Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
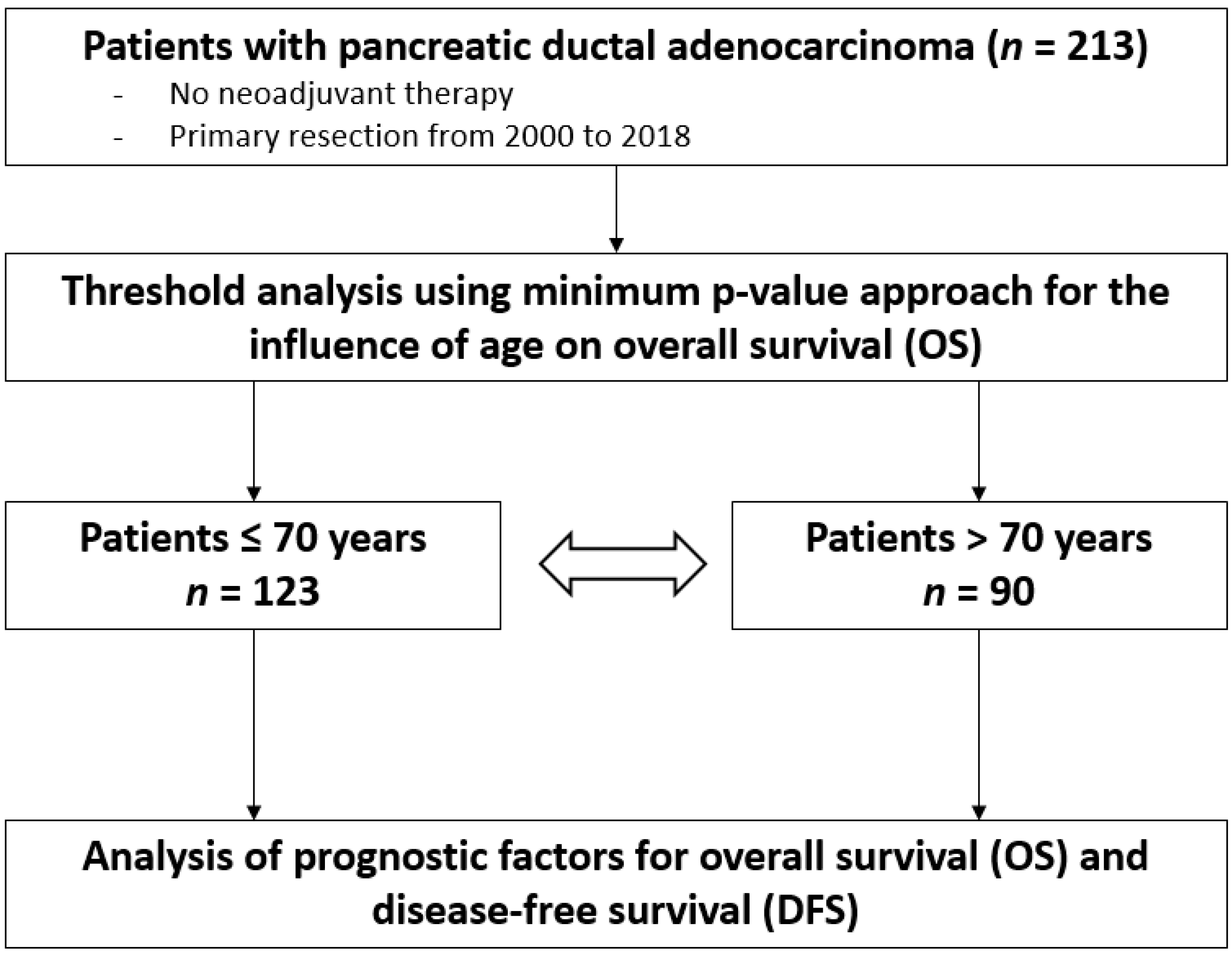
2. Patients and Methods
2.1. Study Design
2.2. Surgical Procedures
2.3. Adjuvant Chemotherapy and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Surgical and Histopathological Details
3.3. Short-Term Postoperative Outcome Parameters
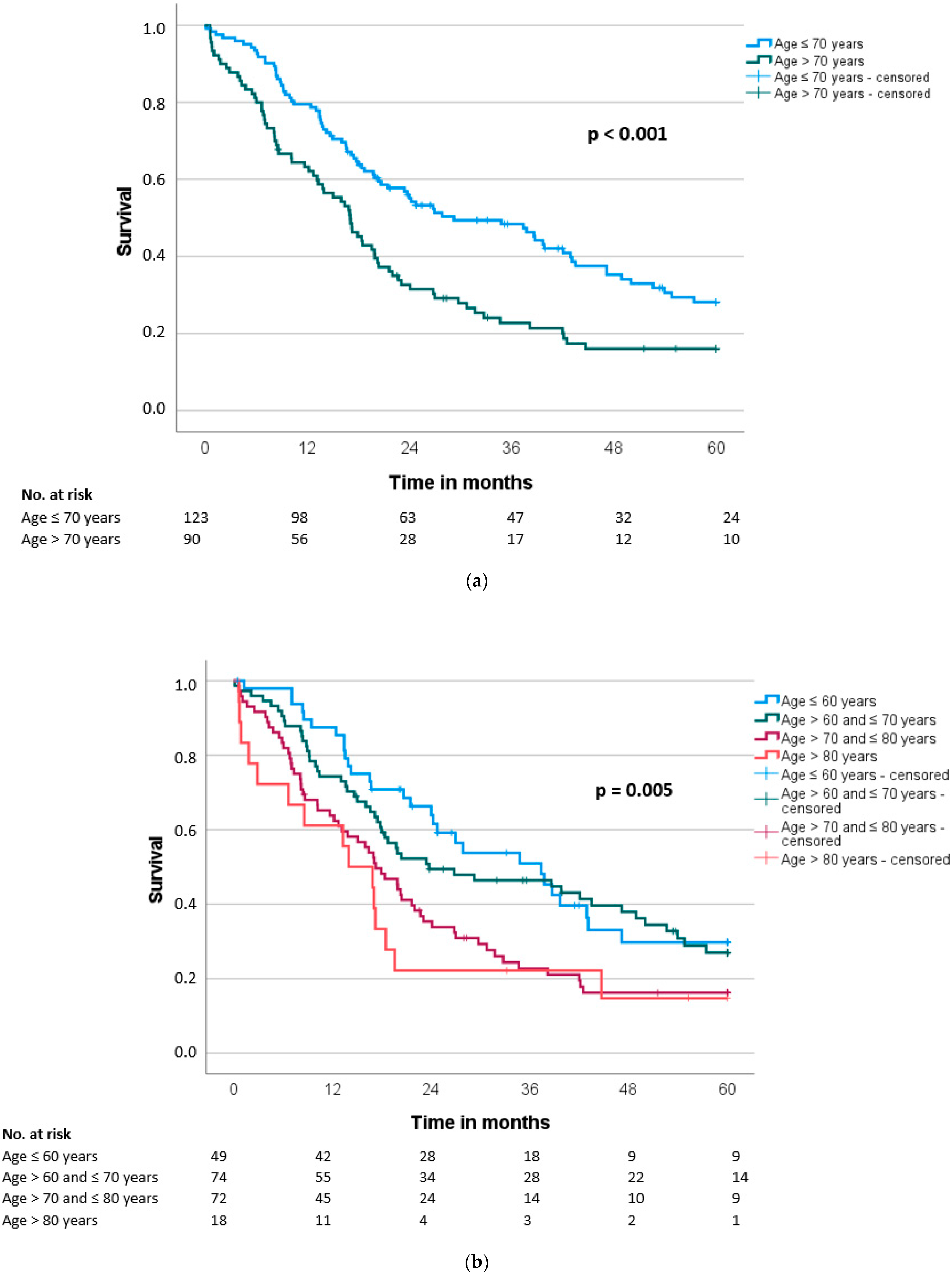
3.4. Overall and Disease-Free Survival
3.5. Prognostic Factors for Overall and Disease-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. The global burden of pancreatic cancer. Arch. Med. Sci. AMS 2020, 16, 820. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Higuera, O.; Ghanem, I.; Nasimi, R.; Prieto, I.; Koren, L.; Feliu, J. Management of pancreatic cancer in the elderly. World J. Gastroenterol. 2016, 22, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Cameron, J.L.; He, J. Two thousand consecutive pancreaticoduodenectomies. J. Am. Coll. Surg. 2015, 220, 530–536. [Google Scholar] [CrossRef]
- Gooiker, G.; Lemmens, V.; Besselink, M.; Busch, O.; Bonsing, B.; Molenaar, I.Q.; Tollenaar, R.; De Hingh, I.; Wouters, M. Impact of centralization of pancreatic cancer surgery on resection rates and survival. J. Br. Surg. 2014, 101, 1000–1005. [Google Scholar] [CrossRef]
- Kaman, L.; Chakarbathi, K.; Gupta, A.; Dahiya, D.; Singh, K.; Ramavath, K.; Behera, A.; Kajal, K. Impact of enhanced recovery after surgery protocol on immediate surgical outcome in elderly patients undergoing pancreaticoduodenectomy. Updates Surg. 2019, 71, 653–657. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Winter, J.M.; Schafer, M.; Cunningham, S.C.; Cameron, J.L.; Yeo, C.J.; Clavien, P.-A. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann. Surg. 2006, 244, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. Impact of patient age on the postoperative survival in pancreatic head cancer. Ann. Surg. Oncol. 2017, 24, 3220–3228. [Google Scholar] [CrossRef]
