Characteristics, Treatments, and Survival of Uveal Melanoma: A Comparison between Chinese and American Cohorts
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Subject
2.2. Study Variables
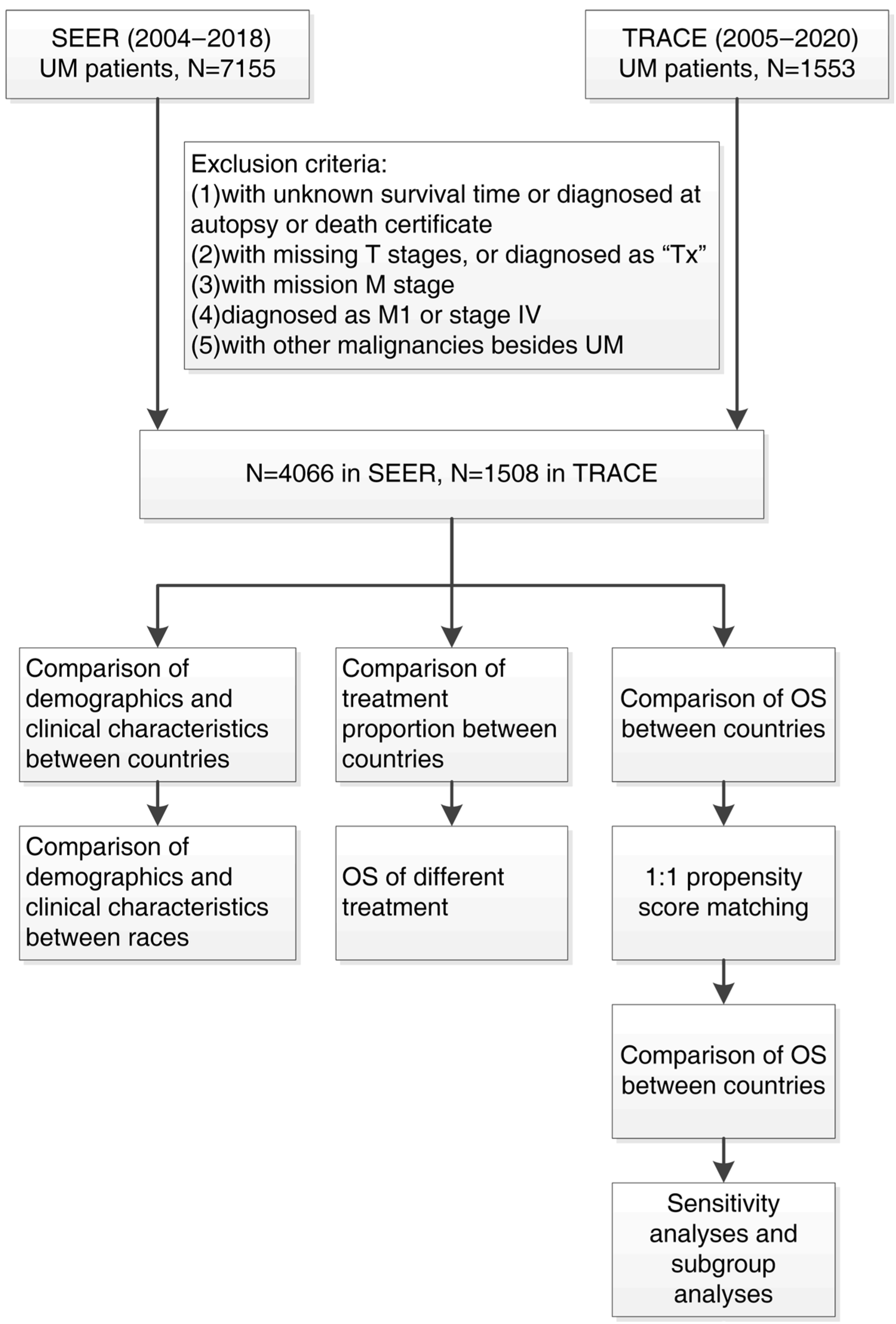
2.3. Study Design
2.4. Statistical Analysis
3. Results
3.1. Population and Tumor Characteristics
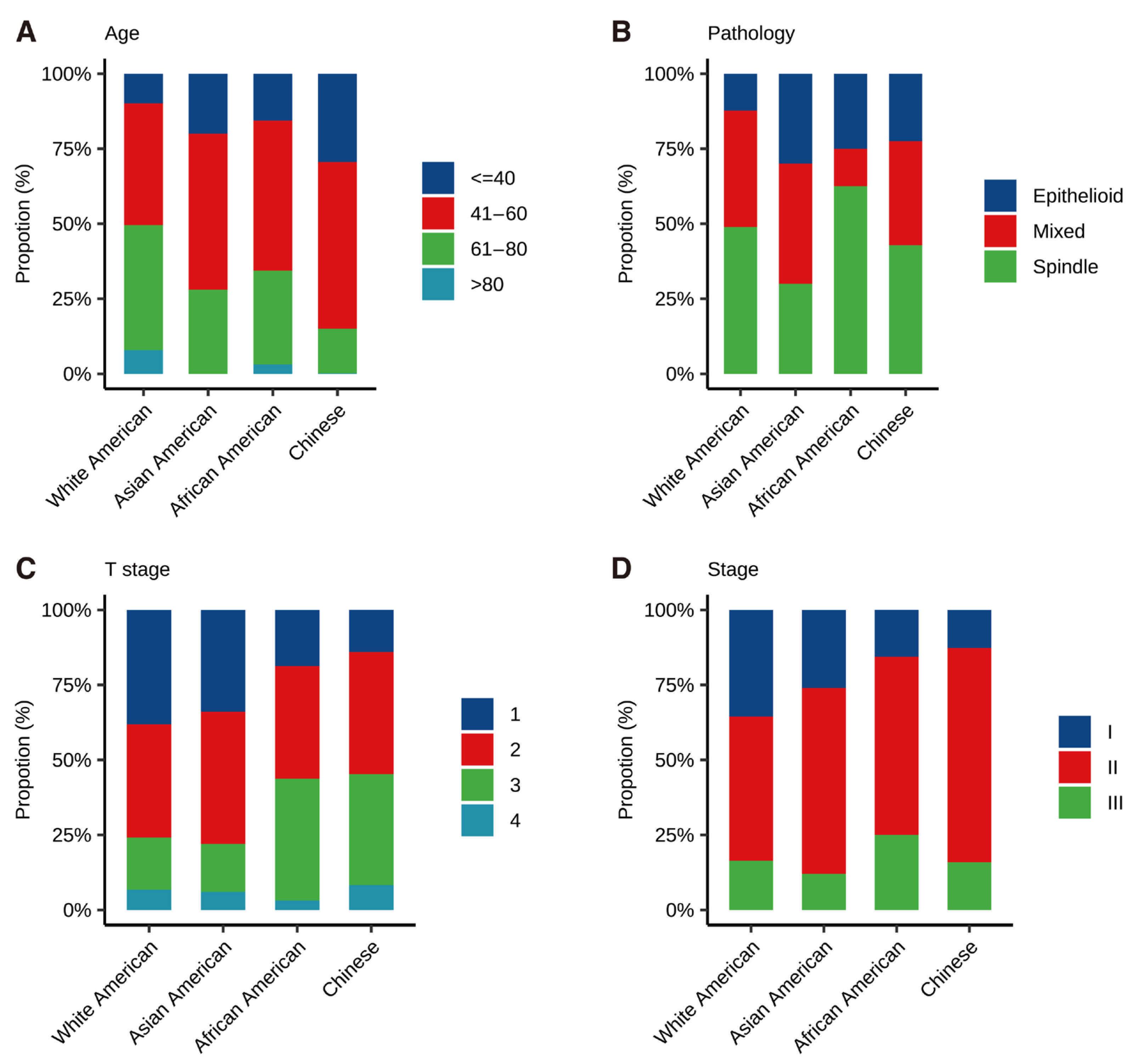
3.2. Tumor Characteristics Disparity in Different Races
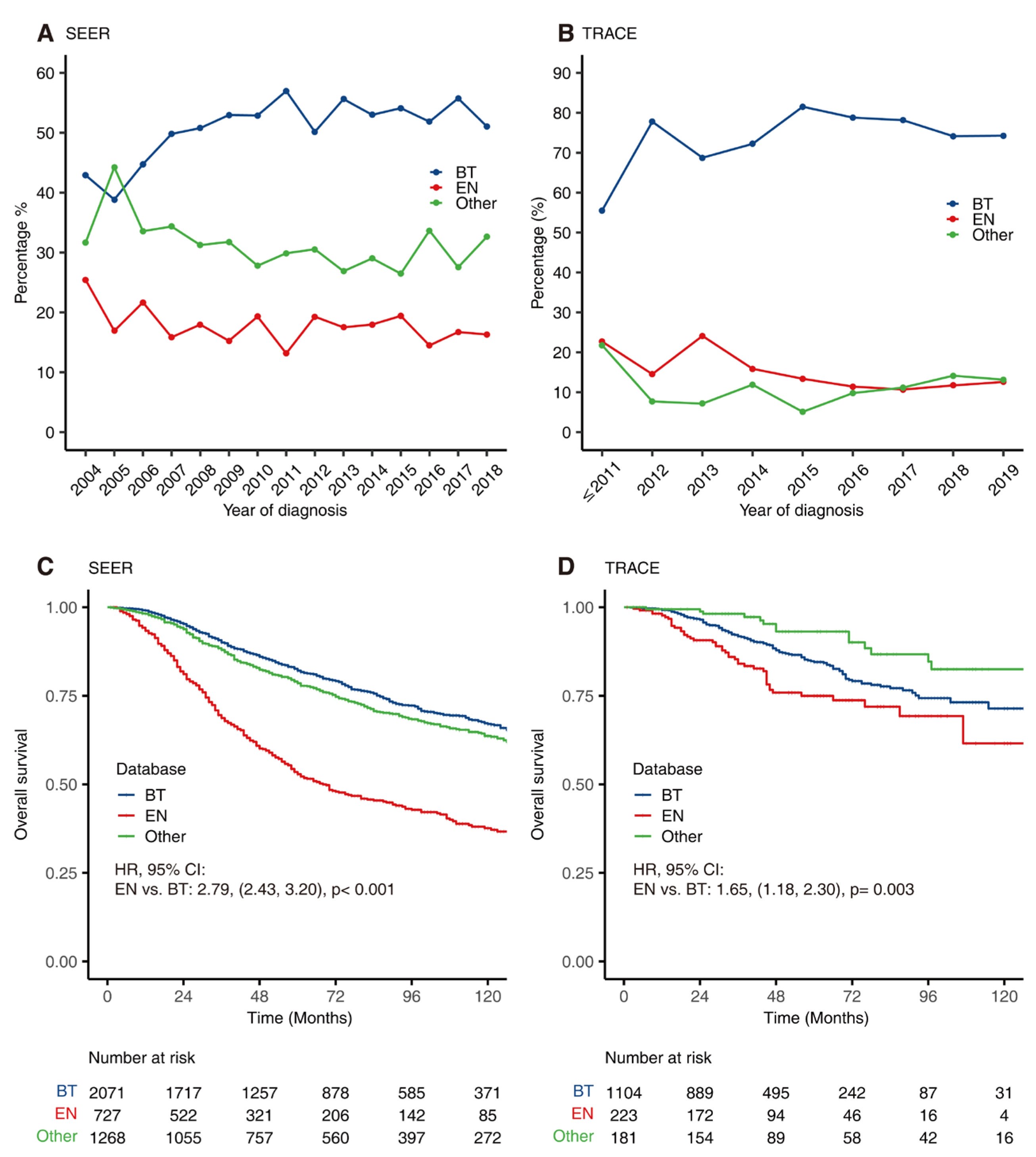
3.3. Trends of Treatment
3.4. Univariate and Multivariate Analysis of Prognosis-Related Factors
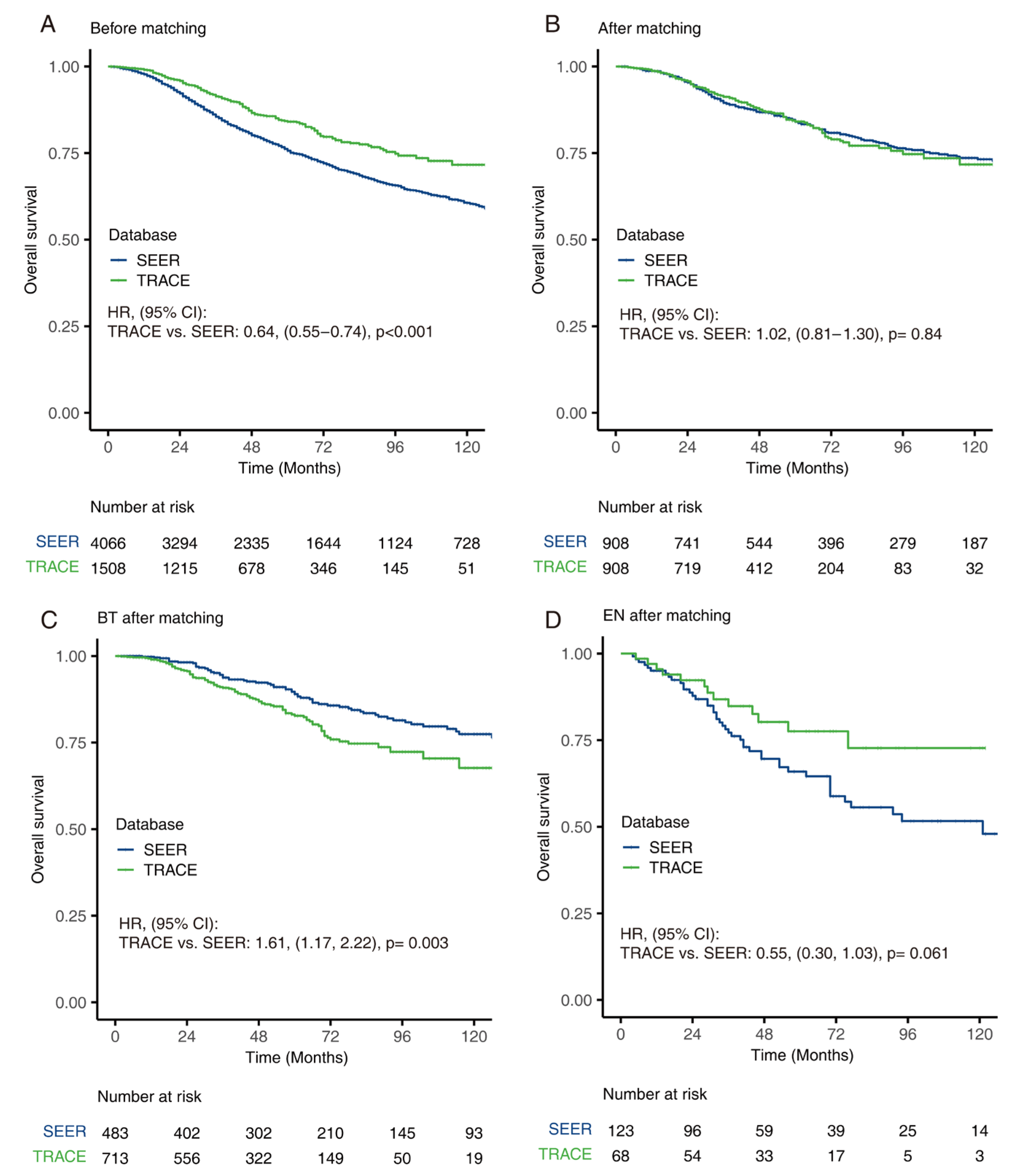
