Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma
Abstract
:Simple Summary
Abstract
1. Epidemiology
2. Brain Imaging Features at Diagnosis
2.1. Magnetic Resonance Imaging (MRI)
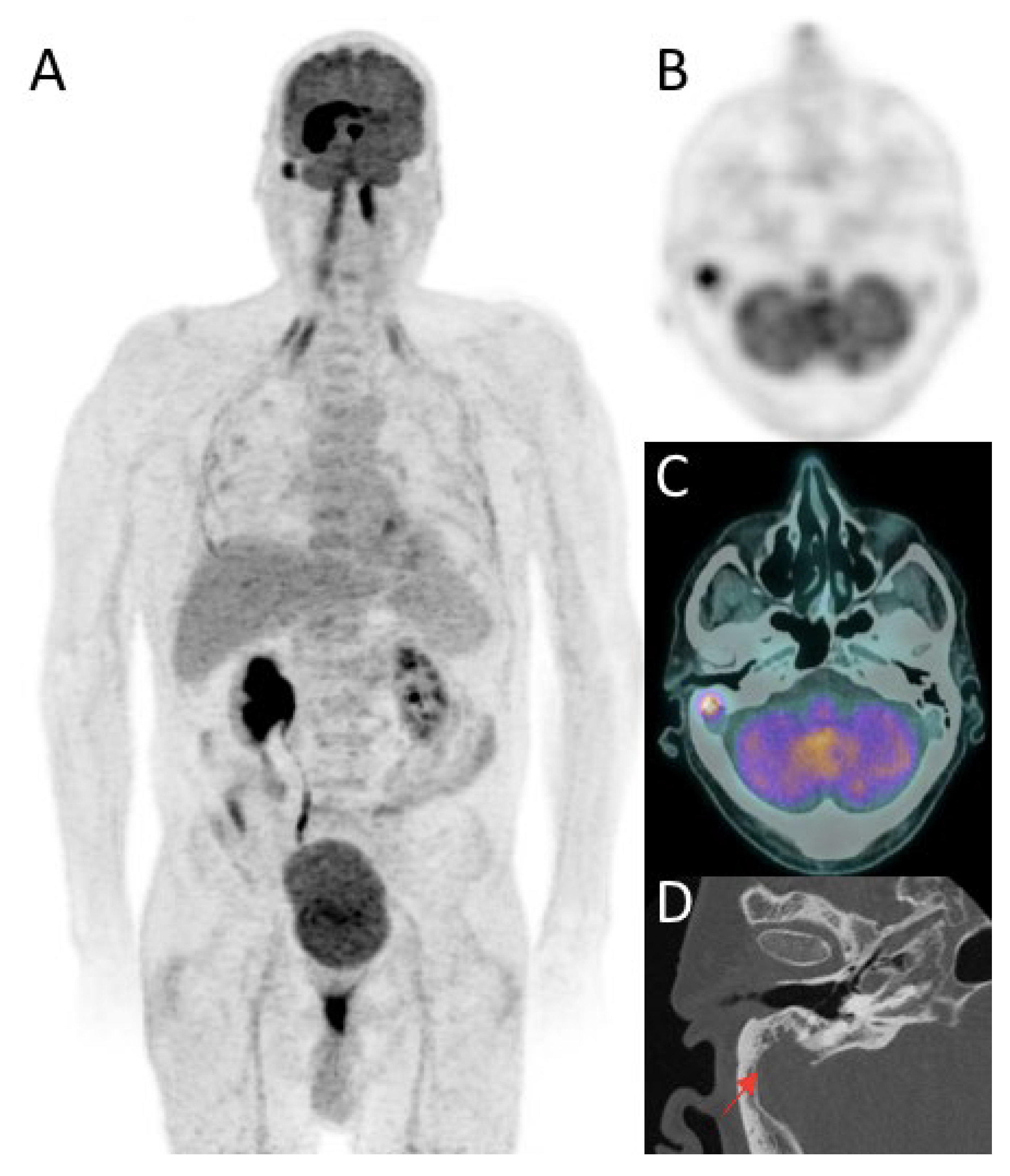
2.2. 18F-FDG Positron Emission Tomography (PET)
2.2.1. Features at Diagnosis
| MRI | PET | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T1+Gd | T2W/FLAIR | DWI | SWI | ADC | Spectroscopy | rCBV | 18F-FDG | 11C-MET | ||
| Biophysical Features | Morphological Features | BBB Disruption | Vasogenic Edema | Cellular Density | Detection of Hemosiderin, Ferritin and Calcium | Cellular Restriction | Detection of Brain Metabolites | Microvascular Blood Volume | Glucidic metabolism | Amino-Acid Analog | |
| IC patients | PCNSL IC | Iso or hypointense Uni or multifocal Profound or periventricular | Homogeneously enhancing parenchymal mass | Tumor is in hyposignal compared to the hypersignal of the adjacent edema FLAIR | Hyperintense | Rare microhemorrhage and calcification | Very low (lower than GBM) | Cho/Cr ⬈ Lipid ⬈ NAA ⬊ (less marked than in glioblastoma) | Low Typical perfusion curve returning above the baseline | High uptake | High uptake |
| Gliobastoma | Hypo to isointense Central hemispheric white matter | Heterogeneous enhanchement with central necrosis | Hyperintense surrounded by vasogenic edema | Hyperintense in the solid portion | Frequent small hemorrhages | Low | Cho/Cr ⬈ Lactate ⬈ Lipids ⬈ NAA ⬊ | High | High uptake (but less pronounced than in PSCNSL) | High uptake | |
| IS patients | PCNCL ID | Iso or hypointense or Multifocal lesions Basal ganglia and corpus callosum | Nodular, or ringlike patterns | Hypointense to slightly hyperintense Small amount of edema and mass effect | Variable | Spontaneous hemorrhage more frequent than for PCNSL IC | Usually rADC <1.6 but overlapping ratio with toxoplasma lesions | Choline ⬈ NAA ⬊ | Low | High uptake | High uptake |
| Toxoplasmosis | Iso to hypointense Multifocal Basal ganglia, corticomedullary junction of the cerebral hemispheres | Ringlike or nodular enhancement patterns | Low hypointense to hyperintense Large edema and mass effect | Variable | Occasional hemorrhages | Usually rADC >1.6 but overlapping ratio with PCNSL | Choline mild ⬈ NAA ⬊ Lactate ⬈ Lipids ⬈ | Low | Hypometabolic | Low to high uptake | |
| Progressive Multifocal Leukoencephalopathy | Hypointense Multifocal and asymetric involvement Subcortical white matter and centrum semi ovale | Non nenhancing or, rarely, mildly enhancing. | Hyperintense Multiple small punctate lesions outside the main PML lesions | Central core with low signal surrounded by a rim of high signal intensity | Typical leukocortical band/rim | Variable/Low ADC reflects active lesions | Choline ⬈ NAA ⬊ Lactate ⬈ Lipids ⬈ | Low | Low uptake | Low to high uptake | |
