Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
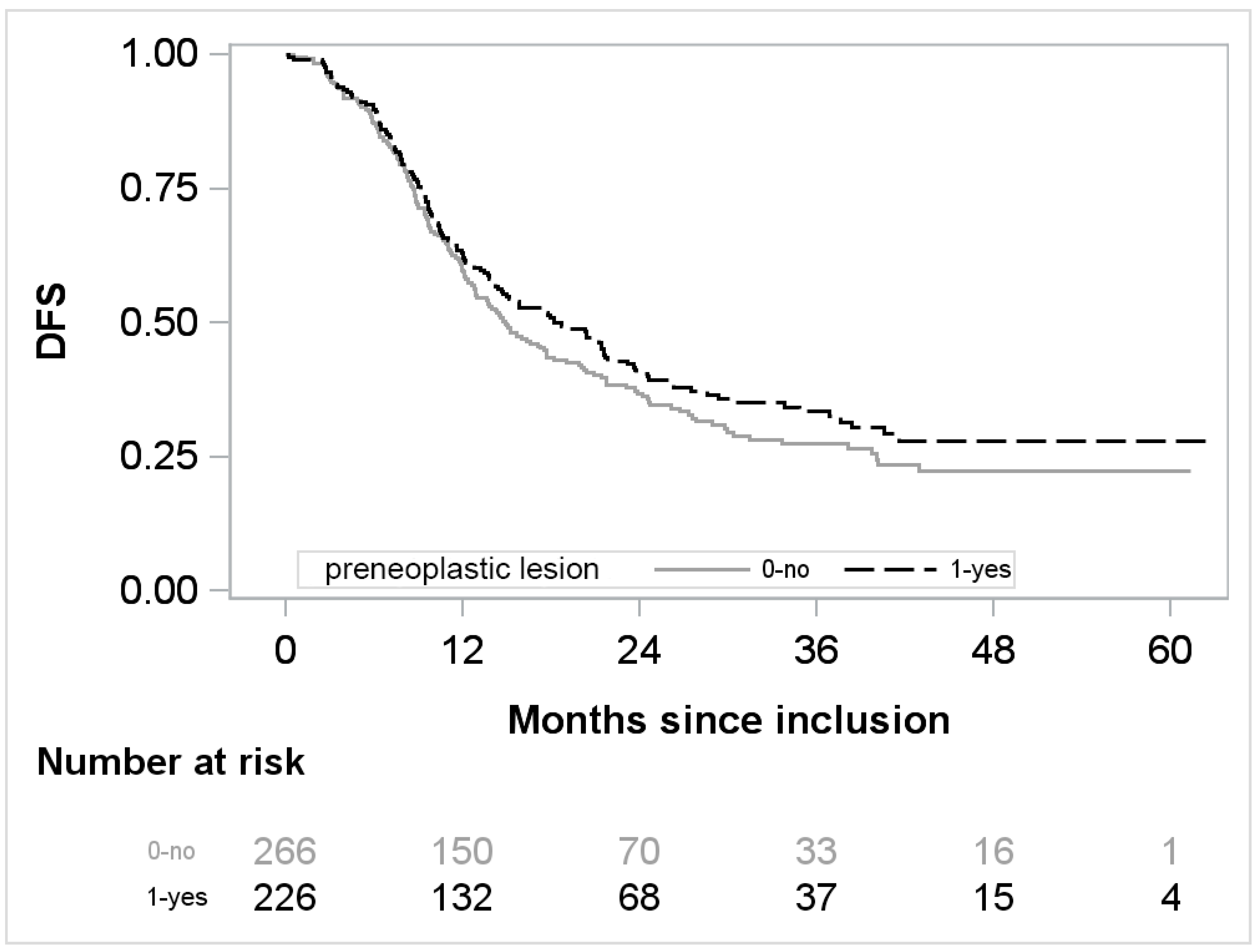
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Opitz, F.V.; Haeberle, L.; Daum, A.; Esposito, I. Tumor Microenvironment in Pancreatic Intraepithelial Neoplasia. Cancers 2021, 13, 6188. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Miller, F.H.; Lopes Vendrami, C.; Recht, H.S.; Wood, C.G.; Mittal, P.; Keswani, R.N.; Gabriel, H.; Borhani, A.A.; Nikolaidis, P.; Hammond, N.A. Pancreatic Cystic Lesions and Malignancy: Assessment, Guidelines, and the Field Defect. RadioGraphics 2022, 42, 87–105. [Google Scholar] [CrossRef]
- Distler, M.; Aust, D.; Weitz, J.; Pilarsky, C.; Grützmann, R. Precursor Lesions for Sporadic Pancreatic Cancer: PanIN, IPMN, and MCN. BioMed. Res. Int. 2014, 2014, 474905. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Frankel, T.L.; LaFemina, J.; Bamboat, Z.M.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Kingham, T.P.; Jarnagin, W.R.; Allen, P.J. Dysplasia at the Surgical Margin Is Associated with Recurrence after Resection of Non-Invasive Intraductal Papillary Mucinous Neoplasms. HPB 2013, 15, 814–821. [Google Scholar] [CrossRef]
- Leng, K.-M.; Wang, Z.-D.; Zhao, J.-B.; Cui, Y.-F.; Zhong, X.-Y. Impact of Pancreatic Margin Status and Lymph Node Metastases on Recurrence after Resection for Invasive and Noninvasive Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Meta-Analysis. Dig. Surg. 2012, 29, 213–225. [Google Scholar] [CrossRef]
- Basturk, O.; Hong, S.-M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Grambsch, P.; Therneau, T. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Barnard, J.; Meng, X.-L. Applications of Multiple Imputation in Medical Studies: From AIDS to NHANES. Stat. Methods Med. Res. 1999, 8, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Palmer, D.; Jackson, R.; Cox, T.; Neoptolemos, J.P.; Ghaneh, P.; Rawcliffe, C.L.; Bassi, C.; Stocken, D.D.; Cunningham, D.; et al. Optimal Duration and Timing of Adjuvant Chemotherapy after Definitive Surgery for Ductal Adenocarcinoma of the Pancreas: Ongoing Lessons from the ESPAC-3 Study. J. Clin. Oncol. 2014, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant Chemotherapy With Fluorouracil Plus Folinic Acid vs Gemcitabine Following Pancreatic Cancer Resection: A Randomized Controlled Trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant Chemotherapy with Gemcitabine and Long-Term Outcomes among Patients with Resected Pancreatic Cancer: The CONKO-001 Randomized Trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.-E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.A.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef]
