IgG-Based Bispecific Anti-CD95 Antibodies for the Treatment of B Cell-Derived Malignancies and Autoimmune Diseases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. Generation and Purification of Recombinant Antibodies
2.3. Induction of Apoptosis and Caspase-3 Activation in Lymphoma Cells
2.4. Depletion of Activated B Cells and Inhibition of IgG and Cytokine Production
2.5. Animal Experiments
2.6. Statistics
3. Results
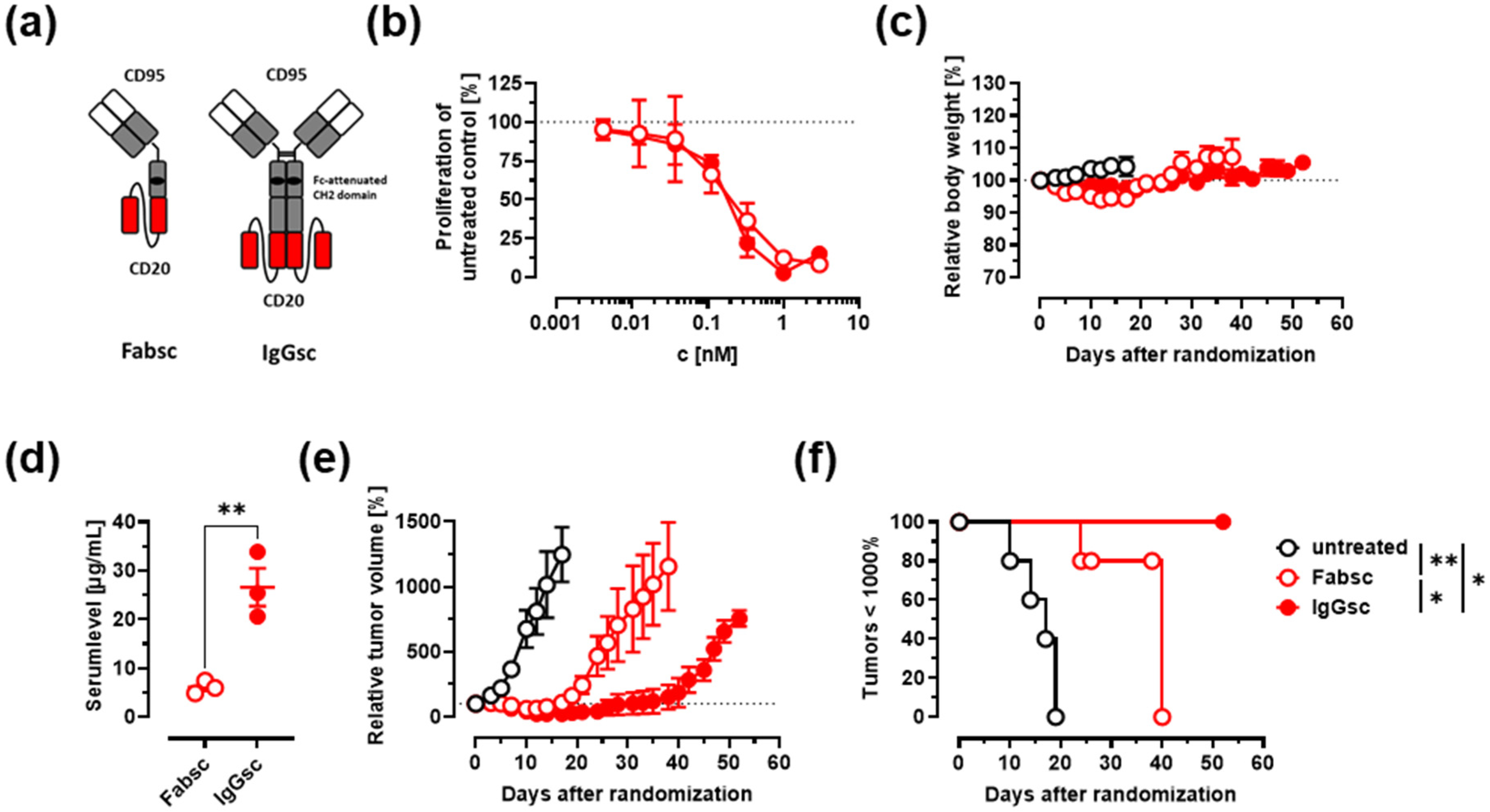
3.1. Construction of Improved Anti-CD95 bsAbs Targeting CD20 or CD19
3.2. In Vitro Activity against Malignant B Cells
3.3. In Vivo Activity against Malignant B Cells
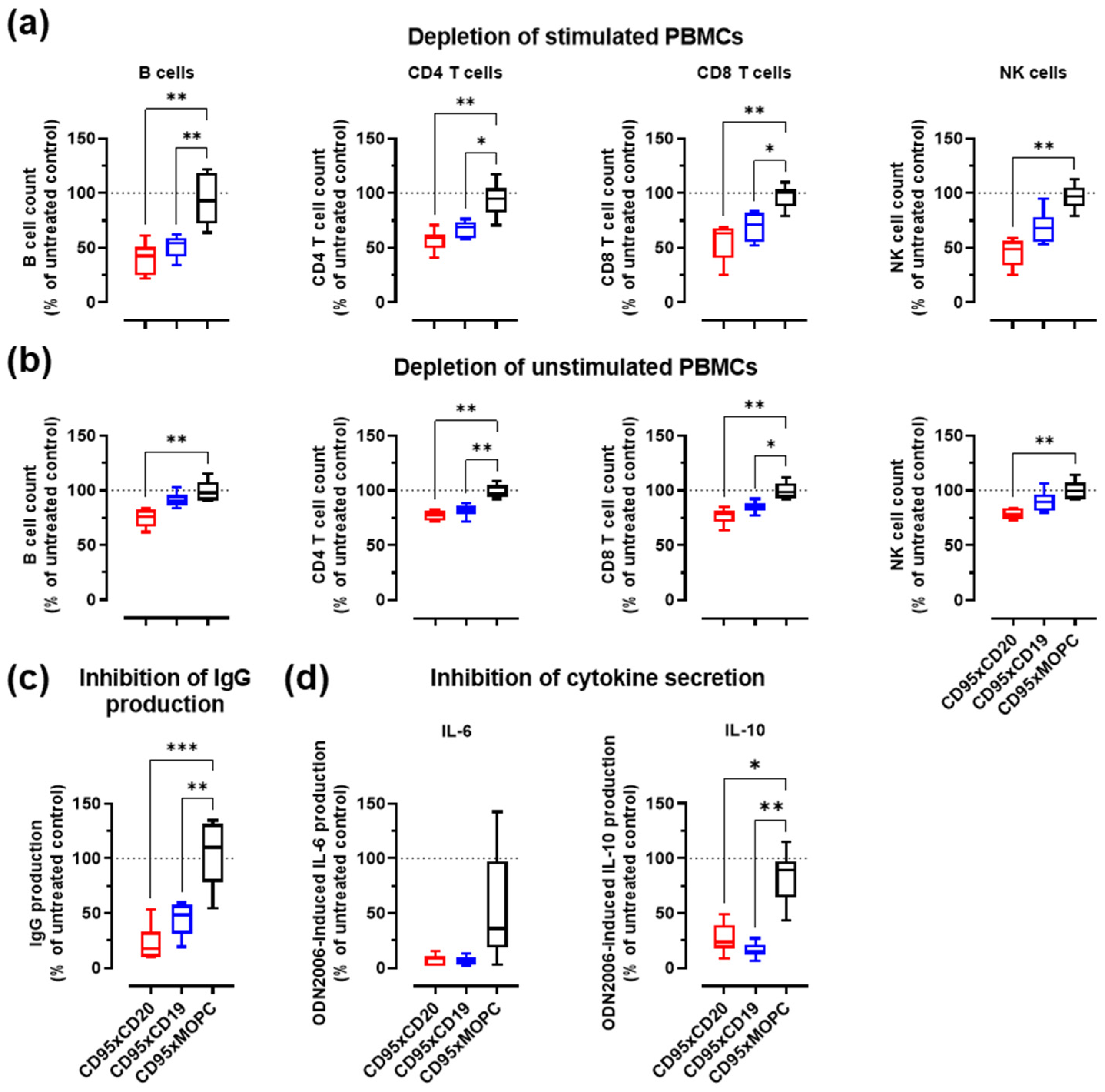
3.4. Depletion of Activated B Cells and Reduction of IgG and Cytokine Levels
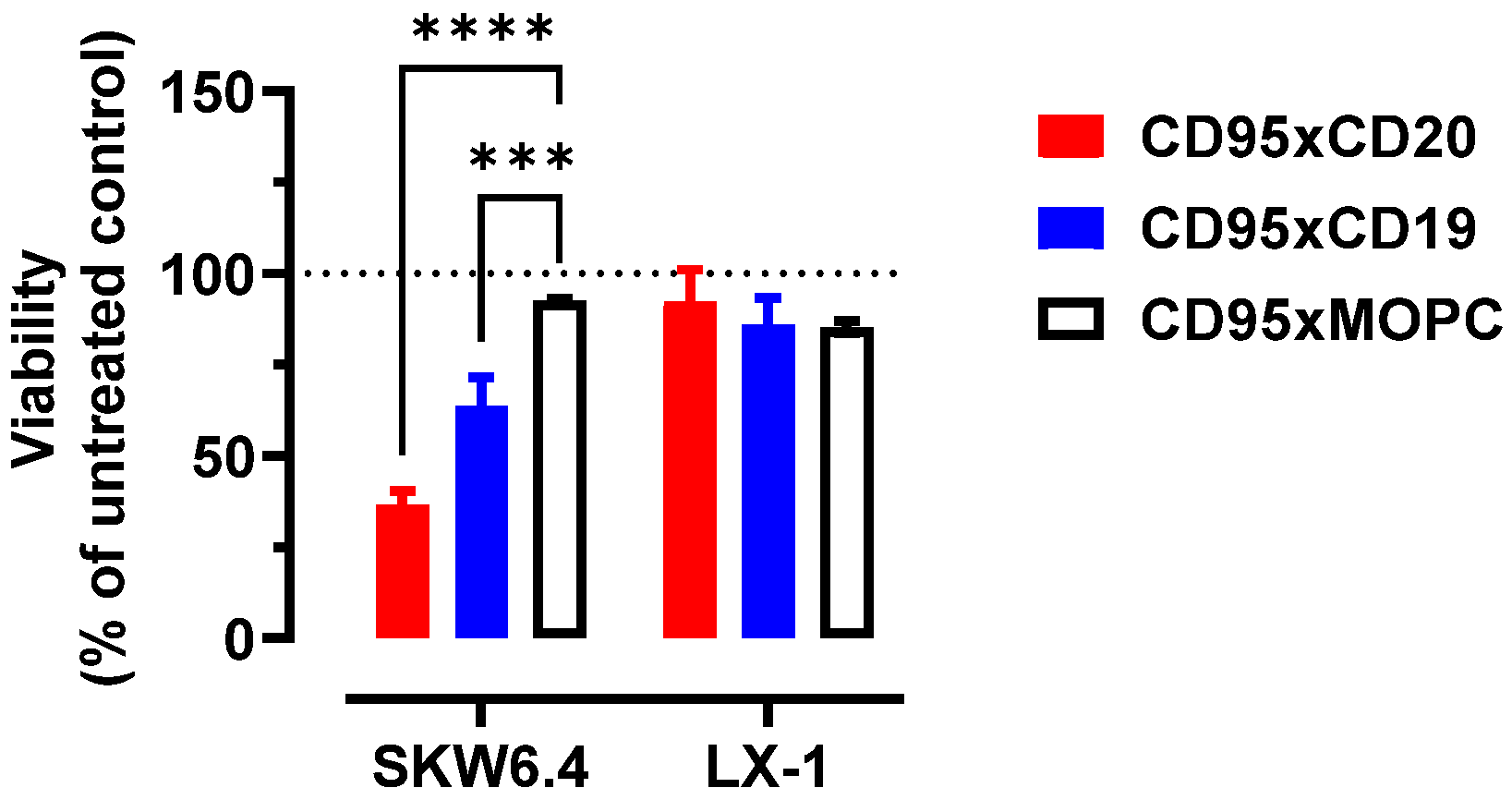
3.5. Anti-CD95 bsAbs in the IgGsc Format Do Not Induce Apoptosis in Hepatocytes
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina, A. A Decade of Rituximab: Improving Survival Outcomes in Non-Hodgkin’s Lymphoma. Annu. Rev. Med. 2008, 59, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Levy, R. Translational Medicine in Action: Anti-CD20 Therapy in Lymphoma. J. Immunol. 2014, 193, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Jayne, D.R.W. Anti-B Cell Antibody Therapies for Inflammatory Rheumatic Diseases. Annu. Rev. Med. 2014, 65, 263–278. [Google Scholar] [CrossRef]
- Jones, R.B.; Cohen Tervaert, J.W.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; van Paassen, P.; et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. N. Engl. J. Med. 2010, 363, 211–220. [Google Scholar] [CrossRef]