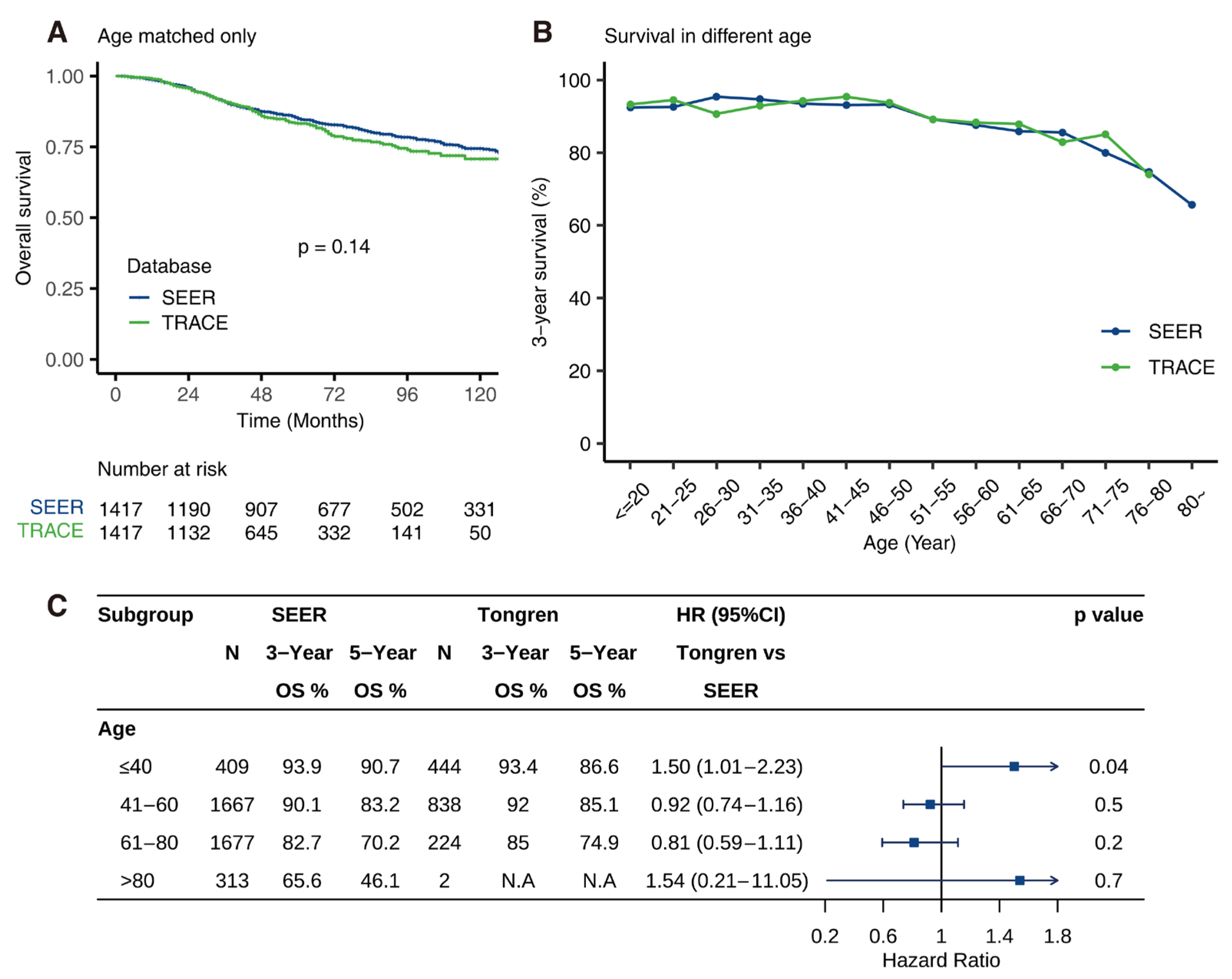
3.5. Comparison of OS between SEER and TRACE
3.6. Age-Dependent Survival Characteristic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jager, M.J.; Shields, C.L.; Cebulla, C.M.; Abdel-Rahman, M.H.; Grossniklaus, H.E.; Stern, M.; Carvajal, R.D.; Belfort, R.N.; Jia, R.; Shields, J.A.; et al. Uveal melanoma. Nat. Rev. Dis. Primers 2020, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.N.; Yu, G.P.; McCormick, S.A.; Schneider, S.; Finger, P.T. Population-based incidence of uveal melanoma in various races and ethnic groups. Am. J. Ophthalmol. 