Quantitative Analysis of Colonic Perfusion Using ICG Fluorescence Angiography and Its Consequences for Anastomotic Healing in a Rat Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
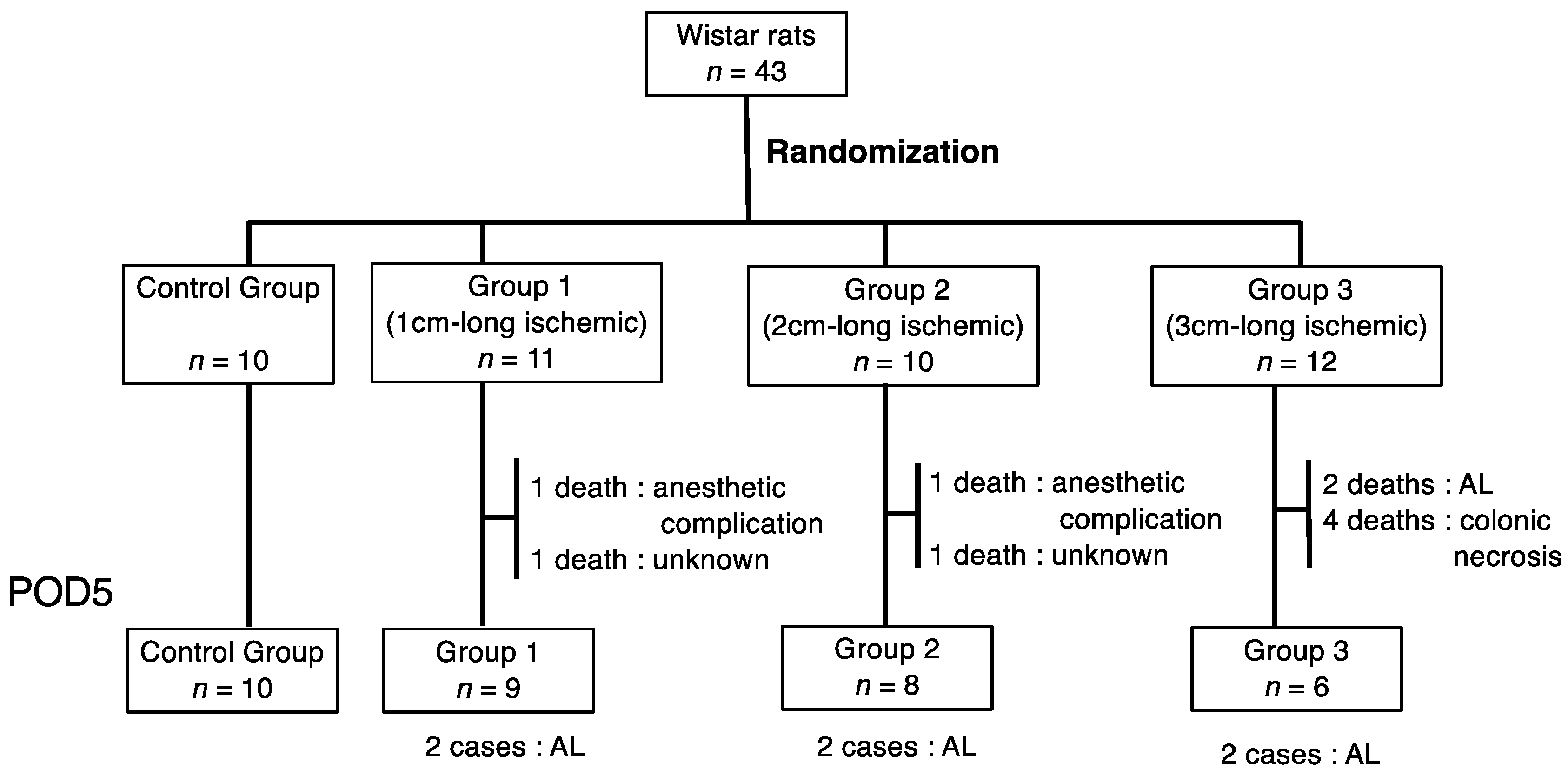
2.1. Animals and Ethical Considerations
2.2. Anesthesia and Surgical Procedures
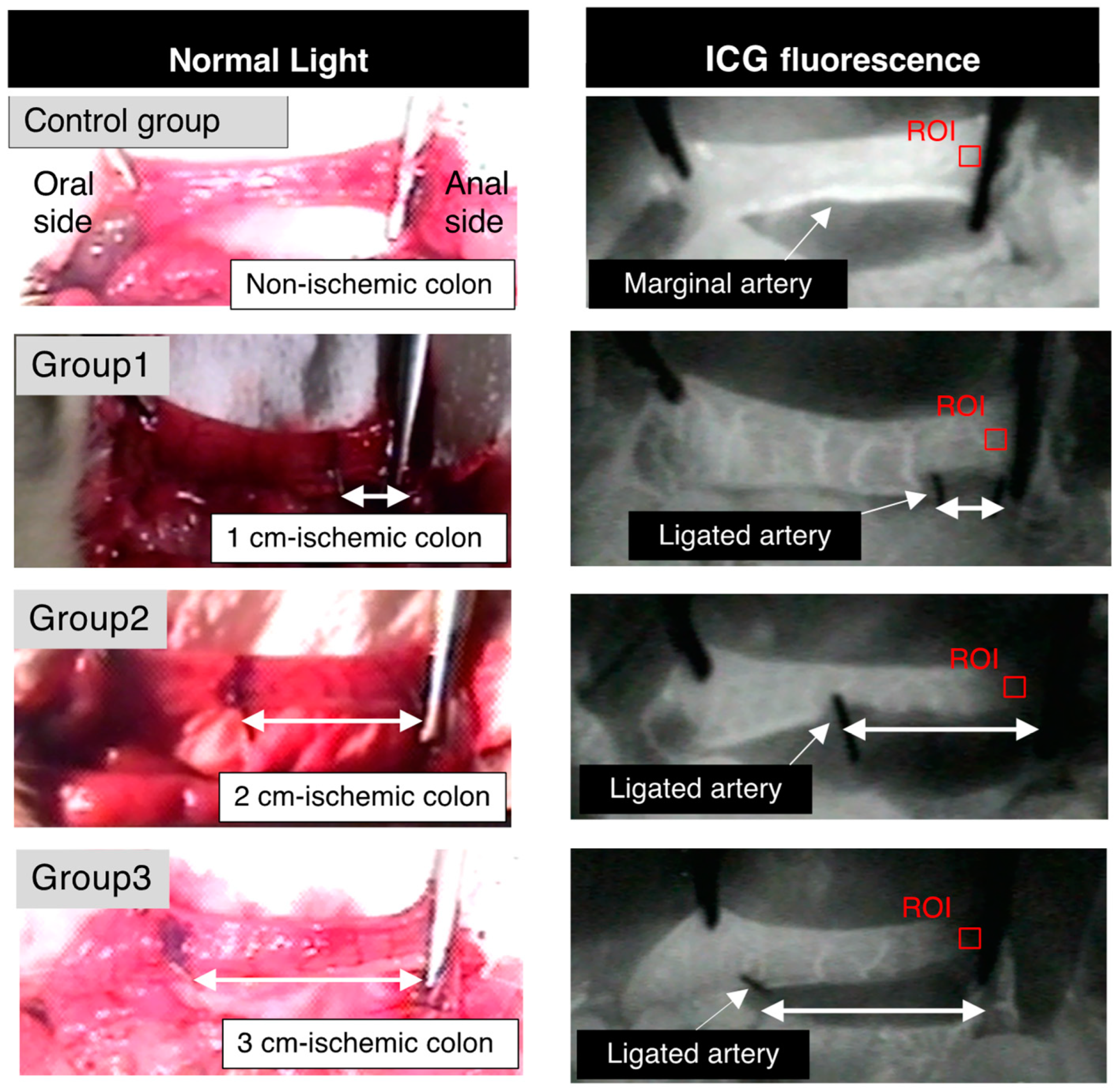
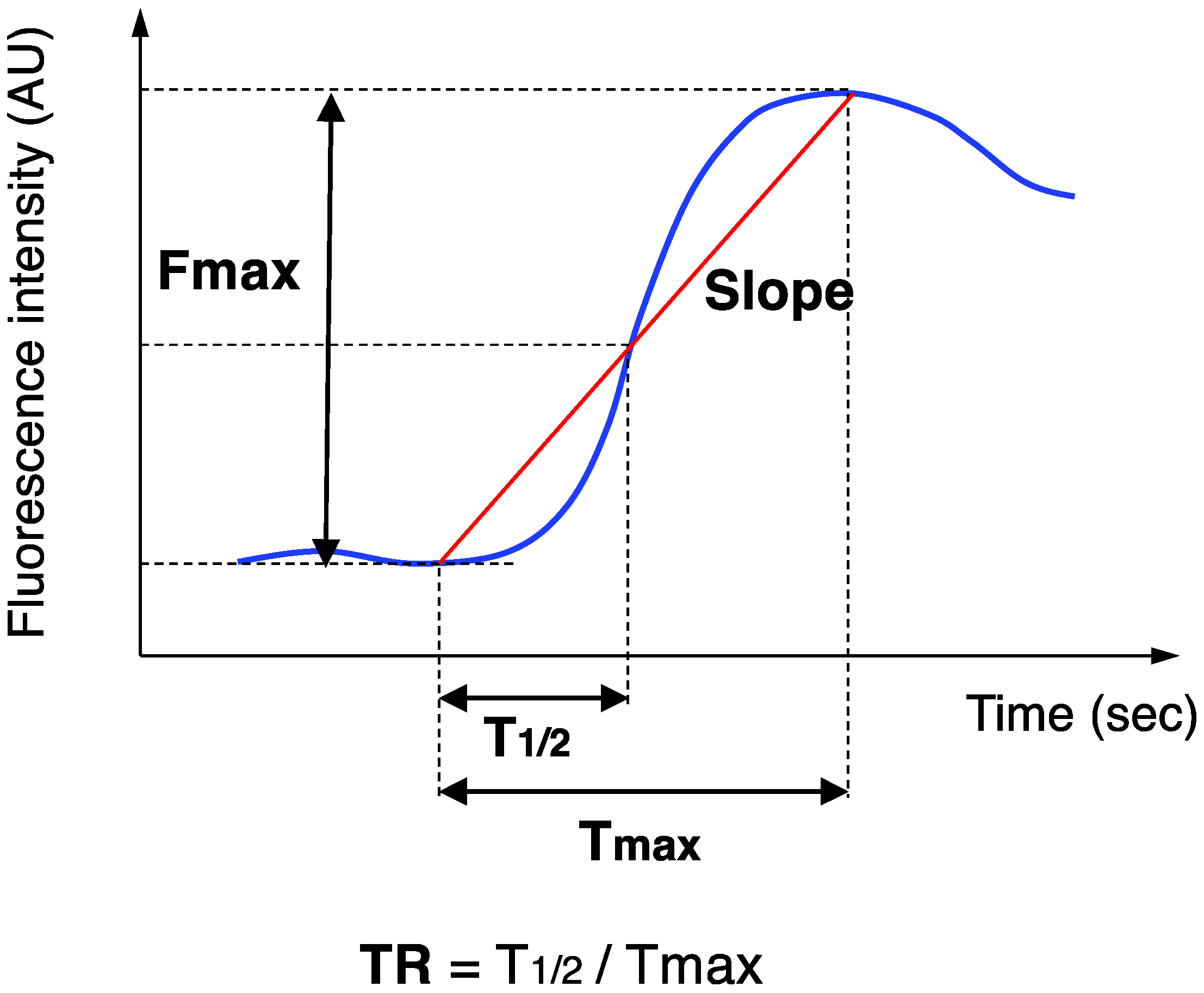
2.3. ICG Fluorescence Angiography
2.4. Bursting Pressure Measurements
2.5. Histopathological Assessment
2.6. Statistical Analysis
3. Results
3.1. Anastomotic Leakage (AL)
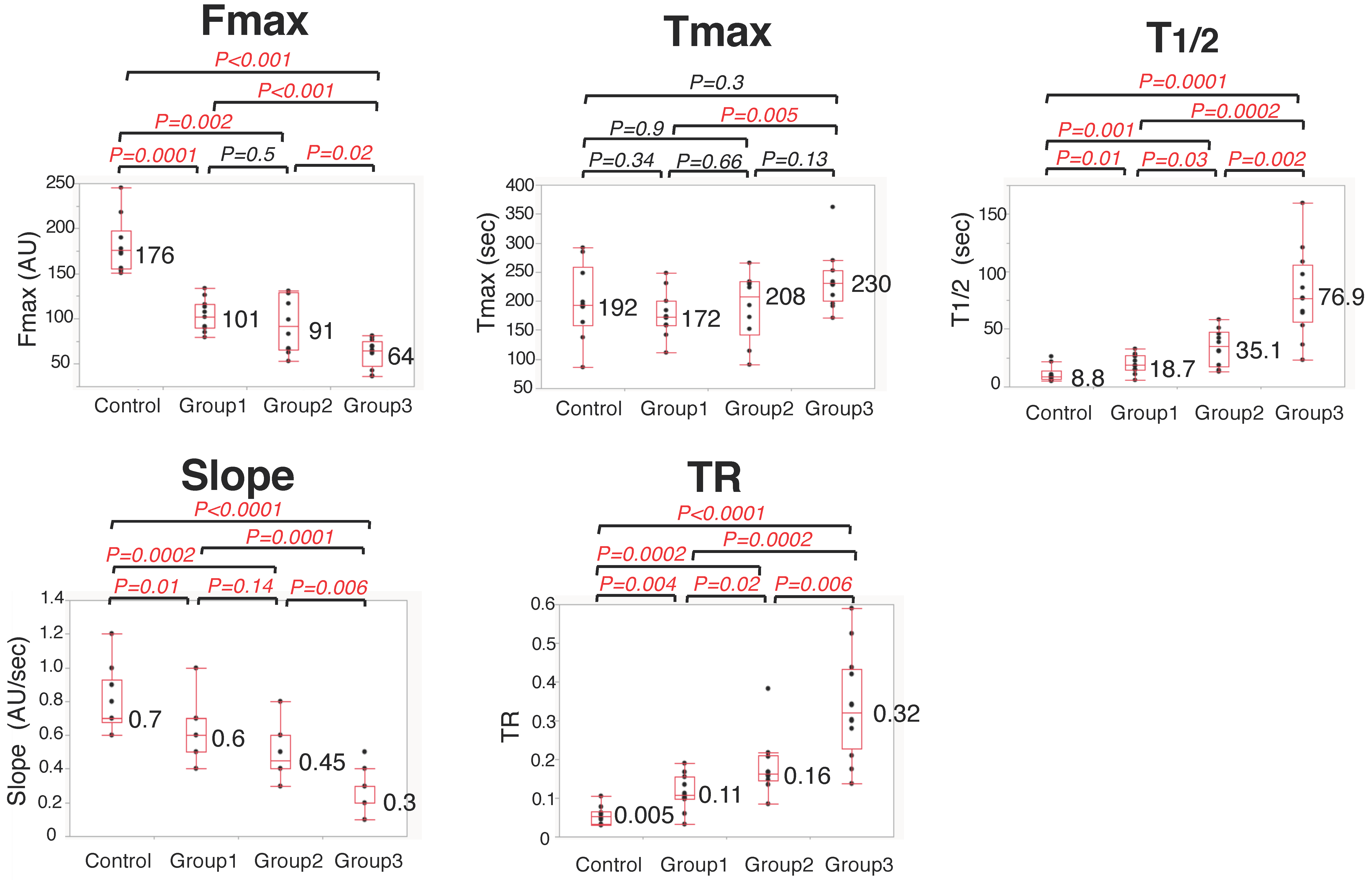
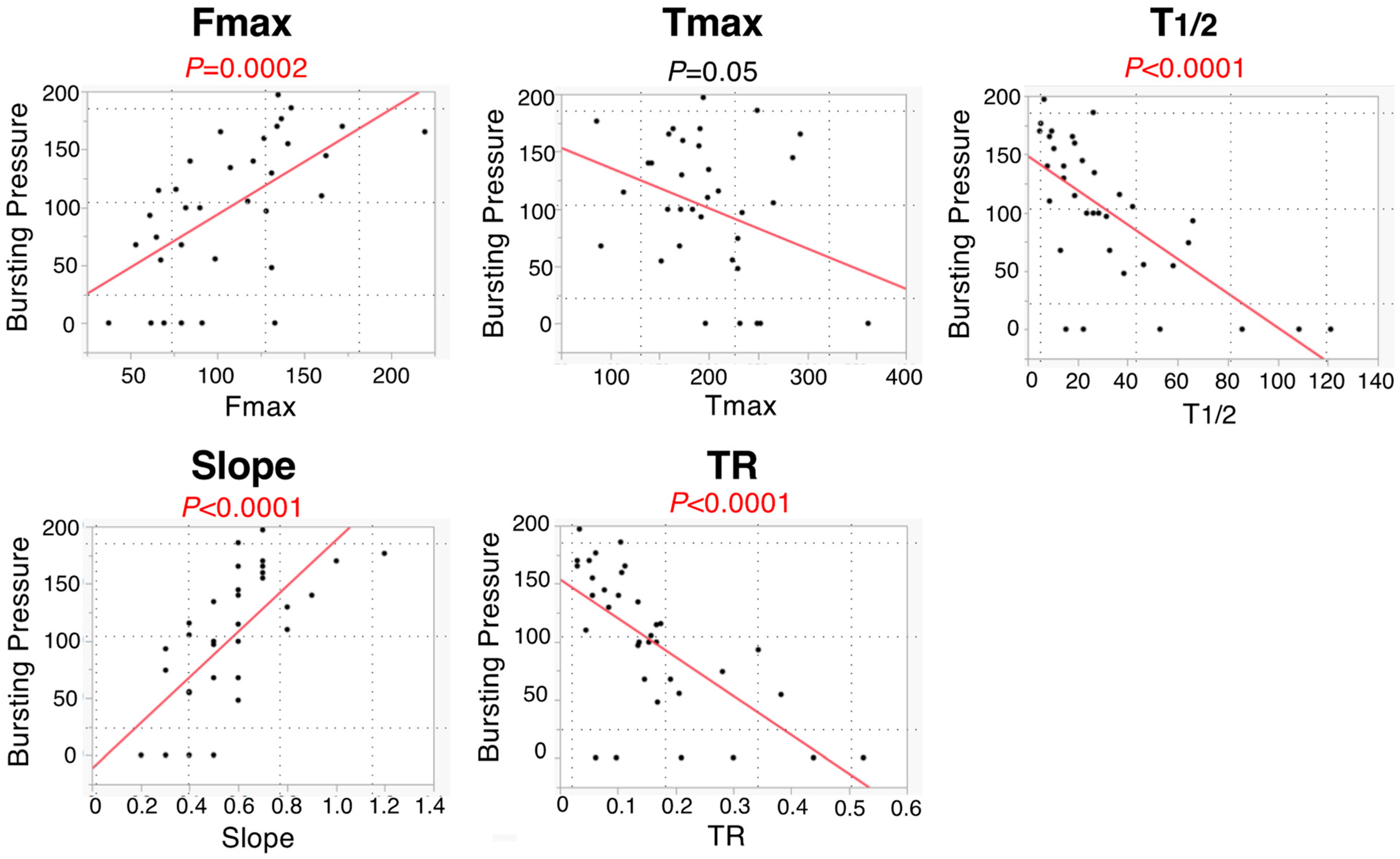
3.2. Colonic Blood Flow Measured by ICG Fluorescence Angiography
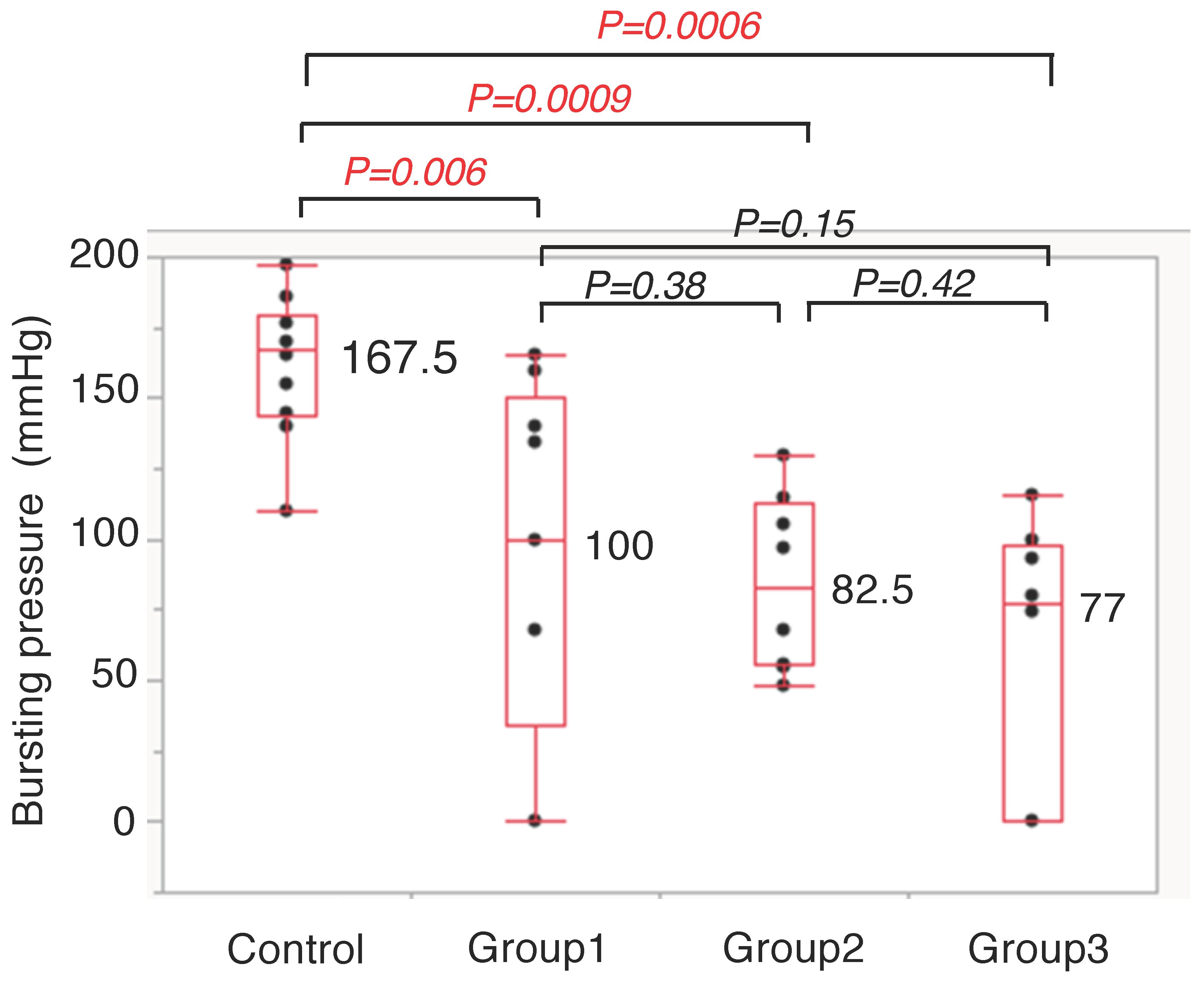
3.3. Anastomotic Bursting Pressure
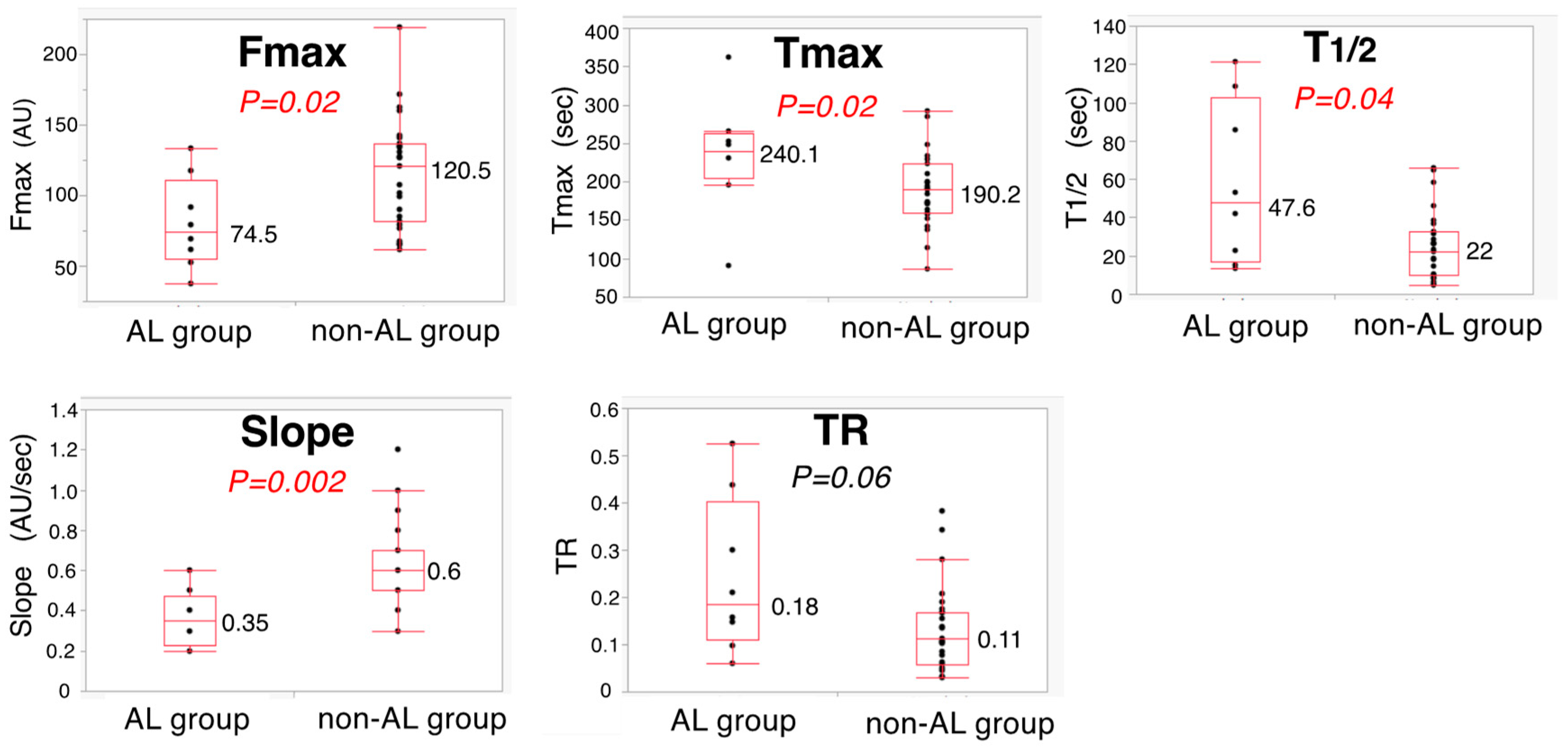
3.4. Five ICG Fluorescence-Related Parameters between AL Group and Non-AL Group
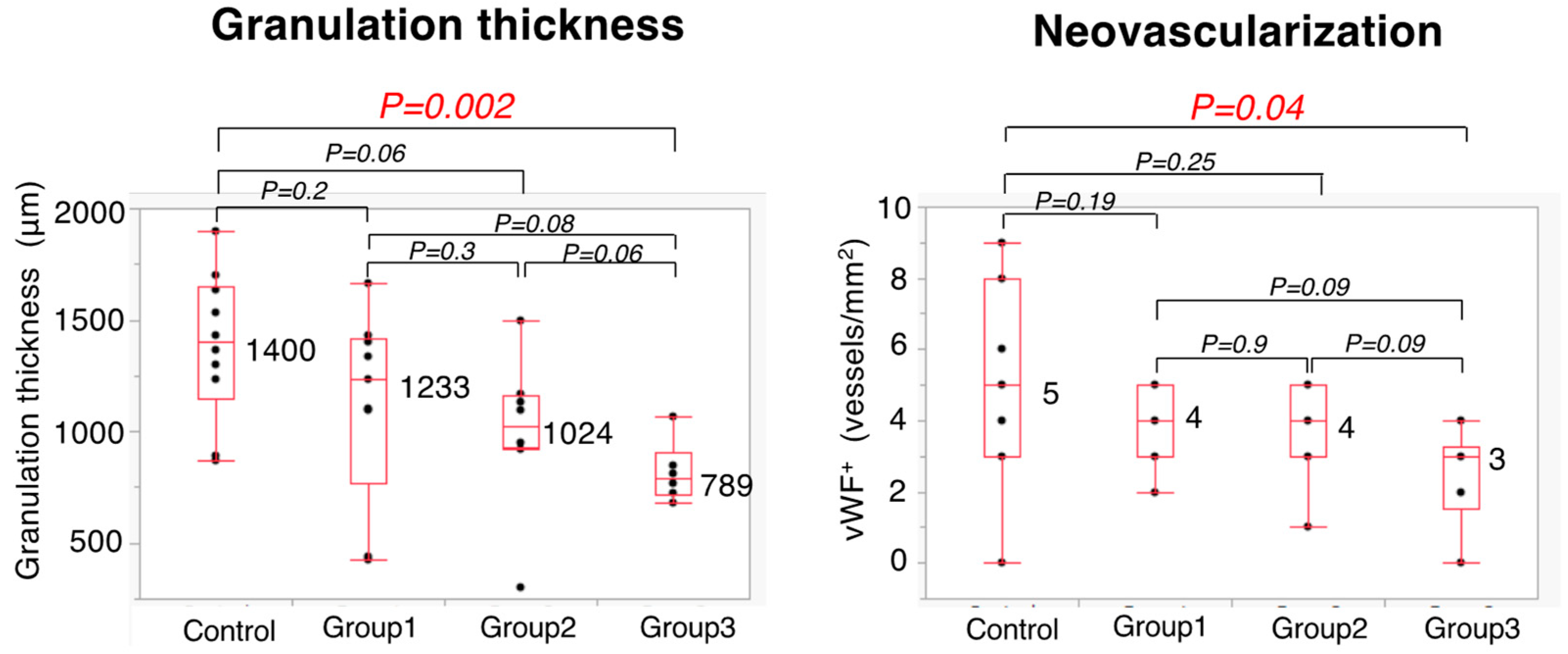
