 63, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.; Fojo, T.; Bates, S. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.P.; Hsu, W.L.; Chiu, C.Y.; Wong, M.L.; Chang, S.C. Overexpression of P-glycoprotein, STAT3, phospho-STAT3 and KIT in spontaneous canine cutaneous mast cell tumours before and after prednisolone treatment. Vet. J. 2012, 193, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Hwang, Y.H.; Im, K.S.; Kim, J.H.; Chon, S.K.; Kim, H.Y.; Sur, J.H. P-glycoprotein expression in canine mammary gland tumours related with myoepithelial cells. Res. Vet. Sci. 2012, 93, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Hifumi, T.; Miyoshi, N.; Kawaguchi, H.; Nomura, K.; Yasuda, N. Immunohistochemical detection of proteins associated with multidrug resistance to anti-cancer drugs in canine and feline primary pulmonary carcinoma. J. Vet. Med. Sci. 2010, 72, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E.; Schrickx, J.A. Multi-drug resistance in a canine lymphoid cell line due to increased P-glycoprotein expression, a potential model for drug-resistant canine lymphoma. Toxicol. In Vitro 2014, 28, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Hughes, C.S.; Fine, R.L.; Page, R.L. P-glycoprotein expression in canine lymphoma: A relevant, intermediate model of multidrug resistance. Cancer 1996, 77, 1892–1898. [Google Scholar] [CrossRef]
- Dhaliwal, R.S.; Kitchell, B.E.; Ehrhart, E.; Valli, V.E.; Dervisis, N.G. Clinicopathologic significance of histologic grade, P-gp, and p53 expression in canine lymphoma. J. Am. Anim. Hosp. Assoc. 2013, 49, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, J.; Urbańska, K.; Giziński, S.; Zabielska, K.; Lechowski, R. Immunohistochemical detection of P-glycoprotein in various subtypes of canine lymphomas. Pol. J. Vet. Sci. 2015, 18, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.S.; Leveille, C.R.; Reimann, K.A.; Shu, H.; Arias, I.M. The expression of P-glycoprotein in canine lymphoma and its association with multidrug resistance. Cancer Investig. 1995, 13, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Zandvliet, M.; Teske, E.; Schrickx, J.A.; Mol, J.A. A longitudinal study of ABC transporter expression in canine multicentric lymphoma. Vet. J. 2015, 205, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Schleis, S.E.; LeBlanc, A.K.; Neilsen, N.R.; LeBlanc, C.J. Flow cytometric evaluation of multidrug resistance proteins on grossly normal canine nodal lymphocyte membranes. Am. J. Vet. Res. 2008, 69, 1310–1315. [Google Scholar] [CrossRef]
- Kosztyu, P.; Dolezel, P.; Vaclavikova, R.; Mlejnek, P. Can the assessment of ABCB1 gene expression predict its function in vitro? Eur. J. Haematol. 2015, 95, 150–159. [Google Scholar] [CrossRef]
- Karászi, E.; Jakab, K.; Homolya, L.; Szakács, G.; Holló, Z.; Telek, B.; Kiss, A.; Rejtô, L.; Nahajevszky, S.; Sarkadi, B.; et al. Calcein assay for multidrug resistance reliably predicts therapy response and survival rate in acute myeloid leukaemia. Br. J. Haematol. 2001, 112, 308–314. [Google Scholar] [CrossRef]
- Sonneveld, P.; List, A.F. Chemotherapy resistance in acute myeloid leukaemia. Best Pract. Res. Clin. Haematol. 2001, 14, 211–233. [Google Scholar] [CrossRef]
- Legrand, O.; Simonin, G.; Beauchamp-Nicoud, A.; Zittoun, R.; Marie, J.P. Simultaneous activity of MRP1 and P-gp is correlated with in vitro resistance to daunorubicin and with in vivo resistance in adult acute myeloid leukemia. Blood 1999, 94, 1046–1056. [Google Scholar] [CrossRef]