- Nimptsch, U.; Krautz, C.; Weber, G.F.; Mansky, T.; Grützmann, R. Nationwide in-hospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann. Surg. 2016, 264, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Winter, J.M.; Cameron, J.L.; Campbell, K.A.; Chang, D.; Cunningham, S.C.; Riall, T.S.; Yeo, C.J. Pancreaticoduodenectomy in the very elderly. J. Gastrointest. Surg. 2006, 10, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sclabas, G.; Lombardo, K.R.; Sarr, M.G.; Nagorney, D.; Kendrick, M.L.; Donohue, J.H.; Que, F.G.; Farnell, M.B. Pancreatoduodenectomy for ductal adenocarcinoma in the very elderly; is it safe and justified? J. Gastrointest. Surg. 2010, 14, 1826–1831. [Google Scholar]
- Turrini, O.; Paye, F.; Bachellier, P.; Sauvanet, A.; Cunha, A.S.; Le Treut, Y.; Adham, M.; Mabrut, J.; Chiche, L.; Delpero, J. Pancreatectomy for adenocarcinoma in elderly patients: Postoperative outcomes and long term results: A study of the French Surgical Association. Eur. J. Surg. Oncol. 2013, 39, 171–178. [Google Scholar] [CrossRef]
- Eguchi, H.; Yamaue, H.; Unno, M.; Mizuma, M.; Hamada, S.; Igarashi, H.; Kuroki, T.; Satoi, S.; Shimizu, Y.; Tani, M. Clinicopathological characteristics of young patients with pancreatic cancer: An analysis of data from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2016, 45, 1411–1417. [Google Scholar] [CrossRef]
- van Dongen, J.C.; van der Geest, L.G.; de Meijer, V.E.; van Santvoort, H.C.; de Vos-Geelen, J.; Besselink, M.G.; Groot Koerkamp, B.; Wilmink, J.W.; van Eijck, C.H.; Group, D.P.C. Age and prognosis in patients with pancreatic cancer: A population-based study. Acta Oncol. 2022, 61, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Oguro, S.; Shimada, K.; Kishi, Y.; Nara, S.; Esaki, M.; Kosuge, T. Perioperative and long-term outcomes after pancreaticoduodenectomy in elderly patients 80 years of age and older. Langenbeck’s Arch. Surg. 2013, 398, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; DiNorcia, J.; Reavey, P.L.; Holden, M.M.; Genkinger, J.M.; Lee, J.A.; Schrope, B.A.; Chabot, J.A.; Allendorf, J.D. Pancreaticoduodenectomy can be performed safely in patients aged 80 years and older. J. Gastrointest. Surg. 2010, 14, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Marmor, S.; Burke, E.E.; Virnig, B.A.; Jensen, E.H.; Tuttle, T.M. A comparative analysis of survival outcomes between pancreatectomy and chemotherapy for elderly patients with adenocarcinoma of the pancreas. Cancer 2016, 122, 3378–3385. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Pal, S. The International Study Group of Pancreatic Surgery definition of delayed gastric emptying and the effects of various surgical modifications on the occurrence of delayed gastric emptying after pancreatoduodenectomy. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 353–363. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Federal Statistical Office. Federal Health Reporting Germany. Available online: https://www.gbe-bund.de/gbe/pkg_olap_tables.prc_sort_ind?p_uid=gast&p_aid=9024890&p_sprache=D&p_help=2&p_indnr=524&p_ansnr=79815113&p_version=3&p_sortorder=d&p_dim_1=D.000&p_dw_1=112370 (accessed on 19 July 2022).
- Tani, M.; Kawai, M.; Hirono, S.; Ina, S.; Miyazawa, M.; Nishioka, R.; Shimizu, A.; Uchiyama, K.; Yamaue, H. A pancreaticoduodenectomy is acceptable for periampullary tumors in the elderly, even in patients over 80 years of age. J. Hepato-Biliary-Pancreat. Surg. 2009, 16, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Riall, T.S.; Reddy, D.M.; Nealon, W.H.; Goodwin, J.S. The effect of age on short-term outcomes after pancreatic resection: A population-based study. Ann. Surg. 2008, 248, 459–467. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Han, S.; Jin, D.; Xu, X.; Kuang, T.; Wu, W.; Wang, D. Prognostic effect of age in resected pancreatic cancer patients: A propensity score matching analysis. Front. Oncol. 2022, 12, 789351. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, E.; Fan, Z.; Birkmeyer, J.D. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J. Am. Coll. Surg. 2007, 205, 729–734. [Google Scholar] [CrossRef]
- Ordonez, J.E.; Hester, C.A.; Zhu, H.; Augustine, M.; Porembka, M.R.; Wang, S.C.; Yopp, A.C.; Mansour, J.C.; Zeh, H.J.; Polanco, P.M. Clinicopathologic features and outcomes of early-onset pancreatic adenocarcinoma in the United States. Ann. Surg. Oncol. 2020, 27, 1997–2006. [Google Scholar] [CrossRef]