- Gavazzi, F.; Capretti, G.; Giordano, L.; Ridolfi, C.; Spaggiari, P.; Sollai, M.; Carrara, S.; Nappo, G.; Bozzarelli, S.; Zerbi, A. Pancreatic Ductal Adenocarcinoma and Invasive Intraductal Papillary Mucinous Tumor: Different Prognostic Factors for Different Overall Survival. Dig. Liver Dis. 2022, 54, 826–833. [Google Scholar] [CrossRef]
- Lim, J.E.; Chien, M.W.; Earle, C.C. Prognostic Factors Following Curative Resection for Pancreatic Adenocarcinoma: A Population-Based, Linked Database Analysis of 396 Patients. Ann. Surg. 2003, 237, 74–85. [Google Scholar] [CrossRef]
- Benassai, G.; Mastrorilli, M.; Quarto, G.; Cappiello, A.; Giani, U.; Forestieri, P.; Mazzeo, F. Factors Influencing Survival after Resection for Ductal Adenocarcinoma of the Head of the Pancreas. J. Surg. Oncol. 2000, 73, 212–218. [Google Scholar] [CrossRef]
- You, M.S.; Lee, S.H.; Choi, Y.H.; Shin, B.; Paik, W.H.; Ryu, J.K.; Kim, Y.-T.; Jang, D.K.; Lee, J.K.; Kwon, W.; et al. Lymph Node Ratio as Valuable Predictor in Pancreatic Cancer Treated with R0 Resection and Adjuvant Treatment. BMC Cancer 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Ohuchida, J.; Ohtsuka, T.; Nakano, K.; Tanaka, M. Intraductal Papillary-Mucinous Tumor of the Pancreas Concomitant with Ductal Carcinoma of the Pancreas. Pancreatology 2002, 2, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, M.; Noë, M.; Masica, D.L.; Hosoda, W.; Chianchiano, P.; Fischer, C.G.; Lionheart, G.; Brosens, L.A.A.; Pea, A.; Yu, J.; et al. IPMNs with Co-Occurring Invasive Cancers: Neighbours but Not Always Relatives. Gut 2018, 67, 1652–1662. [Google Scholar] [CrossRef]
- Khoury, R.E.; Kabir, C.; Maker, V.K.; Banulescu, M.; Wasserman, M.; Maker, A.V. What Is the Incidence of Malignancy in Resected Intraductal Papillary Mucinous Neoplasms? An Analysis of Over 100 US Institutions in a Single Year. Ann. Surg. Oncol. 2018, 25, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kanemitsu, S.; Hatori, T.; Maguchi, H.; Shimizu, Y.; Tada, M.; Nakagohri, T.; Hanada, K.; Osanai, M.; Noda, Y.; et al. Pancreatic Ductal Adenocarcinoma Derived from IPMN and Pancreatic Ductal Adenocarcinoma Concomitant with IPMN. Pancreas 2011, 40, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Yopp, A.C.; Katabi, N.; Janakos, M.; Klimstra, D.S.; D’Angelica, M.I.; DeMatteo, R.P.; Fong, Y.; Brennan, M.F.; Jarnagin, W.R.; Allen, P.J. Invasive Carcinoma Arising in Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Matched Control Study with Conventional Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2011, 253, 968–974. [Google Scholar] [CrossRef]
- Flattet, Y.; Yamaguchi, T.; Andrejevic-Blant, S.; Halkic, N. Pancreatic Adenocarcinoma: The Impact of Preneoplastic Lesion Pattern on Survival. Biosci. Trends 2015, 9, 402–406. [Google Scholar] [CrossRef][Green Version]