2.2.2. Outcome Prediction
3. Role of 18F-FDG PET in the Management of PCNSL
3.1. Systemic Assessment
3.2. Therapeutic Evaluation
4. Others PET Tracers in PCNSL
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, D.C.; Hochberg, F.H.; Harris, N.L.; Gruber, M.L.; Louis, D.N.; Cohen, H. Pathology with Clinical Correlations of Primary Central Nervous System Non-Hodgkin’s Lymphoma. The Massachusetts General Hospital Experience 1958–1989. Cancer 1994, 74, 1383–1397. [Google Scholar] [CrossRef]
- Camilleri-Broët, S.; Martin, A.; Moreau, A.; Angonin, R.; Hénin, D.; Gontier, M.F.; Rousselet, M.C.; Caulet-Maugendre, S.; Cuillière, P.; Lefrancq, T.; et al. Primary Central Nervous System Lymphomas in 72 Immunocompetent Patients: Pathologic Findings and Clinical Correlations. Groupe Ouest Est d’étude Des Leucénies et Autres Maladies Du Sang (GOELAMS). Am. J. Clin. Pathol. 1998, 110, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Grogg, K.L.; Miller, R.F.; Dogan, A. HIV Infection and Lymphoma. J. Clin. Pathol. 2007, 60, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The Elderly Left Behind-Changes in Survival Trends of Primary Central Nervous System Lymphoma over the Past 4 Decades. Neuro-Oncology 2018, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, Gender, and Racial Differences in Incidence and Survival in Primary CNS Lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathi, S.; Wilson, J.D. Primary Central Nervous System Lymphoma. Arch. Pathol. Lab. Med. 2008, 132, 1830–1834. [Google Scholar] [CrossRef]
- Morales-Martinez, A.; Lozano-Sanchez, F.; Duran-Peña, A.; Hoang-Xuan, K.; Houillier, C. Primary Central Nervous System Lymphoma in Elderly Patients: Management and Perspectives. Cancers 2021, 13, 3479. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and Outcome of Primary CNS Lymphoma in the Modern Era: An LOC Network Study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and Treatment of Primary CNS Lymphoma in Immunocompetent Patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef]