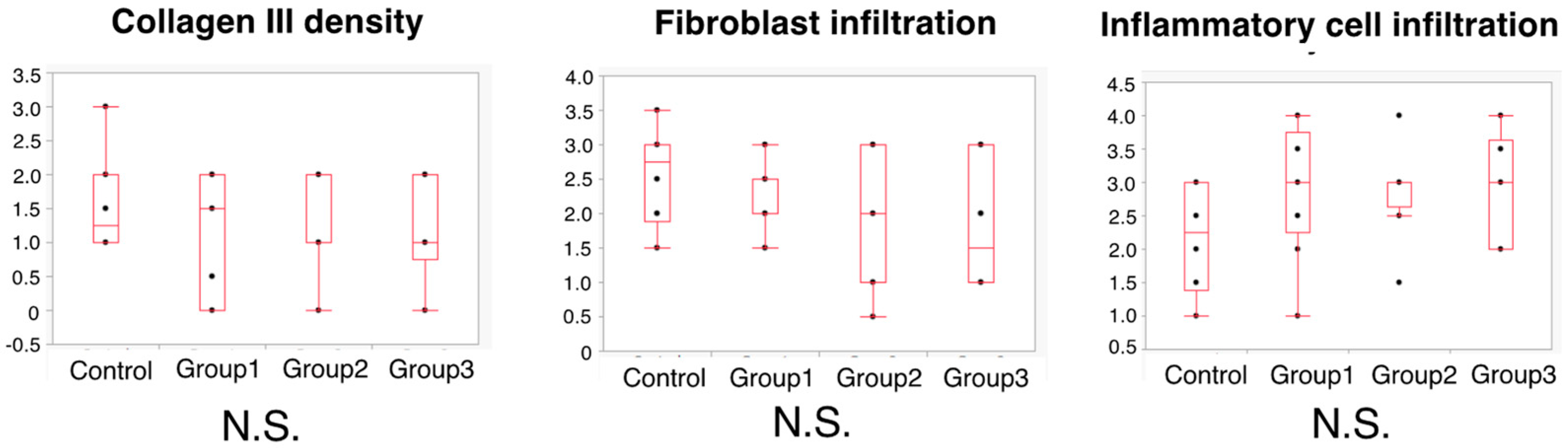
3.5. Histopathological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, C.Y.; Halabi, W.J.; Chaudhry, O.O.; Nguyen, V.; Pigazzi, A.; Carmichael, J.C.; Millis, S.; Stamos, M.J. Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg. 2013, 148, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, N.; Miyata, H.; Gotoh, M.; Tomita, N.; Baba, H.; Kimura, W.; Nakagoe, T.; Simada, M.; Kitagawa, Y.; Sugihara, K.; et al. Mortality after common rectal surgery in Japan: A study on low anterior resection from a newly established nationwide large-scale clinical database. Dis. Colon Rectum 2014, 57, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Posma, L.A.; Hendriks, T.; Verhofstad, A.A.; de Man, B.M.; Lomme, R.M.; Bleichrodt, R.P. Reduction of oxygenation and blood flow in pedicled bowel segments in the rat and its consequences for anastomotic healing. Dis. Colon Rectum 2010, 53, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kawada, K.; Hirai, K.; Toda, K.; Iwamoto, M.; Hasegawa, S.; Sakai, Y. Enhanced anastomotic healing by Daikenchuto (TJ-100) in rats. Sci. Rep. 2018, 18, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, K.; Hasegawa, S.; Wada, T.; Takahashi, R.; Hisamori, S.; Hida, K.; Sakai, Y. Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg. Endosc. 2017, 31, 1061–1069. [Google Scholar] [CrossRef]
- Kudszus, S.; Roesel, C.; Schachtrupp, A.; Höer, J.J. Intraoperative laser fluorescence angiography in colorectal surgery: A noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch. Surg. 2010, 395, 1025–1030. [Google Scholar] [CrossRef]
- Jafari, M.D.; Lee, K.H.; Halabi, W.J.; Mills, S.D.; Carmichael, J.C.; Stamos, M.J.; Pigazzi, A. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg. Endosc. 2013, 27, 3003–3008. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, J.L.; Park, S.H. Interpretative Guidelines and Possible Indications for Indocyanine Green Fluorescence Imaging in Robot-Assisted Sphincter-Saving Operations. Dis. Colon Rectum 2017, 60, 376–384. [Google Scholar] [CrossRef]
- Watanabe, J.; Ishibe, A.; Suwa, Y.; Suwa, H.; Ota, M.; Kunisaki, C.; Endo, I. Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: A propensity score-matched cohort study. Surg. Endosc. 2020, 34, 202–208. [Google Scholar]
- Boni, L.; Fingerhut, A.; Marzorati, A.; Rausei, S.; Dionigi, G.; Cassinotti, E. Indocyanine green fluorescence angiography during laparoscopic low anterior resection: Results of a case-matched study. Surg. Endosc. 2016, 31, 1836–1840. [Google Scholar] [CrossRef]
- Wada, T.; Kawada, K.; Hoshino, N.; Inamoto, S.; Yoshitomi, M.; Hida, K.; Sakai, Y. The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: A propensity score-matched study. Int. J. Clin. Oncol. 