- Abdulahad, W.H.; Meijer, J.M.; Kroese, F.G.M.; Meiners, P.M.; Vissink, A.; Spijkervet, F.K.L.; Kallenberg, C.G.M.; Bootsma, H. B Cell Reconstitution and t Helper Cell Balance after Rituximab Treatment of Active Primary Sjögren’s Syndrome. Arthritis Rheum. 2011, 63, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Trivino, T.; Braithwaite, D.; Bacchetti, P.; Waubant, E. Rituximab in Relapsing and Progressive Forms of Multiple Sclerosis: A Systematic Review. PLoS ONE 2013, 8, e0066308. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Ravaud, P.; Bardin, T.; Cacoub, P.; Cantagrel, A.; Combe, B.; Dougados, M.; Flipo, R.M.; Godeau, B.; Guillevin, L.; et al. Risk Factors for Severe Infections in Patients with Rheumatoid Arthritis Treated with Rituximab in the Autoimmunity and Rituximab Registry. Arthritis Rheum. 2010, 62, 2625–2632. [Google Scholar] [CrossRef]
- Hoy, S.M. Tafasitamab: First Approval. Drugs 2020, 80, 1731–1737. [Google Scholar] [CrossRef]
- Newman, M.J.; Benani, D.J. A Review of Blinatumomab, a Novel Immunotherapy. J. Oncol. Pharm. Pract. 2016, 22, 639–645. [Google Scholar] [CrossRef]
- Portell, C.A.; Wenzell, C.M.; Advani, A.S. Clinical and Pharmacologic Aspects of Blinatumomab in the Treatment of B-Cell Acute Lymphoblastic Leukemia. Clin. Pharmacol. Adv. Appl. 2013, 5, 5–11. [Google Scholar] [CrossRef]
- Makita, S.; Yoshimura, K.; Tobinai, K. Clinical Development of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Cancer Sci. 2017, 108, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. Principles and Mechanisms of CD95 Activation. Biol. Chem. 2014, 395, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Wang, R.; Zhang, L.; Yin, D.; Luo, X.; Solomon, J.; Jiang, R.; Markos, K.; Davidson, W.; Scott, D.; et al. Death the Fas Way: Regulation and Pathophysiology of CD95 and Its Ligand. Pharmacol. Ther. 2000, 88, 333–347. [Google Scholar] [CrossRef]
