Predictive Factors of Long-Term Survival after Neoadjuvant Radiotherapy and Chemotherapy in High-Risk Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Endpoint Definition
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. PCR as a Prognostic Factor
4.2. Chemotherapy Regimens
4.3. The Role of Radiotherapy
4.4. Main Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | adriamycin, cyclophosphamide |
| ALND | axillary lymph node dissection |
| BCS | breast-conserving surgery |
| CTx | chemotherapy |
| CI | confidence interval |
| CMF | cyclophosphamide, methotrexate, fluorouracil |
| DFS | disease free survival |
| DIEP | deep inferior epigastric perforator |
| EC | epirubicin, cyclophosphamide |
| ER | endocrine receptor |
| EQD2 | 2 Gy equivalent dose |
| FU | follow-up |
| HER2 | human epidermal growth factor receptor 2 |
| HR | hazard ratio |
| ICI | immune checkpoint inhibitor |
| IMN | internal mammary nodes |
| MTx | mastectomy |
| naCT | neoadjuvant chemotherapy |
| naRCT | neoadjuvant radiochemotherapy |
| naRT | neoadjuvant radiotherapy |
| naST | neoadjuvant systemic therapy |
| OS | overall survival |
| pCR | pathologic complete response |
| RCB | residual cancer burden |
| RNI | regional nodal irradiation |
| RT | radiotherapy |
| SLND | sentinel lymph node dissection |
| ST | systemic therapy |
| TN | triple negative |
| ypN | postoperative nodal stage |
| ypT | postoperative primary tumor stage |
References
- Kaufmann, M.; von Minckwitz, G.; Mamounas, E.P.; Cameron, D.; Carey, L.A.; Cristofanilli, M.; Denkert, C.; Eiermann, W.; Gnant, M.; Harris, J.R.; et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann. Surg. Oncol. 2011, 19, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2017, 19, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Berruti, A.; Amoroso, V.; Gallo, F.; Bertaglia, V.; Simoncini, E.; Pedersini, R.; Ferrari, L.; Bottini, A.; Bruzzi, P.; Sormani, M.P. Pathologic Complete Response As a Potential Surrogate for the Clinical Outcome in Patients With Breast Cancer After Neoadjuvant Therapy: A Meta-Regression of 29 Randomized Prospective Studies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3883–3891. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Makhoul, I.; Kiwan, E. Neoadjuvant systemic treatment of breast cancer. J. Surg. Oncol. 2011, 103, 348–357. [Google Scholar] [CrossRef]