2005, 140, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Manchegowda, P.; Singh, A.D.; Shields, C.; Kaliki, S.; Shah, P.; Gopal, L.; Rishi, P. Uveal Melanoma in Asians: A Review. Ocul. Oncol. Pathol. 2021, 7, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Qian, J.; Yuan, Y.; Zhang, R.; Bi, Y.; Meng, F.; Xuan, Y. Clinicopathological Characteristics and Prognosis for Survival after Enucleation of Uveal Melanoma in Chinese Patients: Long-term Follow-up. Curr. Eye Res. 2017, 42, 759–765. [Google Scholar] [CrossRef]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Cohen, M.N.; Shields, P.W.; Furuta, M.; Shields, J.A. Prognosis of uveal melanoma based on race in 8100 patients: The 2015 Doyne Lecture. Eye 2015, 29, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, T.D.; Lane, A.M.; Morrison, M.; Gragoudas, E.S.; Kim, I.K. Long-term Outcomes After Proton Beam Irradiation in Patients With Large Choroidal Melanomas. JAMA Ophthalmol. 2017, 135, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Rajeshuni, N.; Zubair, T.; Ludwig, C.A.; Moshfeghi, D.M.; Mruthyunjaya, P. Evaluation of Racial, Ethnic, and Socioeconomic Associations With Treatment and Survival in Uveal Melanoma, 2004–2014. JAMA Ophthalmol. 2020, 138, 876–884. [Google Scholar] [CrossRef]
- Rossi, E.; Croce, M.; Reggiani, F.; Schinzari, G.; Ambrosio, M.; Gangemi, R.; Tortora, G.; Pfeffer, U.; Amaro, A. Uveal Melanoma Metastasis. Cancers 2021, 13, 5684. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wang, Y.N.; Chen, M.X.; Zhang, K.; Chen, R.T.; Fang, R.; Wang, H.; Zhang, H.H.; Huang, Y.N.; Feng, Y.; et al. Machine learning models for outcome prediction of Chinese uveal melanoma patients: A 15-year follow-up study. Cancer Commun. 2022, 42, 273–276. [Google Scholar] [CrossRef] [PubMed]
- About the SEER Program. SEER. Available online: https://seer.cancer.gov/about/overview.html (accessed on 20 April 2022).