- Kaiser, J.; Scheifele, C.; Hinz, U.; Leonhardt, C.-S.; Hank, T.; Koenig, A.-K.; Tjaden, C.; Hackert, T.; Bergmann, F.; Büchler, M.W.; et al. IPMN-Associated Pancreatic Cancer: Survival, Prognostic Staging and Impact of Adjuvant Chemotherapy. Eur. J. Surg. Oncol. 2022, 48, 1309–1320. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Schmidt, C.M.; White, P.B.; Waters, J.A.; Yiannoutsos, C.T.; Cummings, O.W.; Baker, M.; Howard, T.J.; Zyromski, N.J.; Nakeeb, A.; DeWitt, J.M.; et al. Intraductal Papillary Mucinous Neoplasms: Predictors of Malignant and Invasive Pathology. Ann. Surg. 2007, 246, 644–651; discussion 651–654. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bassi, C.; Salvia, R.; Malleo, G.; Marchegiani, G.; Rebours, V.; Levy, P.; Partelli, S.; Suleiman, S.L.; Banks, P.A.; et al. Low Progression of Intraductal Papillary Mucinous Neoplasms with Worrisome Features and High-Risk Stigmata Undergoing Non-Operative Management: A Mid-Term Follow-up Analysis. Gut 2017, 66, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Turpin, A.; Belletier, C.; Wei, A.; Mitry, E.; Lopez, A.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer PRODIGE 24/CCTG PA6trial: Updated Results of a Multicenter International Randomized Phase III Trial of Adjuvant MFOLFIRINOX (MFFX) versus Gemcitabine (Gem) in Patients (Pts) with Resected Pancreatic Ductal Adenocarcinomas (PDAC). Ann. Oncol. 2021, 32 (Suppl. S5), S1283–S1346. [Google Scholar] [CrossRef]
| Characteristics | No. (%) |
|---|---|
| Study arm | |
| Gemcitabine | 246/493 (49.9) |
| mFOLFIRINOX | 247/493 (50.1) |
| Age (years) ≥ 65 | 201/493 (40.8) |
| Male sex | 277/493 (56.2) |
| WHO performance-status score | |
| 0 | 249/487 (51.1) |
| 1 | 238/487 (48.9) |
| Diabetes mellitus | 126/487 (25.9) |
| Location of the tumor | |
| Head of the pancreas | 372/492 (75.6) |
| Other | 120/492 (24.4) |
| Tumor histologic findings | |
| Ductal adenocarcinoma | 486/492 (98.8) |
| Nonductal adenocarcinoma | 6/492 (1.2) |
| Tumor grade | |
| Well differentiated | 149/462 (32.2) |
| Moderately differentiated | 249/462 (54.0) |
| Poorly differentiated or undifferentiated | 64/462 (13.8) |
| Primary tumor status | |
| pT1 or pT2 | 56/493 (11.4) |
| pT3 or pT4 | 437/493 (88.6) |
| Lymph node status | |
| pN0 | 116/493 (23.5) |
| pN1 | 377/493 (76.5) |
| Tumor stage | |
| IA or IB | 26/493 (5.3) |
| IIA or IIB | 452/493 (91.7) |
| III or IV | 15/493 (3.0) |
| Surgical margins | |
| R0 | 282/493 (57.2) |
| R1 | 211/493 (42.8) |
| Lymphovascular invasion | 289/423 (68.3) |
| Perineural invasion | 412/452 (91.2) |
| Capsular rupture | 75/432 (17.4) |
| Vascular resection | |
| Venous resection | 122/490 (24.9) |
| Portal vein resection | 74/493 (15.0) |
| Superior mesenteric vein resection | 44/493 (8.9) |
| Arterial resection | 15/492 (3.1) |
| Postoperative CA 19-9 level | |
| ≤90 U/mL | 457/493 (92.7) |