- SPY2 Trial Consortium. Association of Event-Free and Distant Recurrence–Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1355–1362. [Google Scholar] [CrossRef]
- Wallgren, A.; Arner, O.; Bergström, J.; Blomstedt, B.; Granberg, P.O.; Karnström, L.; Räf, L.; Silfverswärd, C. Preoperative radiotherapy in operable breast cancer: Results in the Stockholm Breast Cancer Trial. Cancer 1978, 42, 1120–1125. [Google Scholar] [CrossRef]
- Wallgren, A.; Arner, O.; Bergström, J.; Blomstedt, B.; Granberg, P.-O.; Räf, L.; Silfverswäd, C.; Einhorn, J. Radiation therapy in operable breast cancer: Results from the Stockholm trial on adjuvant radiotherapy. Int. J. Radiat. Oncol. 1986, 12, 533–537. [Google Scholar] [CrossRef]
- Poleszczuk, J.; Luddy, K.; Chen, L.; Lee, J.K.; Harrison, L.B.; Czerniecki, B.J.; Soliman, H.; Enderling, H. Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival. Breast Cancer Res. 2017, 19, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halloran, N.O.; McVeigh, T.; Martin, J.; Keane, M.; Lowery, A.; Kerin, M. Neoadjuvant chemoradiation and breast reconstruction: The potential for improved outcomes in the treatment of breast cancer. Ir. J. Med. Sci. 2018, 188, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.L.; Audretsch, W.; Bojar, H.; Lang, I.; Willers, R.; Budach, W. Retrospective Study of Neoadjuvant Versus Adjuvant Radiochemotherapy in Locally Advanced Noninflammatory Breast Cancer: Survival advantage in cT2 category by neoadjuvant radiochemotherapy. Strahlenther. Onkol. 2010, 186, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuschek, C.; Bölke, E.; Roth, S.L.; Orth, K.; Lang, I.; Bojar, H.; Janni, J.; Audretsch, W.; Nestle-Kraemling, C.; Lammering, G.; et al. Long-term outcome after neoadjuvant radiochemotherapy in locally advanced noninflammatory breast cancer and predictive factors for a pathologic complete remission: Results of a multivariate analysis. Strahlenther. Onkol. 2012, 188, 777–781. [Google Scholar] [CrossRef]
- Hartmann, K.A.; Audretsch, W.; Carl, U.M.; Gripp, S.; Kolotas, C.; Muskalla, K.; Rezai, M.; Schnabel, T.; Waap, I.; Zamboglou, N.; et al. Preoperative irradiation and interstitial radiotherapy-hyperthermia boost in breast tumors > or = 3 cm. The Düsseldorf experience. Strahlenther. Onkol. 1997, 173, 519–523. [Google Scholar] [CrossRef]