- Sho, M.; Murakami, Y.; Kawai, M.; Motoi, F.; Satoi, S.; Matsumoto, I.; Honda, G.; Uemura, K.; Yanagimoto, H.; Kurata, M. Prognosis after surgical treatment for pancreatic cancer in patients aged 80 years or older: A multicenter study. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Lee, J.H.; Choi, J.K.; Hwang, H.K.; Chung, J.U.; Lee, W.J.; Kwon, K.H. Can we recommend surgical treatment to the octogenarian with periampullary cancer?: National database analysis in South Korea. Eur. J. Cancer 2021, 144, 81–90. [Google Scholar] [CrossRef]
- Furbetta, N.; Comandatore, A.; Gianardi, D.; Palmeri, M.; Di Franco, G.; Guadagni, S.; Caprili, G.; Bianchini, M.; Fatucchi, L.M.; Picchi, M. Perioperative Nutritional Aspects in Total Pancreatectomy: A Comprehensive Review of the Literature. Nutrients 2021, 13, 1765. [Google Scholar] [CrossRef]
- Kim, E.; Kang, J.S.; Han, Y.; Kim, H.; Kwon, W.; Kim, J.R.; Kim, S.-W.; Jang, J.-Y. Influence of preoperative nutritional status on clinical outcomes after pancreatoduodenectomy. HPB 2018, 20, 1051–1061. [Google Scholar] [CrossRef]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Hilal, M.A. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef]
- White, M.N.; Dotan, E.; Catalano, P.J.; Cardin, D.B.; Berlin, J.D. Advanced pancreatic cancer clinical trials: The continued underrepresentation of older patients. J. Geriatr. Oncol. 2019, 10, 540–546. [Google Scholar] [CrossRef]
- Hayman, T.J.; Strom, T.; Springett, G.M.; Balducci, L.; Hoffe, S.E.; Meredith, K.L.; Hodul, P.; Malafa, M.; Shridhar, R. Outcomes of resected pancreatic cancer in patients age≥ 70. J. Gastrointest. Oncol. 2015, 6, 498–504. [Google Scholar]
- Nagrial, A.M.; Chang, D.K.; Nguyen, N.Q.; Johns, A.L.; Chantrill, L.A.; Humphris, J.L.; Chin, V.T.; Samra, J.S.; Gill, A.J.; Pajic, M. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br. J. Cancer 2014, 110, 313–319. [Google Scholar] [CrossRef]
- Perri, G.; Prakash, L.; Qiao, W.; Varadhachary, G.R.; Wolff, R.; Fogelman, D.; Overman, M.; Pant, S.; Javle, M.; Koay, E.J. Response and survival associated with first-line FOLFIRINOX vs gemcitabine and nab-paclitaxel chemotherapy for localized pancreatic ductal adenocarcinoma. JAMA Surg. 2020, 155, 832–839. [Google Scholar] [CrossRef]