| >90 U/mL | 36/493 (7.3) |
| Results presented as no./total no. (%) | |
| All N = 493 | Gemcitabine Arm N = 246 | mFOLFIRINOX Arm N = 247 | |
|---|---|---|---|
| Preneoplastic lesion (n0, %) | 226/492 α (45.9) | 118/246 (48) | 108/246 α (43.9) |
| Location of preneoplastic lesions | |||
| Tumor margin | 20/492 (4.1) | 12/246 (4,9) | 8/246 (3.3) |
| Parenchyma | 161/492 (32.7) | 82/246 (33,3) | 79/246 (32.1) |
| Tumor margin and parenchyma | 45/492 (9.2) | 24/246 (9.8) | 21/246 (8.5) |
| IPMN lesions | 43/492 (8.7) | 27/246 (11.0) | 16/246 (6.5) |
| Grading IPMN | |||
| Low- or intermediate-grade | 10/491 (2) | 7/245 (2.9) | 3/246 (1.2) |
| High-grade | 32/491 (6.5) | 19/245 (7.8) | 13/246 (5.3) |
| PanIN lesions | 193/492 (39.2) | 97/246 (39.4) | 96/246 (39.0) |
| Grading PanIN | |||
| Low- or intermediate-grade | 86/485 (17.7) | 41/242 (16.9) | 45/243 (18.5) |
| High-grade | 100/485 (20.6) | 52/242 (21.5) | 48/243 (19.8) |
| MCN lesions | 3/492(0.6) | 1/246 (0.4) | 2/246 (0.8) |
| Grading MCN | |||
| Low- or intermediate-grade | 1/492 (0.2) | 1/246 (0.4) | 0 |
| High-grade | 2/492 (0.4) | 0 | 2/246 (0.8) |
| Characteristics | HR and 95% CI | p-Value | |
|---|---|---|---|
| Age | ≥65 vs. <65 | 1 [0.8; 1.25] | 1 |
| Gender | Male vs. Female | 1 [0.80; 1.25] | 1 |
| WHO performance status score | 1 vs. 0 | 1.09 [0.88; 1.37] | 0.4 |
| Diabetes mellitus | Yes vs. No | 1.11 [0.86; 1.42] | 0.4 |
| Tumor location | Head vs. Other | 1.13 [0.87; 1.47] | 0.3 |
| Tumor histologic findings | Ductal adenocarcinoma | 1 | |
| Nonductal carcinoma | 0.94 [0.35; 2.54] | 0.9 | |
| Tumor grade | Well differentiated | 1 | |
| Moderately differentiated | 1.3 [1.01; 1.68] | 0.043 | |
| Poorly differentiated or undifferentiated | 1.79 [1.26; 2.55] | 0.001 | |
| Primary tumor pT stage | pT3 or pT4 vs. pT1 or pT2 | 1.28 [0.89; 1.84] | 0.18 |
| Nodal status | pN1 vs. pN0 | 1.76 [1.32; 2.35] | <0.001 |
| Tumor stage | IA or IB | 1 | |
| IIA or IIB | 2.09 [1.14; 3.81] | 0.017 | |
| III or IV | 6.62 [2.72; 16.14] | <0.001 | |
| Status of surgical margins | R1 vs. R0 | 1.47 [1.18; 1.84] | <0.001 |
| Lymphovascular invasion | Yes vs. No | 1.40 [1.09; 1.80] | 0.009 |
| Perineural invasion | Yes vs. No | 2.52 [1.57; 4.05] | <0.001 |
| Capsular rupture | Yes vs. No | 1.47 [1.10; 1.95] | 0.008 |
| Venous resection | Yes vs. No | 1.41 [1.10; 1.80] | 0.007 |
| Portal-vein resection | Yes vs. No | 1.41 [1.06; 1.88] | 0.020 |
| Superior-mesenteric vein resection | Yes vs. No | 1.44 [0.99; 2.10] | 0.053 |
| Arterial resection | Yes vs. No | 0.78 [0.40; 1.52] | 0.5 |
| Postoperative CA 19-9 level | >90 U/mL vs. ≤90 U/mL | 1.39 [0.93; 2.1] | 0.11 |
| Preneoplastic lesion | Yes vs. No | 0.82 [0.66; 1.03] | 0.088 |
| Location of preneoplastic lesions | Tumor margin | 1.02 [0.58; 1.80] | 0.9 |