- Gerlach, B.; Audretsch, W.; Gogolin, F.; Königshausen, T.; Rohn, R.; Schmitt, G.; Dimmerling, P.; Gripp, S.; Hartmann, K.A. Remission Rates in Breast Cancer Treated with Preoperative Chemotherapy and Radiotherapy. Strahlenther. Onkol. 2003, 179, 306–311. [Google Scholar] [CrossRef]
- Aryus, B.; Audretsch, W.; Gogolin, F.; Gripp, S.; Königshausen, T.; Lammering, G.; Rohn, R.; Hartmann, K.A. Remission rates following preoperative chemotherapy and radiation therapy in patients with breast cancer. Strahlenther. Onkol. 2000, 176, 411–415. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Albert, J.M.; Gonzalez-Angulo, A.M.; Guray, M.; Sahin, A.; Tereffe, W.; Woodward, W.A.; Strom, E.A.; Hunt, K.K.; Tucker, S.L.; Buchholz, T.A. Patients with only 1 positive hormone receptor have increased locoregional recurrence compared with patients with estrogen receptor-positive progesterone receptor-positive disease in very early stage breast cancer. Cancer 2010, 117, 1595–1601. [Google Scholar] [CrossRef]
- Prat, A.; Cheang, M.C.U.; Martín, M.; Parker, J.S.; Carrasco, E.; Caballero, R.; Tyldesley, S.; Gelmon, K.; Bernard, P.S.; Nielsen, T.O.; et al. Prognostic Significance of Progesterone Receptor–Positive Tumor Cells Within Immunohistochemically Defined Luminal A Breast Cancer. J. Clin. Oncol. 2013, 31, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Yoon, C.I.; Lee, J.H.; Lee, H.S.; Park, S.E.; Cha, Y.J.; Cha, C.; Bae, S.J.; Lee, K.-A.; Jeong, J. Low PR in ER (+)/HER2 (−) breast cancer: High rates of TP53 mutation and high SUV. Endocr.-Relat. Cancer 2019, 26, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.M.; Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Viale, G.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative early breast cancer: TEXT and SOFT trials. J. Clin. Oncol. 2016, 34, 2221. [Google Scholar] [CrossRef]
- Chevallier, B.; Roche, H.; Olivier, J.P.; Chollet, P.; Hurteloup, P. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am. J. Clin. Oncol. 1993, 16, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, C.; Nestle-Kraemling, C.; Haussmann, J.; Bölke, E.; Wollandt, S.; Speer, V.; Njanang, F.J.D.; Tamaskovics, B.; Gerber, P.A.; Orth, K.; et al. Long-term cosmetic outcome after preoperative radio-/chemotherapy in locally advanced breast cancer patients. Strahlenther. Onkol. 2019, 195, 615–628. [Google Scholar] [CrossRef]