- Daniel, P.T.; Krammer, P.H. Activation Induces Sensitivity toward APO-1 (CD95)-Mediated Apoptosis in Human B Cells. J. Immunol. 1994, 152, 5624–5632. [Google Scholar] [PubMed]
- Chen, L.; Park, S.M.; Tumanov, A.V.; Hau, A.; Sawada, K.; Feig, C.; Turner, J.R.; Fu, Y.X.; Romero, I.L.; Lengyel, E.; et al. CD95 Promotes Tumour Growth. Nature 2010, 465, 492–496. [Google Scholar] [CrossRef]
- Xerri, L.; Bouabdallah, R.; Devilard, E.; Hassoun, J.; Stoppa, A.M.; Birg, F. Sensitivity to Fas-Mediated Apoptosis Is Null or Weak in B-Cell Non-Hodgkin’s Lymphomas and Is Moderately Increased by CD40 Ligation. Br. J. Cancer 1998, 78, 225–232. [Google Scholar] [CrossRef]
- Plumas, J.; Jacob, M.C.; Chaperot, L.; Molens, J.P.; Sotto, J.J.; Bensa, J.C. Tumor B Cells from Non-Hodgkin’s Lymphoma Are Resistant to CD95 (Fas/Apo-1)-Mediated Apoptosis. Blood 1998, 91, 2875–2885. [Google Scholar] [CrossRef]
- Trauth, B.C.; Klas, C.; Peters, A.M.J.; Matzku, S.; Möller, P.; Falk, W.; Debatin, K.M.; Krammer, P.H. Monoclonal Antibody-Mediated Tumor Regression by Induction of Apoptosis. Science 1989, 245, 301–305. [Google Scholar] [CrossRef]
- Ichikawa, K.; Yoshida-Kato, H.; Ohtsuki, M.; Ohsumi, J.; Yamaguchi, J.; Takahashi, S.; Tani, Y.; Watanabe, M.; Shiraishi, A.; Nishioka, K.; et al. A Novel Murine Anti-Human Fas MAb Which Mitigates Lymphadenopathy without Hepatotoxicity. Int. Immunol. 2000, 12, 555–562. [Google Scholar] [CrossRef]
- Galle, P.R.; Krammer, P.H. CD95-Induced Apoptosis in Human Liver Disease. Semin. Liver Dis. 1998, 18, 141–151. [Google Scholar] [CrossRef]