2019, 24, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Noll, E.; Diemunsch, P.; Dallemagne, B.; Benahmed, M.A.; Agnus, V.; Soler, L.; Barry, B.; Namer, I.J.; Demartines, N.; et al. Enhanced-reality video fluorescence: A real-time assessment of intestinal viability. Ann. Surg. 2014, 259, 700–707. [Google Scholar] [CrossRef]
- Gosvig, K.; Jensen, S.S.; Qvist, N.; Agnus, V.; Jensen, T.S.; Lindner, V.; Marescaux, J.; Diana, M.; Ellebæk, M.B. Remote computer-assisted analysis of ICG fluorescence signal for evaluation of small intestinal anastomotic perfusion: A blinded, randomized, experimental trial. Surg. Endosc. 2020, 34, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kawada, K.; Takahashi, R.; Yoshitomi, M.; Hida, K.; Hasegawa, S.; Sakai, Y. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg. Endosc. 2017, 31, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Unno, N.; Miyazaki, S.; Sano, M.; Kikuchi, H.; Hiramatsu, Y.; Ohta, M.; Yamatodani, T.; Mineta, H.; Konno, H. Quantitative assessment of the free jejunal graft perfusion. J. Surg. Res. 2015, 194, 394–399. [Google Scholar] [CrossRef]