| Age ≤ 70 Years | Age > 70 Years | p-Value | |
|---|---|---|---|
| Number | 123 | 90 | |
| Age (years), median [range] | 63 [45–70] | 76 [71–89] | <0.001 |
| Gender, n (%) | 0.782 | ||
| Female | 56 (46) | 43 (48) | |
| Male | 67 (54) | 47 (52) | |
| ASA (n = 201) *, n (%) | 0.094 | ||
| I | 3 (3) | 1 (1) | |
| II | 78 (68) | 47 (55) | |
| III | 34 (30) | 38 (44) | |
| BMI (kg/m2), median (IQR) | 25.6 (5.5) | 25.6 (4.3) | 0.968 |
| Alcohol abuse (n = 187) *, n (%) | 61 (57) | 41 (51) | 0.461 |
| Nicotine abuse (n = 208) *, n (%) | 44 (37) | 3 (3) | <0.001 |
| Comorbidity, n (%) | |||
| Hypertension | 56 (46) | 62 (69) | 0.002 |
| Diabetes | 33 (27) | 25 (28) | 1.000 |
| Cardiovascular | 11 (9) | 15 (17) | 0.095 |
| Pulmonary | 13 (11) | 6 (7) | 0.345 |
| Cerebrovascular | 3 (2) | 9 (10) | 0.031 |
| Liver disease | 7 (6) | 9 (10) | 0.297 |
| Preoperative biliary stenting, n (%) | 62 (53) | 50 (56) | 0.673 |
| Preoperative hemoglobin (g/dL), median (IQR) | 13.0 (2.3) | 12.7 (2.3) | 0.189 |
| Preoperative WBC (109/L), median (IQR) | 7.2 (3.9) | 6.7 (3.0) | 0.308 |
| Preoperative albumin (g/L), median (IQR) | 41.2 (7.9) | 39.7 (6.0) | 0.021 |
| Preoperative CRP (mg/L), median (IQR) | 7 (19) | 5 (12) | 0.441 |
| Preoperative CA19-9 (U/mL) (n = 191) *, median (IQR) | 73 (254) | 108 (415) | 0.097 |
| Preoperative CEA (ng/mL) (n =155) *, median (IQR) | 2.3 (3.4) | 2.9 (3.0) | 0.540 |
| Age ≤ 70 Years (n = 123) | Age > 70 Years (n = 90) | p-Value | |
|---|---|---|---|
| Kind of surgery | 0.153 | ||
| Pancreatic head resection | 88 (72) | 74 (82) | |
| Distal pancreatectomy | 31 (25) | 13 (14) | |
| Total pancreatectomy | 4 (3) | 3 (3) | |
| Portal vein resection, n (%) | 29 (24) | 29 (32) | 0.212 |
| Arterial resection, n (%) | 2 (2) | 2 (2) | 1.000 |
| Multivisceral resection, n (%) | 20 (16) | 18 (20) | 0.587 |
| Operative time (min), median (IQR) | 269 (97) | 292 (111) | 0.075 |
| Intraoperative blood loss (ml), median (IQR) | 500 (675) | 600 (650) | 0.712 |
| Intraoperative blood transfusion, n (%) | 27 (30) | 22 (24) | 0.439 |
| T category | 0.658 | ||