- Schneider, P.; Holler, N.; Bodmer, J.L.; Hahne, M.; Frei, K.; Fontana, A.; Tschopp, J. Conversion of Membrane-Bound Fas(CD95) Ligand to Its Soluble Form Is Associated with Downregulation of Its Proapoptotic Activity and Loss of Liver Toxicity. J. Exp. Med. 1998, 187, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Grosse-Hovest, L.; Krammer, P.H.; Rammensee, H.G. Target Cell-Restricted Triggering of the CD95 (APO-1/Fas) Death Receptor with Bispecific Antibody Fragments. Cancer Res. 2001, 61, 1846–1848. [Google Scholar] [PubMed]
- Nalivaiko, K.; Hofmann, M.; Kober, K.; Teichweyde, N.; Krammer, P.H.; Rammensee, H.-G.; Grosse-Hovest, L.; Jung, G. A Recombinant Bispecific CD20×CD95 Antibody With Superior Activity Against Normal and Malignant B-Cells. Mol. Ther. 2016, 24, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Zekri, L.; Vogt, F.; Osburg, L.; Müller, S.; Kauer, J.; Manz, T.; Pflügler, M.; Maurer, A.; Heitmann, J.S.; Hagelstein, I.; et al. An IgG-based Bispecific Antibody for Improved Dual Targeting in PSMA-positive Cancer. EMBO Mol. Med. 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hörner, S.; Ghosh, M.; Kauer, J.; Spät, P.; Rammensee, H.; Jung, G.; Pflügler, M. Mass Spectrometry for Quality Control of Bispecific Antibodies after SDS-PAGE In-gel Digestion. Biotechnol. Bioeng. 2021, 118, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, K.; Herman, J.; Boon, L.; Waer, M.; Sprangers, B.; Louat, T. Comparative in Vitro Immune Stimulation Analysis of Primary Human B Cells and B Cell Lines. J. Immunol. Res. 2016, 2016, 5281823. [Google Scholar] [CrossRef]