- Barajas, R.F.; Politi, L.S.; Anzalone, N.; Schöder, H.; Fox, C.P.; Boxerman, J.L.; Kaufmann, T.J.; Quarles, C.C.; Ellingson, B.M.; Auer, D.; et al. Consensus Recommendations for MRI and PET Imaging of Primary Central Nervous System Lymphoma: Guideline Statement from the International Primary CNS Lymphoma Collaborative Group (IPCG). Neuro-Oncology 2021, 23, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Chiavazza, C.; Pellerino, A.; Ferrio, F.; Cistaro, A.; Soffietti, R.; Rudà, R. Primary CNS Lymphomas: Challenges in Diagnosis and Monitoring. BioMed Res. Int. 2018, 2018, 3606970. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Heiland, S.; Harting, I.; Tronnier, V.M.; Sommer, C.; Ludwig, R.; Sartor, K. Distinguishing of Primary Cerebral Lymphoma from High-Grade Glioma with Perfusion-Weighted Magnetic Resonance Imaging. Neurosci. Lett. 2003, 338, 119–122. [Google Scholar] [CrossRef]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef]
- Yamasaki, F.; Takayasu, T.; Nosaka, R.; Amatya, V.J.; Doskaliyev, A.; Akiyama, Y.; Tominaga, A.; Takeshima, Y.; Sugiyama, K.; Kurisu, K. Magnetic Resonance Spectroscopy Detection of High Lipid Levels in Intraaxial Tumors without Central Necrosis: A Characteristic of Malignant Lymphoma. J. Neurosurg. 2015, 122, 1370–1379. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Kråkenes, J.; Krossnes, B.K.; Mella, O.; Espeland, A. CT and MR Imaging Features of Primary Central Nervous System Lymphoma in Norway, 1989-2003. AJNR Am. J. Neuroradiol. 2009, 30, 744–751. [Google Scholar] [CrossRef]
- Küker, W.; Nägele, T.; Korfel, A.; Heckl, S.; Thiel, E.; Bamberg, M.; Weller, M.; Herrlinger, U. Primary Central Nervous System Lymphomas (PCNSL): MRI Features at Presentation in 100 Patients. J. Neurooncol. 2005, 72, 169–177. [Google Scholar] [CrossRef]
- Terae, S.; Ogata, A. Nonenhancing Primary Central Nervous System Lymphoma. Neuroradiology 1996, 38, 34–37. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, Y.; Monden, T.; Sasakawa, Y.; Kawai, N.; Satoh, K.; Ohkawa, M. Diagnostic Value of Kinetic Analysis Using Dynamic FDG PET in Immunocompetent Patients with Primary CNS Lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 78–86. [Google Scholar] [CrossRef]
- Bataille, B.; Delwail, V.; Menet, E.; Vandermarcq, P.; Ingrand, P.; Wager, M.; Guy, G.; Lapierre, F. Primary Intracerebral Malignant Lymphoma: Report of 248 Cases. J. Neurosurg. 2000, 92, 261–266. [Google Scholar] [CrossRef]
- Krebs, S.; Barasch, J.G.; Young, R.J.; Grommes, C.; Schöder, H. Positron Emission Tomography and Magnetic Resonance Imaging in Primary Central Nervous System Lymphoma-a Narrative Review. Ann. Lymphoma 2021, 5, 15. [Google Scholar] [CrossRef]
- Brandsma, D.; Bromberg, J.E.C. Primary CNS Lymphoma in HIV Infection. Handb. Clin. Neurol. 2018, 152, 177–186. [Google Scholar] [CrossRef]