- Haussmann, J.; Nestle-Kraemling, C.; Bölke, E.; Wollandt, S.; Speer, V.; Njanang, F.-J.D.; Tamaskovics, B.; Gerber, P.A.; Orth, K.; Ruckhaeberle, E.; et al. Long-term quality of life after preoperative radiochemotherapy in patients with localized and locally advanced breast cancer. Strahlenther. Onkol. 2020, 196, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; De La Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Riet, F.; Fayard, F.; Arriagada, R.; Santos, M.; Bourgier, C.; Ferchiou, M.; Heymann, S.; Delaloge, S.; Mazouni, C.; Dunant, A.; et al. Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. Eur. J. Cancer 2017, 76, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.-S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol. 2021, 23, 149–160. [Google Scholar] [CrossRef]
- Formenti, S.C.; Volm, M.; Skinner, K.A.; Spicer, D.; Cohen, D.; Perez, E.; Bettini, A.C.; Groshen, S.; Gee, C.; Florentine, B.; et al. Preoperative Twice-Weekly Paclitaxel with Concurrent Radiation Therapy Followed by Surgery and Postoperative Doxorubicin-Based Chemotherapy in Locally Advanced Breast Cancer: A Phase I/II Trial. J. Clin. Oncol. 2003, 21, 864–870. [Google Scholar] [CrossRef]
- Shanta, V.; Swaminathan, R.; Rama, R.; Radhika, R. Retrospective Analysis of Locally Advanced Noninflammatory Breast Cancer From Chennai, South India, 1990–1999. Int. J. Radiat. Oncol. 2008, 70, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Conzen, S.D.; Jaskowiak, N.T.; Song, D.H.; Recant, W.; Singh, R.; Masters, G.A.; Fleming, G.F.; Heimann, R. Concomitant radiation therapy and paclitaxel for unresectable locally advanced breast cancer: Results from two consecutive Phase I/II trials. Int. J. Radiat. Oncol. 2005, 61, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Conzen, S.; Fleming, G.; Recant, W.; Heimann, R. In response to Dr. Formenti et al. (Int J Radiat Oncol Biol Phys 2005, 63:1275–1276). Int. J. Radiat. Oncol. 2006, 64, 658. [Google Scholar] [CrossRef]
- Semiglazov, V.E.; Topuzov, E.E.; Bavli, J.L.; Moiseyenko, V.M.; Ivanova, O.A.; Seleznev, I.K.; Orlov, A.A.; Barash, N.Y.; Golubeva, O.M.; Chepic, O.F. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Ann. Oncol. 1994, 5, 591–595. [Google Scholar] [CrossRef]