- Jakab, K.; Gopcsa, L.; Adam, E.; Domjan, G.; Sarkadi, B.; Paloczi, K. Application of flow cytometry immunophenotyping and multidrug resistance assay in B-cell acute lymphoid leukemia and multiple myeloma. Neoplasma 2005, 52, 36–42. [Google Scholar]
- Vajdovich, P.; Koltai, Z.; Dékay, V.; Kungl, K.; Harnos, A. Evaluation of P-gp (MDR1) immunohistochemistry in canine lymphoma—prognostic and clinical aspects. Acta Vet. Hung. 2018, 66, 309–328. [Google Scholar] [CrossRef]
- Bergman, P.J. Multidrug resistance. In Kirk’s Current Veterinary Therapy XIII: Small Animal Practice, 13th ed.; Bonagura, J.D., Ed.; WB Saunders: Philadelphia, PA, USA, 2000; pp. 479–482. [Google Scholar]
- Karai, E.; Szebényi, K.; Windt, T.; Fehér, S.; Szendi, E.; Dékay, V.; Vajdovich, P.; Szakács, G.; Füredi, A. Celecoxib Prevents Doxorubicin-Induced Multidrug Resistance in Canine and Mouse Lymphoma Cell Lines. Cancers 2020, 12, 1117. [Google Scholar] [CrossRef]
- Zandvliet, M.; Teske, E.; Chapuis, T.; Fink-Gremmels, J.; Schrickx, J.A. Masitinib reverses doxorubicin resistance in canine lymphoid cells by inhibiting the function of P-glycoprotein. J. Vet. Pharmacol. Ther. 2013, 36, 583–587. [Google Scholar] [CrossRef]
- Ma, L.; Pratt, S.E.; Cao, J.; Dantzig, A.H.; Moore, R.E.; Slapak, C.A. Identification and characterization of the canine multidrug resistance-associated protein. Mol. Cancer Ther. 2002, 1, 1335–1342. [Google Scholar]
- Tomiyasu, H.; Tsujimoto, H. Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma. Vet. Sci. 2015, 2, 185–205. [Google Scholar] [CrossRef]
- Flatland, B.; Freeman, K.P.; Vap, L.M.; Harr, K.E.; ASVCP. ASVCP guidelines: Quality assurance for point-of-care testing in veterinary medicine. Vet. Clin. Pathol. 2013, 42, 405–423. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary cooperative oncology group—common terminology criteria for adverse events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Kiupel, M.; Teske, E.; Bostock, D. Prognostic factors for treated canine malignant lymphoma. Vet. Pathol. 1999, 36, 292–300. [Google Scholar] [CrossRef]
- Harris, N.L.; Jaffe, E.S.; Stein, H.; Banks, P.M.; Chan, J.K.; Cleary, M.L.; Delsol, D.; De Wolf-Peeters, C.; Falini, B.; Gatter, K.C.; et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994, 84, 1361–1392. [Google Scholar] [CrossRef]
- Valli, V.E.; Kass, P.H.; San Myint, M.; Scott, F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 2013, 50, 738–748. [Google Scholar] [CrossRef]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Tarasova, N.I.; Seth, R.; Tarasov, S.G.; Kosakowska-Cholody, T.; Hrycyna, C.A.; Gottesman, M.M.; Michejda, C.J. Transmembrane inhibitors of P-glycoprotein, an ABC transporter. J. Med. Chem. 2005, 48, 3768–3775. [Google Scholar] [CrossRef]
- Holló, Z.; Homolya, L.; Hegedûs, T.; Müller, M.; Szakács, G.; Jakab, K.; Antal, F.; Sarkadi, B. Parallel functional and immunological detection of human multidrug resistance proteins, P-glycoprotein and MRP1. Anticancer Res. 1998, 18, 2981–2987. [Google Scholar]
- Holló, Z.; Homolya, L.; Davis, C.W.; Sarkadi, B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim. Biophys. Acta. 1994, 1191, 384–388. [Google Scholar] [CrossRef]
- Poggi, A.; Miniscalco, B.; Morello, E.; Gattino, F.; Delaude, A.; Ferrero Poschetto, L.; Aresu, L.; Gelain, M.E.; Martini, V.; Comazzi, S.; et al. Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma. Vet. Comp. Oncol. 2017, 15, 431–440. [Google Scholar] [CrossRef]