- Anonymous. Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report no. 1. Arch. Ophthalmol. 1990, 108, 1268–1273, Erratum in Arch. Ophthalmol. 1990, 108, 1708. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.A.; Lu, Y.; Edwards, K.; Jakowatz, J.; Meyskens, F.L.; Liu-Smith, F. Race-, Age-, and Anatomic Site-Specific Gender Differences in Cutaneous Melanoma Suggest Differential Mechanisms of Early- and Late-Onset Melanoma. Int. J. Environ. Res. Public Health 2019, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Rennie, I.G.; Seregard, S.; Giblin, M.; McKenzie, J. Sunlight exposure and pathogenesis of uveal melanoma. Surv. Ophthalmol. 2004, 49, 419–428. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Jampol, L.M.; Moy, C.S.; Murray, T.G.; Reynolds, S.M.; Albert, D.M.; Schachat, A.P.; Diddie, K.R.; Engstrom, R.E., Jr.; Finger, P.T.; Hovland, K.R.; et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002, 109, 2197–2206. [Google Scholar] [CrossRef]
- Bergman, L.; Nilsson, B.; Lundell, G.; Lundell, M.; Seregard, S. Ruthenium Brachytherapy for Uveal Melanoma, 1979–2003: Survival and Functional Outcomes in the Swedish Population. Ophthalmology 2005, 112, 834–840. [Google Scholar] [CrossRef]
- Ah-Fat, F.G.; Damato, B.E. Delays in the diagnosis of uveal melanoma and effect on treatment. Eye 1998, 12 Pt 5, 781–782. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Yang, Y.; Zhang, K.; Liu, Y.; Zhao, H.; Dong, L.; Xu, J.; Li, Y.; Wei, W. Prognosis Prediction of Uveal Melanoma After Plaque Brachytherapy Based on Ultrasound With Machine Learning. Front. Med. 2021, 8, 777142. [Google Scholar] [CrossRef]
- Liu, Y.M.; Li, Y.; Wei, W.B.; Xu, X.; Jonas, J.B. Clinical Characteristics of 582 Patients with Uveal Melanoma in China. PLoS ONE 2015, 10, e0144562. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, J.J.; Bechrakis, N.E.; Singh, N.; Bellerive, C.; Singh, A.D. Iodine-125 Brachytherapy for Uveal Melanoma: A Systematic Review of Radiation Dose. Ocul. Oncol. Pathol. 2017, 3, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Naseripour, M.; Cater, J.; Shields, J.; Demirci, H.; Youseff, A.; Freire, J. Plaque radiotherapy for large posterior uveal melanomas (≥8-mm thick) in 354 consecutive patients. Ophthalmology 2002, 109, 1838–1849. [Google Scholar] [CrossRef]
- Hayreh, S.S. Neovascular glaucoma. Prog. Retin Eye Res. 2007, 26, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Salazar, D.; McCannel, C.A.; Kamrava, M.; Demanes, D.J.; Lamb, J.; Caprioli, J.; McCannel, T.A. Glaucoma After Iodine-125 Brachytherapy for Uveal Melanoma: Incidence and Risk Factors. J. Glaucoma 2020, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oellers, P.; Mowery, Y.M.; Perez, B.A.; Stinnett, S.; Mettu, P.; Vajzovic, L.; Light, K.; Steffey, B.A.; Cai, J.; Dutton, J.J.; et al. Efficacy and Safety of Low-Dose Iodine Plaque Brachytherapy for Juxtapapillary Choroidal Melanoma. Am. J. Ophthalmol. 2018, 186, 32–40. [Google Scholar] [CrossRef]
- Naseripour, M.; Jaberi, R.; Sedaghat, A.; Azma, Z.; Nojomi, M.; Falavarjani, K.G.; Nazari, H. Ruthenium-106 brachytherapy for thick uveal melanoma: Reappraisal of apex and base dose radiation and dose rate. J. Contemp. Brachytherapy 2016, 8, 66–73. [Google Scholar] [CrossRef]