- Coloma, M.J.; Morrison, S.L. Design and Production of Novel Tetravalent Bispecific Antibodies. Nat. Biotechnol. 1997, 15, 159–163. [Google Scholar] [CrossRef]
- Liu, A.Y.; Robinson, R.R.; Murray, E.D.; Ledbetter, J.A.; Hellström, I.; Hellström, K.E. Production of a Mouse-Human Chimeric Monoclonal Antibody to CD20 with Potent Fc-Dependent Biologic Activity. J. Immunol. 1987, 139, 3521–3526. [Google Scholar]
- MEEKER, T.C.; MILLER, R.A.; LINK, M.P.; BINDL, J.; WARNKE, R.; LEVY, R. A Unique Human B Lymphocyte Antigen Defined by a Monoclonal Antibody. Hybridoma 1984, 3, 305–320. [Google Scholar] [CrossRef]
- Shen, P.; Fillatreau, S. Antibody-Independent Functions of B Cells: A Focus on Cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Nakayama, J.; Ogawa, Y.; Yoshigae, Y.; Onozawa, Y.; Yonemura, A.; Saito, M.; Ichikawa, K.; Yamoto, T.; Komai, T.; Tatsuta, T.; et al. A Humanized Anti-Human Fas Antibody, R-125224, Induces Apoptosis in Type I Activated Lymphocytes but Not in Type II Cells. Int. Immunol. 2006, 18, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, H.; Zhong, C.; Peng, B.; Zhang, M.; Li, B.; Hou, S.; Guo, Y.; Ding, J. Crystal Structure of Chimeric Antibody C2H7 Fab in Complex with a CD20 Peptide. Mol. Immunol. 2008, 45, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Große-Hovest, L.; Otz, T.; Krammer, P.H.; Rammensee, H.G.; Jung, G. Construction of Optimized Bispecific Antibodies for Selective Activation of the Death Receptor CD95. Cancer Res. 2008, 68, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Wörn, A.; Plückthun, A. Stability Engineering of Antibody Single-Chain Fv Fragments. J. Mol. Biol. 2001, 305, 989–1010. [Google Scholar] [CrossRef]