- Iwamoto, H.; Matsuda, K.; Hayami, S.; Tamura, K.; Mitani, Y.; Mizumoto, Y.; Nakamura, Y.; Murakami, D.; Ueno, M.; Yokoyama, S.; et al. Quantitative Indocyanine Green Fluorescence Imaging Used to Predict Anastomotic Leakage Focused on Rectal Stump During Laparoscopic Anterior Resection. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Son, G.M.; Kwon, M.S.; Kim, Y.; Kim, J.; Kim, S.H.; Lee, J.W. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg. Endosc. 2019, 33, 1640–1649. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H.P.; Trarver, H.; Hunt, T.K. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann. Surg. 1973, 177, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Sakai, Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J. Gastroenterol. 2016, 22, 5718–5727. [Google Scholar] [CrossRef] [Green Version]
- Diana, M.; Agnus, V.; Halvax, P.; Liu, Y.Y.; Dallemagne, B.; Schlagowski, A.I.; Geny, B.; Diemunsch, P.; Lindner, V.; Marescaux, J. Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br. J. Surg. 2015, 102, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nerup, N.; Andersen, H.S.; Ambrus, R.; Strandby, R.B.; Svendsen, M.B.S.; Madsen, M.H.; Svendsen, L.B.; Achiam, M.P. Quantification of fluorescence angiography in a porcine model. Langenbecks Arch. Surg. 2017, 402, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Winer, J.H.; Laurence, R.G.; Frangioni, J.V. Predicting the survival of experimental ischaemic small bowel using intraoperative near-infrared fluorescence angiography. Br. J. Surg. 2011, 98, 1725–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Colorectal Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: Results of a multicenter randomized controlled trial. Surg. Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, T.; Kawada, K.; Hanada, K.; Obama, K. Quantitative Analysis of Colonic Perfusion Using ICG Fluorescence Angiography and Its Consequences for Anastomotic Healing in a Rat Model. Cancers 2022, 14, 4024. https://doi.org/10.3390/cancers14164024
Wada T, Kawada K, Hanada K, Obama K. Quantitative Analysis of Colonic Perfusion Using ICG Fluorescence Angiography and Its Consequences for Anastomotic Healing in a Rat Model. Cancers. 2022; 14(16):4024. https://doi.org/10.3390/cancers14164024
Chicago/Turabian StyleWada, Toshiaki, Kenji Kawada, Keita Hanada, and Kazutaka Obama. 2022. "Quantitative Analysis of Colonic Perfusion Using ICG Fluorescence Angiography and Its Consequences for Anastomotic Healing in a Rat Model" Cancers 14, no. 16: 4024. https://doi.org/10.3390/cancers14164024
APA StyleWada, T., Kawada, K., Hanada, K., & Obama, K. (2022). Quantitative Analysis of Colonic Perfusion Using ICG Fluorescence Angiography and Its Consequences for Anastomotic Healing in a Rat Model. Cancers, 14(16), 4024. https://doi.org/10.3390/cancers14164024