- Bollet, M.A.; Sigal-Zafrani, B.; Gambotti, L.; Extra, J.-M.; Meunier, M.; Nos, C.; Dendale, R.; Campana, F.; Kirova, Y.M.; Diéras, V.; et al. Pathological response to preoperative concurrent chemo-radiotherapy for breast cancer: Results of a phase II study. Eur. J. Cancer 2006, 42, 2286–2295. [Google Scholar] [CrossRef]
- Formenti, S.C.; Dunnington, G.; Uzieli, B.; Lenz, H.; Keren-Rosenberg, S.; Silberman, H.; Spicer, D.; Denk, M.; Leichman, G.; Groshen, S.; et al. Original p53 status predicts for pathological response in locally advanced breast cancer patients treated preoperatively with continuous infusion 5-Fluorouracil and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 1059–1068. [Google Scholar] [CrossRef]
- Thiruchelvam, P.T.R.; Leff, D.R.; Godden, A.R.; Cleator, S.; Wood, S.H.; Kirby, A.M.; Jallali, N.; Somaiah, N.; Hunter, J.E.; Henry, F.P.; et al. Primary radiotherapy and deep inferior epigastric perforator flap reconstruction for patients with breast cancer (PRADA): A multicentre, prospective, non-randomised, feasibility study. Lancet Oncol. 2022, 23, 682–690. [Google Scholar] [CrossRef]
- Chidley, P.; Foroudi, F.; Tacey, M.; Khor, R.; Yeh, J.; Bevington, E.; Hyett, A.; Loh, S.W.; Chew, G.; McCracken, J.; et al. Neoadjuvant radiotherapy for locally advanced and high-risk breast cancer. J. Med. Imaging Radiat. Oncol. 2021, 65, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Maire, M.; Debled, M.; Petit, A.; Fournier, M.; Macgrogan, G.; Quenel-Thueux, N.; Charitansky, H.; Mathoulin-Pelissier, S.; Bonnefoi, H.; Tunon de Lara, C. Neoadjuvant chemotherapy and radiotherapy for locally advanced breast cancer: Safety and efficacy of reverse sequence compared to standard technique? Eur. J. Surg. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Barrou, J.; Bannier, M.; Cohen, M.; Lambaudie, E.; Gonçalves, A.; Bertrand, P.; Buttarelli, M.; Opinel, P.; Sterkers, N.; Tallet, A.; et al. Pathological complete response in invasive breast cancer treated by skin sparing mastectomy and immediate reconstruction following neoadjuvant chemotherapy and radiation therapy: Comparison between immunohistochemical subtypes. Breast 2017, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, H.; Zheng, Y.; Zhai, Z.; Wang, M.; Lin, S.; Li, Y.; Wei, B.; Xu, P.; Wu, Y.; et al. Impact of Preoperative vs Postoperative Radiotherapy on Overall Survival of Locally Advanced Breast Cancer Patients. Front. Oncol. 