- Fisher, G.H.; Rosenberg, F.J.; Straus, S.E.; Dale, J.K.; Middelton, L.A.; Lin, A.Y.; Strober, W.; Lenardo, M.J.; Puck, J.M. Dominant Interfering Fas Gene Mutations Impair Apoptosis in a Human Autoimmune Lymphoproliferative Syndrome. Cell 1995, 81, 935–946. [Google Scholar] [CrossRef]
- Agrebi, N.; Ben-Mustapha, I.; Matoussi, N.; Dhouib, N.; Ben-Ali, M.; Mekki, N.; Ben-Ahmed, M.; Larguèche, B.; Ben Becher, S.; Béjaoui, M.; et al. Rare Splicing Defects of FAS Underly Severe Recessive Autoimmune Lymphoproliferative Syndrome. Clin. Immunol. 2017, 183, 17–23. [Google Scholar] [CrossRef]
- Leandro, M.J.; Cambridge, G.; Ehrenstein, M.R.; Edwards, J.C.W. Reconstitution of Peripheral Blood B Cells after Depletion with Rituximab in Patients with Rheumatoid Arthritis. Arthritis Rheum. 2006, 54, 613–620. [Google Scholar] [CrossRef]
- Yazawa, N.; Hamaguchi, Y.; Poe, J.C.; Tedder, T.F. Immunotherapy Using Unconjugated CD19 Monoclonal Antibodies in Animal Models for B Lymphocyte Malignancies and Autoimmune Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 15178–15183. [Google Scholar] [CrossRef]
- Barr, T.A.; Shen, P.; Brown, S.; Lampropoulou, V.; Roch, T.; Lawrie, S.; Fan, B.; O’Connor, R.A.; Anderton, S.M.; Bar-Or, A.; et al. B Cell Depletion Therapy Ameliorates Autoimmune Disease through Ablation of IL-6-Producing B Cells. J. Exp. Med. 2012, 209, 1001–1010. [Google Scholar] [CrossRef]

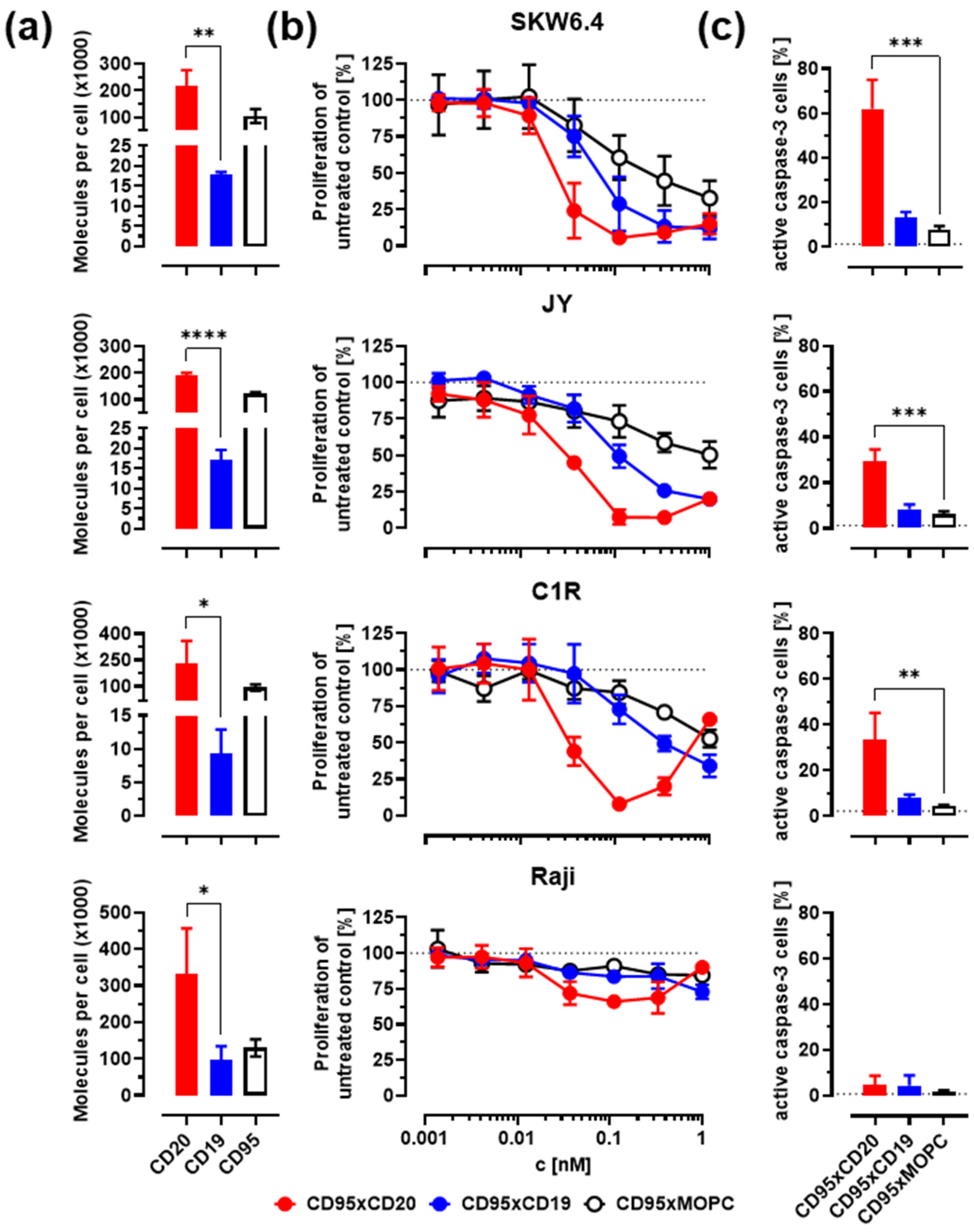
| Molecules/Cell (×1000) | CD95xCD20 | CD95xCD19 | CD95xMOPC | |||
|---|---|---|---|---|---|---|
| Cell Line | CD20 | CD19 | CD95 | abs. IC50 | abs. IC50 | abs. IC50 |
| SKW6.4 | 218 ± 58 | 18 ± 1 | 105 ± 26 | 24 ± 11 | 69 ± 29 | 481 ± 571 |