- Kines, R.C.; Varsavsky, I.; Choudhary, S.; Bhattacharya, D.; Spring, S.; McLaughlin, R.; Kang, S.J.; Grossniklaus, H.E.; Vavvas, D.; Monks, S.; et al. An Infrared Dye–Conjugated Virus-like Particle for the Treatment of Primary Uveal Melanoma. Mol. Cancer Ther. 2018, 17, 565–574. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, L.; Wang, Y.; Miao, Q.; Jin, K.; Chen, M.; Ye, J. Epidemiological Study of Uveal Melanoma from US Surveillance, Epidemiology, and End Results Program (2010–2015). J. Ophthalmol. 2020, 2020, 3614039. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.D.; Olszewski, A.J. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int. J. Cancer 2013, 134, 2961–2971. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, M.T.; Mieler, W.F.; I Leiderman, Y. Epidemiological trends in uveal melanoma. Br. J. Ophthalmol. 2015, 99, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, J.P.; Borah, B.J.; Foote, R.L.; Pulido, J.S.; Shah, N.D. Cost-Effectiveness of Proton Beam Therapy for ntraocular Melanoma. PLoS ONE 2015, 10, e0127814. [Google Scholar] [CrossRef]
| SEER (N = 4066) | TRACE (N = 1508) | p Value | |
|---|---|---|---|
| Age: | 59.7 (14.8) | 47.3 (12.5) | <0.001 |
| Sex: | 0.500 | ||
| Male | 1944 (47.8%) | 737 (48.9%) | |
| Female | 2122 (52.2%) | 771 (51.1%) | |
| Race: | 0.000 | ||
| African | 32 (0.79%) | 0 (0.00%) | |
| Asian | 50 (1.23%) | 1508 (100%) | |
| Other | 67 (1.65%) | 0 (0.00%) | |
| White | 3917 (96.3%) | 0 (0.00%) | |
| Location: | <0.001 | ||
| Choroid | 3612 (88.8%) | 1440 (95.5%) | |
| Ciliary body | 367 (9.03%) | 63 (4.18%) | |
| Iris | 87 (2.14%) | 5 (0.33%) | |
| Laterality: | 0.583 | ||
| Left | 2016 (49.7%) | 736 (48.8%) | |
| Right | 2042 (50.3%) | 772 (51.2%) | |
| Pathology: | <0.001 | ||
| Epithelioid | 108 (12.4%) | 83 (22.5%) | |
| Mixed | 338 (38.9%) | 128 (34.7%) | |
| Spindle | 423 (48.7%) | 158 (42.8%) | |
| T: | <0.001 | ||
| 1 | 1544 (38.0%) | 212 (14.1%) | |
| 2 | 1541 (37.9%) | 614 (40.7%) | |
| 3 | 712 (17.5%) | 557 (36.9%) | |
| 4 | 269 (6.6%) | 125 (8.3%) | |
| Diameter | 11.3 (8.27) | 12.0 (3.54) | |
| Thickness | 4.91 (3.01) | 7.13 (3.28) | |
| M: 0 | 4066 (100%) | 1508 (100%) | |
| Stage: | <0.001 | ||
| I | 1436 (35.3%) | 192 (12.7%) | |
| II | 1966 (48.4%) | 1076 (71.4%) | |
| III | 664 (16.3%) | 240 (15.9%) |
| SEER (N = 4066) | TRACE (N = 1508) | OR | p Value | |
|---|---|---|---|---|
| Brachytherapy: | <0.001 | |||
| No | 1995 (49.1%) | 404 (26.8%) | Ref. | |
| Yes | 2071 (50.9%) | 1104 (73.2%) | 2.63 [2.31; 3.00] | |
| Enucleation: | 0.007 | |||
| No | 3339 (82.1%) | 1285 (85.2%) | Ref. | |
| Yes | 727 (17.9%) | 223 (14.8%) | 0.80 [0.68; 0.94] | |
| Other: | <0.001 | |||
| No | 2798 (68.8%) | 1327 (88.0%) | Ref. | |
| Yes | 1268 (31.2%) | 181 (12.0%) | 0.30 [0.25; 0.36] |
| SEER | TRACE | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | p Value | Multivariable | p Value | Univariable | p Value | Mulitvariable | p Value | |
| Age | ||||||||
| ≤40 | Reference | Reference | Reference | Reference | ||||
| 41–60 | 1.90 (1.39–2.59) | <0.001 | 1.88 (1.37–2.56) | <0.001 | 1.17 (0.85–1.62) | 0.344 | 1.15 (0.83–1.60) | 0.402 |
| 61–80 | 3.55 (2.62–4.81) | <0.001 | 3.53 (2.60–4.79) | <0.001 | 1.99 (1.34–2.98) | 0.001 | 2.10 (1.40–3.14) | <0.001 |
| >80 | 9.02 (6.52–12.48) | <0.001 | 8.62 (6.23–11.94) | <0.001 | 9.46 (1.3–68.77) | 0.026 | 16.03 (2.18–118.16) | 0.006 |
| Sex | ||||||||
| Female | Reference | Reference | ||||||
| Male | 1.11 (0.99–1.25) | 0.078 | 1.12 (0.85–1.47) | 0.431 | ||||
| Laterality | ||||||||
| Left | Reference | Reference | ||||||
| Right | 1.11 (0.99–1.24) | 0.089 | 1.03 (0.78–1.35) | 0.845 | ||||
| Location | ||||||||
| Choroid | Reference | Reference | Reference | Reference | ||||
| Ciliary body | 1.27 (1.06–1.51) | 0.008 | 1.28 (1.06–1.53) | 0.01 | 1.22 (0.64–2.3) | 0.545 | 0.77 (0.37–1.59) | 0.478 |
| Iris | 0.14 (0.04–0.42) | 0.001 | 0.16 (0.05–0.50) | 0.002 | 6.95 (0.96–50.15) | 0.055 | 9.54 (1.28–71.15) | 0.028 |
| Pathology | ||||||||
| Spindle | Reference | Reference | Reference | Reference | ||||