2021, 11, 779185. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yi, J.; Huang, X.; Qu, Y.; Luo, J.; Xiao, J.; Zhang, S.; Tang, Y.; Liu, W.; Xu, G.; et al. Prognostic impact of pathological complete remission after preoperative irradiation in patients with locally advanced head and neck squamous cell carcinoma: Re-analysis of a phase 3 clinical study. Radiat. Oncol. 2019, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Harris, J.; Kraybill, W.G.; Eisenberg, B.L.; Kirsch, D.G.; Kane, J.M.; Ettinger, D.S.; Spiro, I.J.; Trotti, A.; Freeman, C.R.; et al. Pathologic complete response and survival outcomes in patients with localized soft tissue sarcoma treated with neoadjuvant chemoradiotherapy or radiotherapy: Long-term update of NRG Oncology RTOG 9514 and 0630. J. Clin. Oncol. 2017, 35, 11012. [Google Scholar] [CrossRef]
- Erlandsson, J.; Lörinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer—Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Dodwell, D.; Taylor, C.; McGale, P.; Coles, C.; Duane, F.; Gray, R.; Kühn, T.; Hennequin, C.; Oliveros, S.; Wang, Y.; et al. Abstract GS4-02: Regional lymph node irradiation in early stage breast cancer: An EBCTCG meta-analysis of 13,000 women in 14 trials. Cancer Res. 2019, 79, GS4-02. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mukai, H.; Akiyama, F.; Arihiro, K.; Masuda, S.; Kurosumi, M.; Kodama, Y.; Horii, R.; Tsuda, H. Inter-observer agreement among pathologists in grading the pathological response to neoadjuvant chemotherapy in breast cancer. Breast Cancer 2017, 25, 118–125. [Google Scholar] [CrossRef] [PubMed]
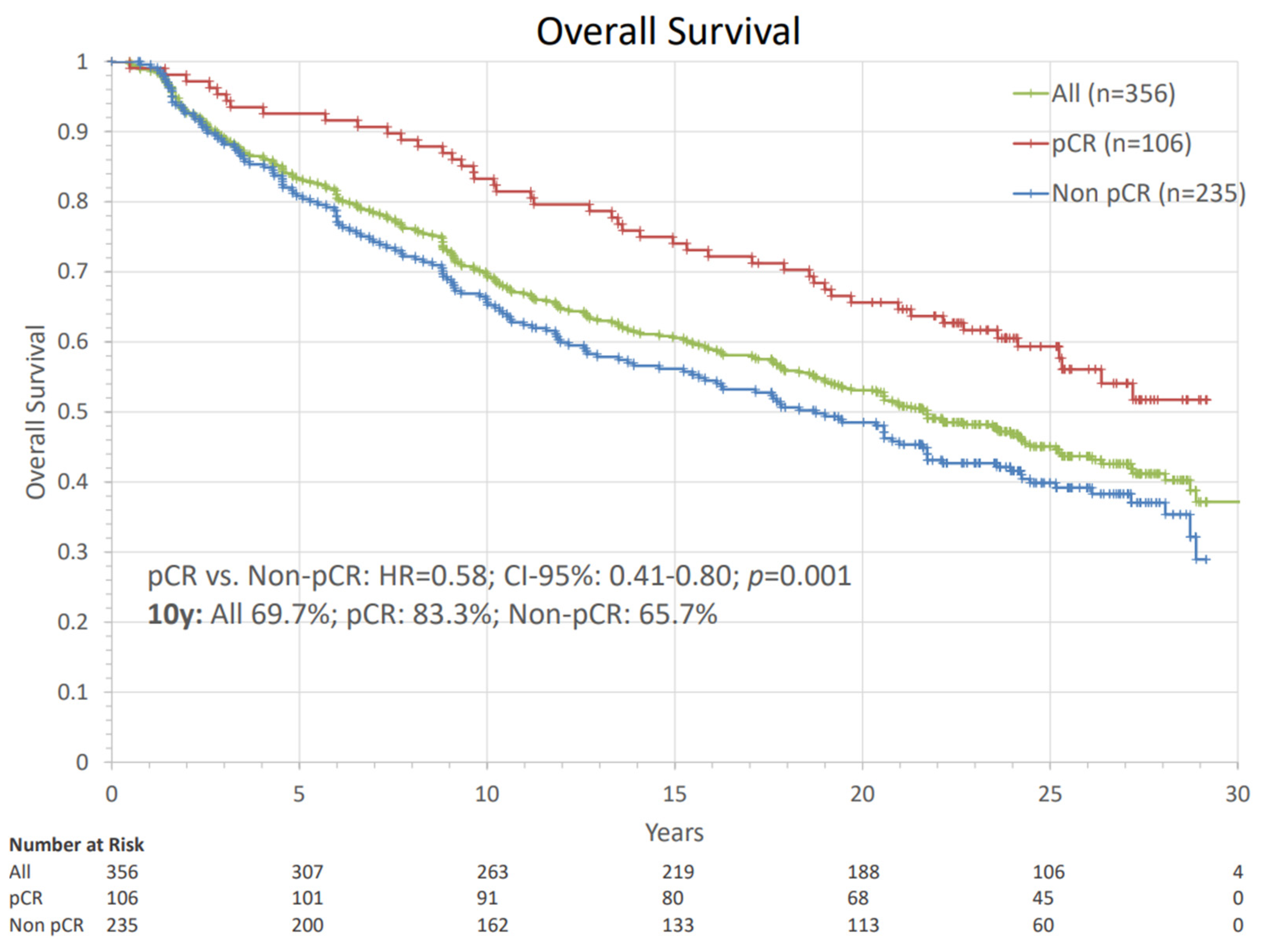
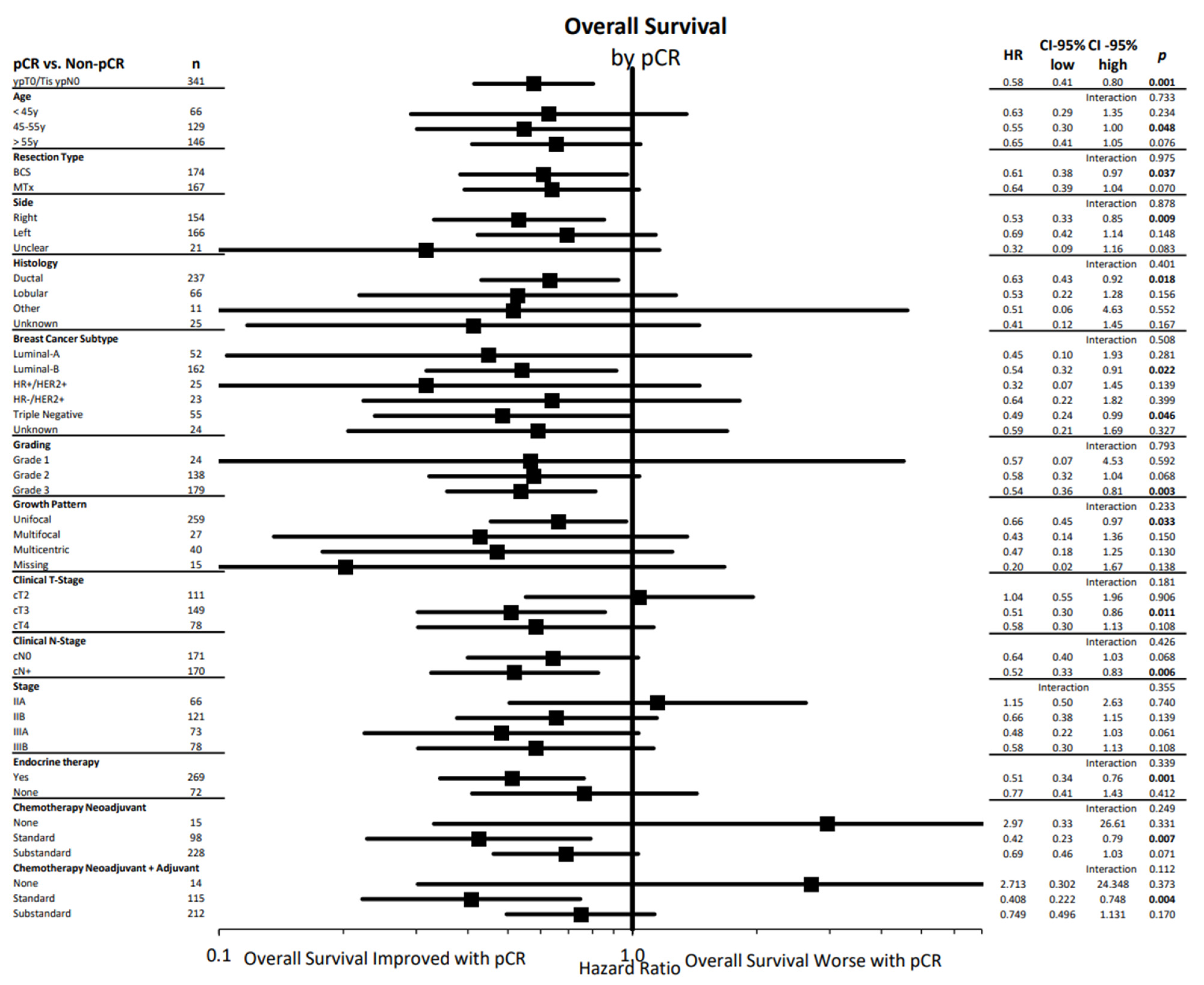




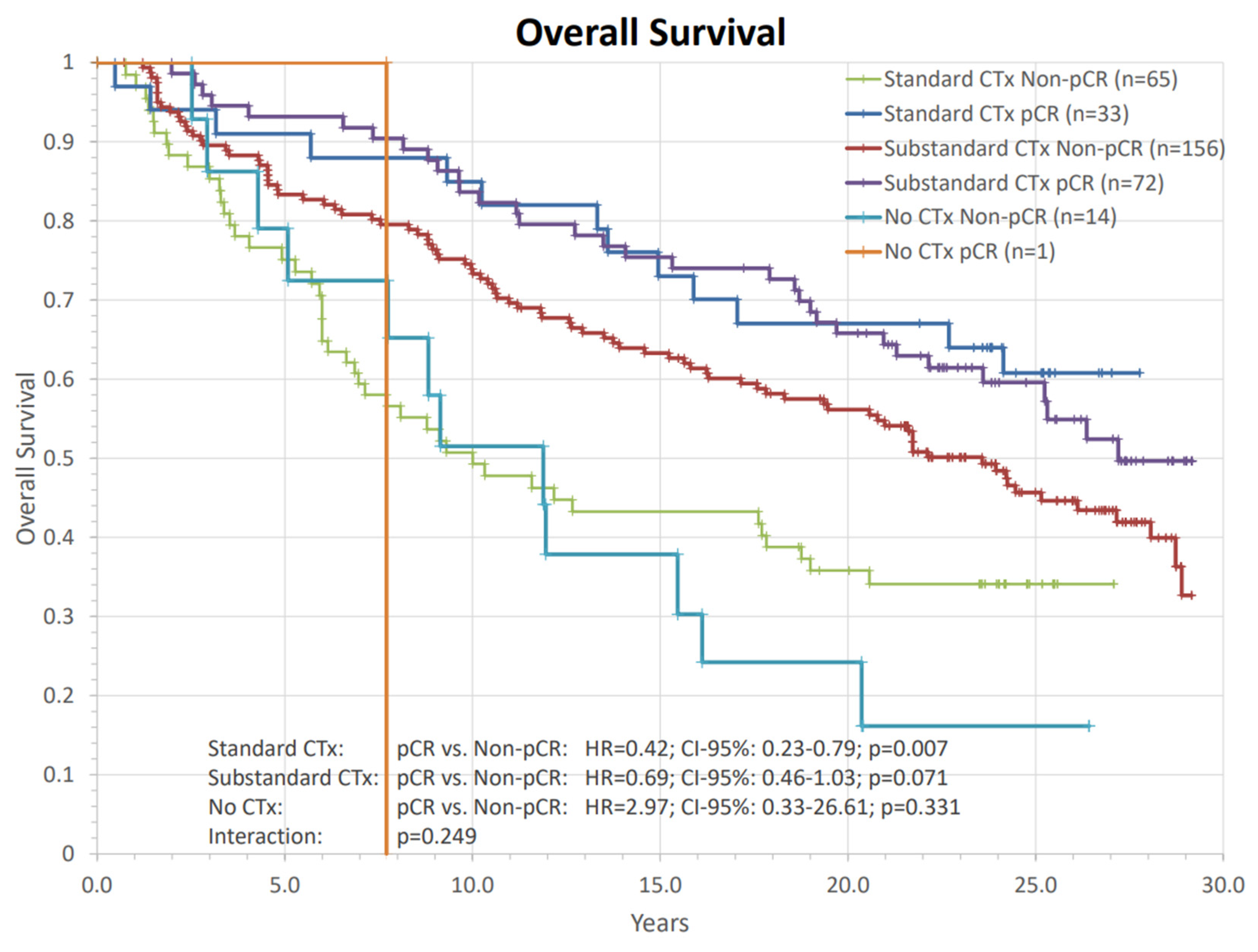

| Characteristics | N (%) | Characteristics | N (%) | Characteristics | N (%) |
|---|---|---|---|---|---|
| Median FU [y] | 20.4 | Clinical Tumor Stage | 356 | Resection type | 356 |
| Median Age [y] | 53.5 | cT1 | 3 (0.8%) | BCS | 178 (50.0%) |
| Age < 45 y | 70 (19.7%) | cT2 | 116 (32.6%) | Mastectomy | 178 (50.0%) |
| Age 45 y–55 y | 135 (37.9%) | cT3 | 155 (43.8%) | Neoadjuvant Systemic Therapy | 356 |
| Age > 55 y | 151 (42.4%) | cT4 | 82 (23.0%) | No Neoadjuvant Systemic Therapy | 15 (4.2%) |