| JY | 190 ± 10 | 17 ± 3 | 122 ± 5 | 35 ± 1 | 107 ± 35 | - |
| C1R | 233 ± 124 | 9 ± 4 | 93 ± 17 | 32 ± 7 | 306 ± 154 | - |
| Raji | 332 ± 125 | 99 ± 36 | 130 ± 24 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörner, S.; Moustafa-Oglou, M.; Teppert, K.; Hagelstein, I.; Kauer, J.; Pflügler, M.; Neumann, K.; Rammensee, H.-G.; Metz, T.; Herrmann, A.; et al. IgG-Based Bispecific Anti-CD95 Antibodies for the Treatment of B Cell-Derived Malignancies and Autoimmune Diseases. Cancers 2022, 14, 3941. https://doi.org/10.3390/cancers14163941
Hörner S, Moustafa-Oglou M, Teppert K, Hagelstein I, Kauer J, Pflügler M, Neumann K, Rammensee H-G, Metz T, Herrmann A, et al. IgG-Based Bispecific Anti-CD95 Antibodies for the Treatment of B Cell-Derived Malignancies and Autoimmune Diseases. Cancers. 2022; 14(16):3941. https://doi.org/10.3390/cancers14163941
Chicago/Turabian StyleHörner, Sebastian, Moustafa Moustafa-Oglou, Karin Teppert, Ilona Hagelstein, Joseph Kauer, Martin Pflügler, Kristina Neumann, Hans-Georg Rammensee, Thomas Metz, Andreas Herrmann, and et al. 2022. "IgG-Based Bispecific Anti-CD95 Antibodies for the Treatment of B Cell-Derived Malignancies and Autoimmune Diseases" Cancers 14, no. 16: 3941. https://doi.org/10.3390/cancers14163941
APA StyleHörner, S., Moustafa-Oglou, M., Teppert, K., Hagelstein, I., Kauer, J., Pflügler, M., Neumann, K., Rammensee, H. -G., Metz, T., Herrmann, A., Salih, H. R., Jung, G., & Zekri, L. (2022). IgG-Based Bispecific Anti-CD95 Antibodies for the Treatment of B Cell-Derived Malignancies and Autoimmune Diseases. Cancers, 14(16), 3941. https://doi.org/10.3390/cancers14163941