| Epithelioid | 3.1 (2.24–4.30) | <0.001 | 1.97 (1.41–2.73) | <0.001 | 2.6 (1.57–4.3) | <0.001 | 2.52 (1.51–4.20) | <0.001 |
| Mixed | 2.72 (2.11–3.50) | <0.001 | 1.84 (1.42–2.37) | <0.001 | 1.37 (0.8–2.33) | 0.251 | 1.15 (0.67–1.98) | 0.611 |
| T | ||||||||
| T1 | Reference | Reference | Reference | Reference | ||||
| T2 | 1.54 (1.33–1.79) | <0.001 | 1.19 (0.71–2.00) | 0.511 | 1.58 (0.85–2.93) | 0.149 | 0.65 (0.18–2.32) | 0.509 |
| T3 | 3.1 (2.63–3.66) | <0.001 | 2.14 (1.24–3.72) | 0.007 | 3.35 (1.84–6.11) | <0.001 | 1.25 (0.35–4.40) | 0.731 |
| T4 | 4.56 (3.7–5.63) | <0.001 | 2.72 (1.50–4.93) | 0.001 | 6.24 (3.22–12.1) | <0.001 | 1.51 (0.37–6.16) | 0.564 |
| Stage | ||||||||
| I | Reference | Reference | Reference | Reference | ||||
| II | 1.64 (1.42–1.90) | <0.001 | 1.25 (0.74–2.11) | 0.406 | 2.59 (1.32–5.01) | 0.006 | 3.09 (0.76–12.65) | 0.116 |
| III | 3.76 (3.19–4.42) | <0.001 | 1.42 (0.80–2.54) | 0.234 | 6.36 (3.15–12.85) | <0.001 | 4.42 (0.95–20.67) | 0.059 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| SEER | TRACE | p Value | SEER | TRACE | p Value | |
| Age | 59.7 (14.8) | 47.3 (12.5) | <0.001 | 51.5 (11.8) | 51.1 (11.4) | 0.559 |
| Sex: | 0.500 | 0.963 | ||||
| Female | 1944 (47.8%) | 737 (48.9%) | 439 (48.3%) | 441 (48.6%) | ||
| Male | 2122 (52.2%) | 771 (51.1%) | 469 (51.7%) | 467 (51.4%) | ||
| Location: | <0.001 | 0.083 | ||||
| Choroid | 3612 (88.8%) | 1440 (95.5%) | 890 (98.0%) | 876 (96.5%) | ||
| Ciliary body | 367 (9.03%) | 63 (4.18%) | 17 (1.87%) | 27 (2.97%) | ||
| Iris | 87 (2.14%) | 5 (0.33%) | 1 (0.11%) | 5 (0.55%) | ||
| Pathology: | <0.001 | 0.328 | ||||
| Epithelioid | 108 (2.66%) | 83 (5.50%) | 14 (1.54%) | 8 (0.88%) | ||
| Mixed | 338 (8.31%) | 128 (8.49%) | 36 (3.96%) | 34 (3.74%) | ||
| Spindle | 423 (10.4%) | 158 (10.5%) | 58 (6.39%) | 73 (8.04%) | ||
| Unknown | 3197 (78.6%) | 1139 (75.5%) | 800 (88.1%) | 793 (87.3%) | ||
| T: | <0.001 | 0.916 | ||||
| 1 | 1544 (38.0%) | 212 (14.1%) | 194 (21.4%) | 185 (20.4%) | ||
| 2 | 1541 (37.9%) | 614 (40.7%) | 457 (50.3%) | 470 (51.8%) | ||
| 3 | 712 (17.5%) | 557 (36.9%) | 217 (23.9%) | 216 (23.8%) | ||
| 4 | 269 (6.62%) | 125 (8.29%) | 40 (4.41%) | 37 (4.07%) | ||
| Stage: | <0.001 | 0.749 | ||||
| I | 1436 (35.3%) | 192 (12.7%) | 190 (20.9%) | 177 (19.5%) | ||
| II | 1966 (48.4%) | 1076 (71.4%) | 639 (70.4%) | 650 (71.6%) | ||
| III | 664 (16.3%) | 240 (15.9%) | 79 (8.70%) | 81 (8.92%) | ||
| Stage: | <0.001 | 0.749 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Zhang, C.; Yang, Y.; Xiu, J.; Zhao, H.; Liang, C.; Feng, Z.; Chen, Y.; Liu, Y.; Li, Y.; et al. Characteristics, Treatments, and Survival of Uveal Melanoma: A Comparison between Chinese and American Cohorts. Cancers 2022, 14, 3960. https://doi.org/10.3390/cancers14163960
Luo J, Zhang C, Yang Y, Xiu J, Zhao H, Liang C, Feng Z, Chen Y, Liu Y, Li Y, et al. Characteristics, Treatments, and Survival of Uveal Melanoma: A Comparison between Chinese and American Cohorts. Cancers. 2022; 14(16):3960. https://doi.org/10.3390/cancers14163960
Chicago/Turabian StyleLuo, Jingting, Chengkai Zhang, Yuhang Yang, Jingying Xiu, Hanqing Zhao, Chuqiao Liang, Zhaoxun Feng, Yuning Chen, Yueming Liu, Yang Li, and et al. 2022. "Characteristics, Treatments, and Survival of Uveal Melanoma: A Comparison between Chinese and American Cohorts" Cancers 14, no. 16: 3960. https://doi.org/10.3390/cancers14163960
APA StyleLuo, J., Zhang, C., Yang, Y., Xiu, J., Zhao, H., Liang, C., Feng, Z., Chen, Y., Liu, Y., Li, Y., & Wei, W. (2022). Characteristics, Treatments, and Survival of Uveal Melanoma: A Comparison between Chinese and American Cohorts. Cancers, 14(16), 3960. https://doi.org/10.3390/cancers14163960