- Rout, E.D.; Labadie, J.D.; Yoshimoto, J.A.; Avery, P.R.; Curran, K.M.; Avery, A.C. Clinical outcome and prognostic factors in dogs with B-cell chronic lymphocytic leukemia: A retrospective study. J. Vet. Intern. Med. 2021, 35, 1918–1928. [Google Scholar] [CrossRef]
- Benjamin, S.E.; Sorenmo, K.U.; Krick, E.L.; Salah, P.; Walsh, K.A.; Weinstein, N.M.; Keuler, N.S.; Avery, A.C.; Atherton, M.J.; Lenz, J.A. Response-based modification of CHOP chemotherapy for canine B-cell lymphoma. Vet. Comp. Oncol. 2021, 19, 541–550. [Google Scholar] [CrossRef]
- Rassnick, K.M.; McEntee, M.C.; Erb, H.N.; Burke, B.P.; Balkman, C.E.; Flory, A.B.; Kiselow, M.A.; Autio, K.; Gieger, T.L. Comparison of 3 Protocols for treatment after induction of remission in dogs with lymphoma. J. Vet. Intern. Med. 2017, 21, 1364–1373. [Google Scholar] [CrossRef]
- Poźniak, B.; Pawlak, A.; Obmińska-Mrukowicz, B. Flow cytometric assessment of P-glycoprotein and multidrug resistance-associated protein activity and expression in canine lymphoma. In Vivo 2015, 29, 149–153. [Google Scholar]
- Culmsee, K.; Gruber, A.D.; von Samson-Himmelstjerna, G.; Nolte, I. Quantification of MDR-1 gene expression in canine tissues by real-time reverse transcription quantitative polymerase chain reaction. Res. Vet. Sci. 2004, 77, 223–229. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef] [PubMed]
- Homolya, L.; Holló, M.; Müller, M.; Mechetner, E.B.; Sarkadi, B. A new method for a quantitative assessment of P-glycoprotein-related multidrug resistance in tumour cells. Br. J. Cancer 1996, 73, 849–855. [Google Scholar] [CrossRef]
- Palacios-Ortega, J.; Rivera-de-Torre, E.; Gavilanes, J.G.; Slotte, J.P.; Martínez-Del-Pozo, Á.; García-Linares, S. Biophysical approaches to study actinoporin-lipid interactions. Methods Enzymol. 2021, 649, 307–339. [Google Scholar] [CrossRef]
- Abbaci, M.; Barberi-Heyob, M.; Blondel, W.; Guillemin, F.; Didelon, J. Advantages and limitations of commonly used methods to assay the molecular permeability of gap junctional intercellular communication. Biotechniques 2008, 45, 3–52. [Google Scholar] [CrossRef]
- Fonseca, P.C.; Nihei, O.K.; Urban-Maldonado, M.; Abreu, S.; Carvalho, A.C.; Spray, D.D.; Savino, W.; Alves, L.A. Characterization of connexin 30.3 and 43 in thymocytes. Immunology 2004, 94, 65–75. [Google Scholar] [CrossRef]
- Wang, S.L.; Lee, J.J.; Liao, A.T. Chemotherapy-induced neutropenia is associated with prolonged remission duration and survival time in canine lymphoma. Vet. J. 2015, 205, 69–73. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical perspective of FDA approved drugs with P-glycoprotein inhibition activities for potential cancer therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef]
- Gramer, I.; Kessler, M.; Geyer, J. Determination of MDR1 gene expression for prediction of chemotherapy tolerance and treatment outcome in dogs with lymphoma. Vet. Comp. Oncol. 2015, 13, 363–372. [Google Scholar] [CrossRef]
- Bansal, T.; Jaggi, M.; Khar, R.K.; Talegaonkar, S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 2009, 12, 46–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.B. Drugs as P-glycoprotein substrates, inhibitors, and inducers. Drug. Metab. Rev. 2002, 34, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Hoshino, H.; Wang, J.; Ono, S.; Iida, Y.; Hata, K.; Huang, S.K.; Colquhoun, S.; Hoon, D.S. MicroRNA-93 activates c-Met/PI3K/ Akt pathway activity in hepatocellular carcinoma by directly inhibiting PTEN and CDKN1A. Oncotarget 2015, 6, 3211–3224. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Nieth, C.; Lage, H. Induction of the ABC-transporters Mdr1/P-gp (Abcb1), mrpl (Abcc1), and bcrp (Abcg2) during establishment of multidrug resistance following exposure to mitoxantrone. J. Chemother. 2005, 17, 215–223. [Google Scholar] [CrossRef]