| pT1 | 9 (7) | 3 (3) | |
| pT2 | 22 (18) | 15 (17) | |
| pT3 | 90 (73) | 70 (878) | |
| pT4 | 2 (2) | 2 (2) | |
| n category | 0.888 | ||
| pN0 | 49 (40) | 37 (41) | |
| pN+ | 74 (60) | 53 (59) | |
| M category | 0.243 | ||
| pM0 | 114 (93) | 79 (88) | |
| pM1 | 9 (7) | 11 (12) | |
| R status | 1.000 | ||
| R0 | 107 (87) | 79 (88) | |
| R1 | 12 (10) | 8 (9) | |
| R2 | 4 (3) | 3 (3) | |
| Differentation | 0.698 | ||
| G1 | 3 (2) | 2 (2) | |
| G2 | 44 (36) | 27 (30) | |
| G3 | 76 (62) | 61 (68) |
| Age ≤ 70 Years (n = 123) | Age > 70 Years (n = 90) | p-Value | |
|---|---|---|---|
| Morbidity, n (%) | 72 (58) | 60 (67) | 0.255 |
| Major morbidity, n (%) | 30 (24) | 30 (33) | 0.167 |
| Mortality, n (%) | 2 (2) | 6 (7) | 0.073 |
| Reoperation, n (%) | 10 (8) | 9 (10) | 0.809 |
| POPF, n (%) | 28 (23) | 13 (14) | 0.160 |
| DGE, n (%) | 31 (25) | 34 (38) | 0.052 |
| PPH, n (%) | 1 (1) | 0 (0) | 1.000 |
| Length of postoperative stay (days), median (IQR) | 17 (9) | 21 (16) | <0.001 |
| Adjuvant chemotherapy, n (%) | 74 (60) | 41 (46) | 0.038 |
| Overall survival (months), median (SD) | 29.2 (6.3) | 17.1 (1.6) | <0.001 |
| Disease-free survival (months), median (SD) | 14.9 (2.2) | 10.4 (1.6) | 0.034 |
| Overall Survival (OS) | Disease-Free Survival (DFS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||
| n | Median | p | HR | 95% CI | p | Median | p | HR | 95% CI | p | |
| Age ≤70 years >70 years | 123 90 | 29.2 17.1 | <0.001 | 2.23 | 1.58–3.15 | <0.001 | 14.9 10.4 | 0.034 | 1.62 | 1.17–2.24 | 0.004 |
| Gender Female Male | 99 114 | 26.8 19.8 | 0.119 | 13.9 12.6 | 0.276 | ||||||
| ASA (n = 201) * I/II III | 129 72 | 24.1 16.9 | 0.020 | 1.36 | 0.96–1.91 | 0.082 | 14.4 10.2 | 0.037 | 1.28 | 0.92–1.77 | 0.140 |
| Arterial hypertension Yes No | 118 95 | 18.5 24.2 | 0.193 | 12.5 16.0 | 0.251 | ||||||
| Cerebrovascular disease Yes No | 12 201 | 8.2 21.5 | 0.230 | 4.7 13.9 | 0.392 | ||||||
| Preoperative albumin <40 g/L ≥40 g/L | 100 113 | 18.8 26.9 | 0.060 | 11.3 16.2 | 0.131 | ||||||
| Ca19-9 (n = 191) * <50 U/mL ≥50 U/ml | 72 119 | 24.2 20.7 | 0.173 | 16.5 12.2 | 0.086 | ||||||