| Side Primary Tumor | 356 | Clinical Nodal Stage | 341 | Agent Concomitant to RT | 122 (34.3%) |
| Right | 159 (44.7%) | cN0 | 171 (50.1%) | “Standard” Regime | 98 (27.5%) |
| Left | 176 (49.4%) | cN+ | 170 (49.9%) | AC/EC + Taxane | 11 (3.1%) |
| Unknown | 21 (5.9%) | Clinical Stage | 356 | AC/EC + CMF | 82 (23.0%) |
| Histological type | 356 | I | 1 (0.3%) | Combination of “Standard” Regime + One Agent | 5 (1.4%) |
| Ductal | 247 (69.4%) | IIA | 68 (19.1%) | “Substandard” Regime | 243 (68.3%) |
| Lobular | 66 (18.5%) | IIB | 124 (34.8%) | Mitoxantrone | 109 (30.6%) |
| Mixed ductal/lobular | 2 (0.6%) | IIIA | 80 (22.5%) | AC/EC (4–6 cylces) | 113 (31.7%) |
| Other | 13 (3.7%) | IIIB | 82 (23.0%) | AC/EC + Mitoxantrone | 4 (1.1%) |
| Unknown | 28 (7.9%) | IIIC | 1 (0.3%) | CMF (3–6 cycles) | 4 (1.1%) |
| Histological Grading | 356 | Growth pattern | 356 | CMF + Mitoxantrone | 7 (2.0%) |
| Grade 1 | 24 (6.7%) | Unifocal | 272 (76.4%) | Other (Taxane; Epirubicine + Taxane; EC + Vinorelbine; Epirubicine + Taxane + CMF) | 6 (1.7%) |
| Grade 2 | 138 (38.8%) | Multifocal | 27 (7.6%) | Additional Adjuvant Systemic Therapy | 44 (12.4%) |
| Grade 3 | 194 (54.5%) | Multicentric | 41 (11.5%) | 2–4x EC | 3 (0.8%) |
| Breast Cancer Subtype | 356 | Unknown | 16 (4.5%) | 3–6x CMF | 27 (7.6%) |
| HR+ Luminal A-like | 52 (14.6%) | Radiation Treatment Details | 356 | 2–4x Taxane | 10 (2.8%) |
| HR+ Luminal B-like | 168 (47.2%) | Mean Time Interval RT to Rx [days] | 193; SD = 80 | Other (CMF + Taxane; Epirubicine + Taxane; EC + Taxane) | 5 (1.4%) |
| HR+/HER2+ | 26 (7.3%) | Mean Tumor Bed Dose as EQD2 (3.7) | 64 Gy; Range: 48.6–75.5 Gy | Neoadjuvant + Adjuvant Systemic Therapy | |
| HR−/HER2+ | 25 (7.0%) | Regional Nodal Irradiation | 302 (84.8%) | “Standard” Regime | 113 (32.0%) |
| Triple Negative | 61 (17.1%) | Brachytherapy Boost + Hyperthermia | 108 (30.3%) | “Substandard” Regime | 229 (64.0%) |
| Unknown | 24 (6.7%) | Tumor Bed Boost | 340 (95.5%) | Endocrine Therapy | 275 (77.2%) |
| Response Parameters | N (%) | Response Parameters | N (%) |
|---|---|---|---|
| Neoadjuvant Therapy followed by breast operation and ALND | 341 (95.8%) | Breast Cancer Subtype | pCR |
| pCR (ypT0/Tis and ypN0) | 106 (31.1%) | • HR+ Luminal A-like | 8 (15.4%) |