- Grandjean, F.; Brémaud, L.; Robert, J.; Ratinaud, M.H. Alterations in the expression of cytochrome c oxidase subunits in doxorubicin-resistant leukemia K562 cells. Biochem. Pharmacol. 2002, 63, 823–831. [Google Scholar] [CrossRef]



| Chemotherapy | Additional Therapy | |
|---|---|---|
| Week 1 | Doxorubicin (30 mg/m2 iv.) | Prednisone (2 mg/kgbw/day po.) |
| Week 2 | Vincristine (0.75 mg/m2 iv.) | Predinsone (1.5 mg/kgbw/day po.) |
| Week 3 | Vincristine (0.75 mg/m2 iv.) | Prednisone (1 mg/kgbw/day po.) |
| Week 4 | Vincristine (0.75 mg/m2 iv.) Cyclophosphamide (250 mg/m2 po.) | Prednisone (0.5 mg/kgbw/day po.) |
| Week 5 | Vincristine (0.75 mg/m2 iv.) | |
| Week 6 | Vincristine (0.75 mg/m2 iv.) | |
| Week 7 | Vincristine (0.75 mg/m2 iv.) Cyclophosphamide (250 mg/m2 po.) | |
| Week 8 | Vincristine (0.75 mg/m2 iv.) | |
| Week 9 | Vincristine (0.75 mg/m2 iv.) | |
| Week 10 | Doxorubicin (30 mg/m2 iv.) | |
| Week 11 | Vincristine (0.75 mg/m2 iv.) | |
| Week 12 | Vincristine (0.75 mg/m2 iv.) | |
| Week 13 | Vincristine (0.75 mg/m2 iv.) Cyclophosphamide (250 mg/m2 po.) | |
| Week 14 | Vincristine (0.75 mg/m2 iv.) | |
| Week 15 | Vincristine (0.75 mg/m2 iv.) | |
| Week 16 | Vincristine (0.75 mg/m2 iv.) Cyclophosphamide (250 mg/m2 po.) | |
| Week 17 | Vincristine (0.75 mg/m2 iv.) | |
| Week 18 | Vincristine (0.75 mg/m2 iv.) | |
| Week 19 | Doxorubicin (30 mg/m2 iv.) |
| Chemotherapy | Additional Therapy | |
|---|---|---|
| Week 1 | L-Asparaginase (400 IU/kgbw im.) Vincristine (0.75 mg/m2 iv.) | Prednisone (2 mg/kgbw/day po.) |
| Week 2 | Cyclophosphamide (250 mg/m2 po.) | Predinsone (1.5 mg/kgbw/day po.) |
| Week 3 | Vincristine (0.75 mg/m2 iv.) | Prednisone (1 mg/kgbw/day po.) |
| Week 4 | Doxorubicin (30 mg/m2 iv.) | Prednisone (0.5 mg/kgbw/day po.) |
| Week 5 | No medication | |
| Week 6 | Vincristine (0.75 mg/m2 iv.) | |
| Week 7 | Cyclophosphamide (250 mg/m2 po.) | |
| Week 8 | Vincristine (0.75 mg/m2 iv.) | |
| Week 9 | Doxorubicin (30 mg/m2 iv.) | |
| Week 10 | No medication | |
| Week 11 | Vincristine (0.75 mg/m2 iv.) | |
| Week 12 | No medication | |
| Week 13 | Cyclophosphamide (250 mg/m2 po.) | |
| Week 14 | No medication | |
| Week 15 | Vincristine (0.75 mg/m2 iv.) | |
| Week 16 | No medication | |
| Week 17 | Doxorubicin (30 mg/m2 iv.) | |
| Week 18 | No medication | |
| Week 19 | Vincristine (0.75 mg/m2 iv.) | |
| Week 20 | No medication | |
| Week 21 | Cyclophosphamide (250 mg/m2 po.) | |
| Week 22 | No medication | |
| Week 23 | Vincristine (0.75 mg/m2 iv.) | |
| Week 24 | No medication | |
| Week 25 | Doxorubicin (30 mg/m2 iv.) |
| Stage | II + III | IV | V | p-Values |
|---|---|---|---|---|
| Number | 13 | 52 | 14 | |
| Observed deaths | 9 | 31 | 12 | |
| Extent of exposure to risk of death | 8.602 | 36.705 | 6.693 | |
| Relative rate | 1.046 | 0.845 | 1.793 | |
| Median OST | 417 | 505 | 309 | |
| II + III vs. IV | 0.5159 | |||
| II + III vs. V | 0.2356 | |||
| IV vs. V | 0.0233 |
| Stage | II + III | IV | V | p-Values |
|---|---|---|---|---|
| Number | 13 | 52 | 14 | |
| Observed deaths | 9 | 32 | 12 | |