| Kind of surgery PHR DP TP | 162 44 7 | 23.4 17.3 6.7 | 0.153 | 14.8 10.2 6.7 | 0.206 | ||||||
| Vascular resection Yes No | 59 154 | 17.2 22.0 | 0.242 | 9.6 14.0 | 0.286 | ||||||
| Multivisceral resection Yes No | 38 175 | 17.0 23.4 | 0.111 | 8.9 14.0 | 0.104 | ||||||
| T category pT1/pT2 pT3/pT4 | 49 164 | 37.8 18.4 | 0.013 | 1.28 | 0.82–2.01 | 0.279 | 20.0 11.7 | 0.006 | 1.27 | 0.84–1.93 | 0.263 |
| N category pN0 pN+ | 86 127 | 39.7 17.8 | <0.001 | 2.01 | 1.39–2.91 | <0.001 | 18.7 11.4 | <0.001 | 1.88 | 1.32–2.69 | <0.001 |
| M category M0 pM1 | 193 20 | 23.1 12.4 | 0.010 | 0.81 | 0.43–1.55 | 0.532 | 14.0 8.1 | 0.077 | |||
| R status R0 R1/R2 | 186 27 | 23.8 8.8 | <0.001 | 2.77 | 1.62–4.73 | <0.001 | 14.7 8.7 | 0.006 | 1.94 | 1.22–3.07 | 0.005 |
| Differentiation G1/G2 G3 | 76 137 | 37.8 17.1 | <0.001 | 1.72 | 1.19–2.50 | 0.004 | 19.7 11.7 | <0.001 | 1.65 | 1.17–2.34 | 0.005 |
| Morbidity Yes No | 132 81 | 19.8 29.2 | 0.376 | 13.1 13.9 | 0.820 | ||||||
| Reoperation Yes No | 19 192 | 17.0 22.0 | 0.131 | 14.0 13.7 | 0.400 | ||||||
| Adjuvant chemotherapy Yes No | 115 98 | 23.4 17.2 | 0.297 | 14.8 11.0 | 0.557 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackner, D.; Hobbs, M.; Merkel, S.; Siepmann, T.; Krautz, C.; Weber, G.F.; Grützmann, R.; Brunner, M. Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 3929. https://doi.org/10.3390/cancers14163929
Hackner D, Hobbs M, Merkel S, Siepmann T, Krautz C, Weber GF, Grützmann R, Brunner M. Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(16):3929. https://doi.org/10.3390/cancers14163929
Chicago/Turabian StyleHackner, Danilo, Mirianna Hobbs, Susanne Merkel, Timo Siepmann, Christian Krautz, Georg F. Weber, Robert Grützmann, and Maximilian Brunner. 2022. "Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 16: 3929. https://doi.org/10.3390/cancers14163929
APA StyleHackner, D., Hobbs, M., Merkel, S., Siepmann, T., Krautz, C., Weber, G. F., Grützmann, R., & Brunner, M. (2022). Impact of Patient Age on Postoperative Short-Term and Long-Term Outcome after Pancreatic Resection of Pancreatic Ductal Adenocarcinoma. Cancers, 14(16), 3929. https://doi.org/10.3390/cancers14163929