| Pathological Tumor Stage | 356 | • HR+ Luminal B-like | 42 (25.0%) |
| ypT0 | 125 (35.1%) | • HR+/HER2+ | 8 (30.8%) |
| ypTis | 16 (4.5%) | • HR−/HER2+ | 10 (40.0%) |
| ypT0/Tis | 141 (39.6%) | • Triple Negative | 25 (41.0%) |
| ypT1 | 144 (40.4%) | • Unknown | 15 (62.5%) |
| ypT2 | 52 (14.6%) | Response to Neoadjuvant Therapy | |
| ypT3 | 6 (1.7%) | Any Primary Tumor Downstaging | 328 (92.1%) |
| ypT4 | 13 (3.7%) | cN0 to ypN0 | 137 (40.2%) |
| Pathological Nodal Stage | 341 | cN+ to ypN0 | 99 (29.0%) |
| ypN0 | 236 (69.2%) | cN0 to ypN+ | 34 (10.0%) |
| ypN1 | 57 (16.7%) | cN+ to ypN+ | 71 (20.8%) |
| ypN2 | 32 (9.4%) | Mean T-Size Reduction [mm] | 45.4 |
| ypN3 | 16 (4.7%) | Mean Residual Tumor Volume [%] | 13.0 |
| Axillary Lymph Node Dissection | Median Residual Tumor Volume [%] | 0.2 | |
| Mean Number of dissected Nodes | 19 | ||
| Mean Number of + Nodes | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haussmann, J.; Budach, W.; Nestle-Krämling, C.; Wollandt, S.; Tamaskovics, B.; Corradini, S.; Bölke, E.; Krug, D.; Fehm, T.; Ruckhäberle, E.; et al. Predictive Factors of Long-Term Survival after Neoadjuvant Radiotherapy and Chemotherapy in High-Risk Breast Cancer. Cancers 2022, 14, 4031. https://doi.org/10.3390/cancers14164031
Haussmann J, Budach W, Nestle-Krämling C, Wollandt S, Tamaskovics B, Corradini S, Bölke E, Krug D, Fehm T, Ruckhäberle E, et al. Predictive Factors of Long-Term Survival after Neoadjuvant Radiotherapy and Chemotherapy in High-Risk Breast Cancer. Cancers. 2022; 14(16):4031. https://doi.org/10.3390/cancers14164031
Chicago/Turabian StyleHaussmann, Jan, Wilfried Budach, Carolin Nestle-Krämling, Sylvia Wollandt, Balint Tamaskovics, Stefanie Corradini, Edwin Bölke, David Krug, Tanja Fehm, Eugen Ruckhäberle, and et al. 2022. "Predictive Factors of Long-Term Survival after Neoadjuvant Radiotherapy and Chemotherapy in High-Risk Breast Cancer" Cancers 14, no. 16: 4031. https://doi.org/10.3390/cancers14164031
APA StyleHaussmann, J., Budach, W., Nestle-Krämling, C., Wollandt, S., Tamaskovics, B., Corradini, S., Bölke, E., Krug, D., Fehm, T., Ruckhäberle, E., Audretsch, W., Jazmati, D., & Matuschek, C. (2022). Predictive Factors of Long-Term Survival after Neoadjuvant Radiotherapy and Chemotherapy in High-Risk Breast Cancer. Cancers, 14(16), 4031. https://doi.org/10.3390/cancers14164031