| Parenchyma | 0.88 [0.69; 1.12] | 0.3 | |
| Tumor margin and parenchyma | 0.58 [0.37; 0.90] | 0.015 | |
| IPMN lesions | Yes vs. No | 0.63 [0.41; 0.97] | 0.038 |
| Grading IPMN | Low or intermediate grade | 0.64 [0.26; 1.58] | 0.4 |
| High-grade—Invasive | 0.63 [0.38; 1.03] | 0.064 | |
| PanIn lesions | Yes vs. No | 0.97 [0.78; 1.22] | 0.8 |
| Grading PanIn | Low or intermediate grade | 1.00 [0.74; 1.36] | 1 |
| High-grade | 1.04 [0.72; 1.26] | 0.7 |
| Full Multivariate Model | Final Multivariate Model * | |||
|---|---|---|---|---|
| HR and 95% CI | p-Value | HR and 95% CI | p-Value | |
| Arm | ||||
| Gemcitabine | 1 | 1 | ||
| mFOLFIRINOX | 0.54 [0.43; 0.69] | <0.001 | 0.56 [0.45; 0.71] | <0.001 |
| Tumor grade | ||||
| Well differentiated | 1 | 1 | ||
| Moderately differentiated | 1.2 [0.93; 1.55] | 0.164 | 1.22 [0.94; 1.57] | 0.131 |
| Poorly differentiated or undifferentiated | 1.86 [1.28; 2.71] | 0.001 | 1.8 [1.26; 2.57] | 0.001 |
| Nodal status | ||||
| pN0 | 1 | 1 | ||
| pN1 | 1.38 [1.01; 1.89] | 0.042 | 1.46 [1.08; 1.97] | 0.014 |
| Status of surgical margins | ||||
| R0 | 1 | 1 | ||
| R1 | 1.31 [1.04; 1.65] | 0.021 | 1.33 [1.06; 1.67] | 0.013 |
| Lymphovascular invasion | ||||
| No | 1 | |||
| Yes | 1.1 [0.84; 1.45] | 0.468 | - | |
| Perineural invasion | ||||
| No | 1 | |||
| Yes | 1.9 [1.16; 3.12] | 0.011 | 1.98 [1.21; 3.23] | 0.006 |
| Location of preneoplastic lesions | ||||
| Tumor margin | 0.91 [0.5; 1.66] | 0.757 | - | |
| Parenchyma | 0.86 [0.66; 1.11] | 0.247 | - | |
| Tumor margin and parenchyma | 0.61 [0.38; 0.98] | 0.041 | - | |
| IPMN lesions | ||||
| No | 1 | |||
| Yes | 0.95 [0.59; 1.53] | 0.838 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legrand, T.; Salleron, J.; Conroy, T.; Marchal, F.; Thomas, J.; Monard, L.; Biagi, J.J.; Lambert, A. Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial. Cancers 2022, 14, 3945. https://doi.org/10.3390/cancers14163945
Legrand T, Salleron J, Conroy T, Marchal F, Thomas J, Monard L, Biagi JJ, Lambert A. Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial. Cancers. 2022; 14(16):3945. https://doi.org/10.3390/cancers14163945
Chicago/Turabian StyleLegrand, Théo, Julia Salleron, Thierry Conroy, Frédéric Marchal, Jacques Thomas, Laure Monard, James Jim Biagi, and Aurélien Lambert. 2022. "Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial" Cancers 14, no. 16: 3945. https://doi.org/10.3390/cancers14163945
APA StyleLegrand, T., Salleron, J., Conroy, T., Marchal, F., Thomas, J., Monard, L., Biagi, J. J., & Lambert, A. (2022). Preneoplastic Lesions in Surgical Specimens Do Not Worsen the Prognosis of Patients Who Underwent Surgery for Pancreatic Adenocarcinoma: Post-Hoc Analysis of the PRODIGE 24-CCTG PA 6 Trial. Cancers, 14(16), 3945. https://doi.org/10.3390/cancers14163945