| Extent of exposure to risk of death | 10.863 | 36.629 | 5.506 | |
| Relative rate | 0.828 | 0.874 | 2.179 | |
| Median RFP | 345 | 301 | 190 | |
| II + III vs. IV | 0.8815 | |||
| II + III vs. V | 0.0206 | |||
| IV vs. V | 0.0062 |
| Stage II + III | Stage IV | Stage V | |
|---|---|---|---|
| Number | 13 | 52 | 14 |
| Mean MAF | 0.205 | 0.236 | 0.233 |
| SD | ±0.109 | ±0.175 | ±0.133 |
| p-value | One way ANOVA global test: p = 0.8231 | ||
| MAF | Substage “a” | Substage “b” |
|---|---|---|
| Number | 58 | 21 |
| Mean | 0.235 | 0.216 |
| Standard deviation | 0.164 | 0.149 |
| p-value | One way ANOVA, global test: p = 0.6559 | |
| Variable (1) | Variable (2) | Pearson R-Value | Linear Regression, Kendall’s Rank Correlation Coefficient | p-Value |
|---|---|---|---|---|
| MAF | Grade of side effects | −0.3347 | −0.258 | 0.0025 |
| MAF | Slipped drug administration time due to side effect | −0.2734 | −0.264 | 0.0052 |
| OST | Grade of side effects | −0.3019 | −0.235 | 0.0052 |
| RFP | Grade of side effects | −0.2232 | 0.214 | 0.0111 |
| Observed Parameters | Group 1 | Group 2 | Group 3 | p-Value |
|---|---|---|---|---|
| Slipped drug administration time | ||||
| Occasion/Number | 12/34 | 17/29 | 4/16 | |
| % | 35.3 | 58.6 | 25.0 | |
| Group 1 vs. Group 2 | 0.0641 | |||
| Group 1 vs. Group 3 | 0.4667 | |||
| Group 2 vs. Group 3 | 0.0304 | |||
| Dogs with dose reduction | ||||
| Occasion/Number | 6/34 | 8/29 | 3/16 | |
| % | 17.64 | 27.58 | 18.75 | |
| Group 1 vs. Group 2 | 0.344254 | |||
| Group 1 vs. Group 3 | 0.924557 | |||
| Group 2 vs. Group 3 | 0.509106 | |||
| Grade of side effects | Group 1 | Group 2 | Group 3 | p-value |
| Number | 34 | 29 | 16 | |
| Mean | 1.38 | 2.21 | 1.13 | |
| ± SD | 1.26 | 1.35 | 0.78 | |
| Group 1 vs. Group 2 | 0.0173 | |||
| Group 1 vs. Group 3 | 0.3925 | |||
| Group 2 vs. Group 3 | 0.0018 | |||
| Dogs with anorexia | ||||
| Occasion/Number | 18/34 | 21/29 | 9/16 | |
| % | 52.94 | 72.41 | 56.25 | |
| Group 1 vs. Group 2 | 0.1126 | |||
| Group 1 vs. Group 3 | 0.8266 | |||
| Group 2 vs. Group 3 | 0.2708 |
| Relapse during the First 19 Weeks | Group 1 | Group 2 | Group 3 | p-Value |
|---|---|---|---|---|
| Occasion/Number | 4/34 | 14/29 | 1/16 | |
| % | 11.7 | 48.3 | 6.25 | |
| Group 1 vs. Group 2 | 0.0014 | |||
| Group 1 vs. Group 3 | 0.5443 | |||
| Group 2 vs. Group 3 | 0.0042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dékay, V.; Karai, E.; Füredi, A.; Szebényi, K.; Szakács, G.; Vajdovich, P. P-Glycoprotein Activity at Diagnosis Does Not Predict Therapy Outcome and Survival in Canine B-Cell Lymphoma. Cancers 2022, 14, 3919. https://doi.org/10.3390/cancers14163919
Dékay V, Karai E, Füredi A, Szebényi K, Szakács G, Vajdovich P. P-Glycoprotein Activity at Diagnosis Does Not Predict Therapy Outcome and Survival in Canine B-Cell Lymphoma. Cancers. 2022; 14(16):3919. https://doi.org/10.3390/cancers14163919
Chicago/Turabian StyleDékay, Valéria, Edina Karai, András Füredi, Kornélia Szebényi, Gergely Szakács, and Péter Vajdovich. 2022. "P-Glycoprotein Activity at Diagnosis Does Not Predict Therapy Outcome and Survival in Canine B-Cell Lymphoma" Cancers 14, no. 16: 3919. https://doi.org/10.3390/cancers14163919
APA StyleDékay, V., Karai, E., Füredi, A., Szebényi, K., Szakács, G., & Vajdovich, P. (2022). P-Glycoprotein Activity at Diagnosis Does Not Predict Therapy Outcome and Survival in Canine B-Cell Lymphoma. Cancers, 14(16), 3919. https://doi.org/10.3390/cancers14163919