- Kawai, N.; Nishiyama, Y.; Miyake, K.; Tamiya, T.; Nagao, S. Evaluation of Tumor FDG Transport and Metabolism in Primary Central Nervous System Lymphoma Using [18F]Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) Kinetic Analysis. Ann. Nucl. Med. 2005, 19, 685–690. [Google Scholar] [CrossRef]
- Gupta, T.; Manjali, J.J.; Kannan, S.; Purandare, N.; Rangarajan, V. Diagnostic Performance of Pretreatment 18F-Fluorodeoxyglucose Positron Emission Tomography With or Without Computed Tomography in Patients With Primary Central Nervous System Lymphoma: Updated Systematic Review and Diagnostic Test Accuracy Meta-Analyses. Clin. Lymphoma Myeloma Leuk. 2021, 21, 497–507. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, T.; Purandare, N.; Rangarajan, V.; Puranik, A.; Moiyadi, A.; Shetty, P.; Epari, S.; Sahay, A.; Mahajan, A.; et al. Utility of Flouro-Deoxy-Glucose Positron Emission Tomography/Computed Tomography in the Diagnostic and Staging Evaluation of Patients with Primary CNS Lymphoma. CNS Oncol. 2019, 8, CNS46. [Google Scholar] [CrossRef]
- Roelcke, U.; Leenders, K.L. Positron Emission Tomography in Patients with Primary CNS Lymphomas. J. Neurooncol. 1999, 43, 231–236. [Google Scholar] [CrossRef]
- Kosaka, N.; Tsuchida, T.; Uematsu, H.; Kimura, H.; Okazawa, H.; Itoh, H. 18F-FDG PET of Common Enhancing Malignant Brain Tumors. AJR Am. J. Roentgenol. 2008, 190, W365–W369. [Google Scholar] [CrossRef]
- Rosenfeld, S.S.; Hoffman, J.M.; Coleman, R.E.; Glantz, M.J.; Hanson, M.W.; Schold, S.C. Studies of Primary Central Nervous System Lymphoma with Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1992, 33, 532–536. [Google Scholar]
- Baraniskin, A.; Deckert, M.; Schulte-Altedorneburg, G.; Schlegel, U.; Schroers, R. Current Strategies in the Diagnosis of Diffuse Large B-Cell Lymphoma of the Central Nervous System. Br. J. Haematol. 2012, 156, 421–432. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hirata, K.; Kobayashi, H.; Shiga, T.; Manabe, O.; Kobayashi, K.; Motegi, H.; Terasaka, S.; Houkin, K. The Diagnostic Role of (18)F-FDG PET for Primary Central Nervous System Lymphoma. Ann. Nucl. Med. 2014, 28, 603–609. [Google Scholar] [CrossRef]
- Albano, D.; Bosio, G.; Bertoli, M.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT in Primary Brain Lymphoma. J. Neurooncol. 2018, 136, 577–583. [Google Scholar] [CrossRef]
- Kawai, N.; Okubo, S.; Miyake, K.; Maeda, Y.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. Use of PET in the Diagnosis of Primary CNS Lymphoma in Patients with Atypical MR Findings. Ann. Nucl. Med. 2010, 24, 335–343. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, J.S.; Kim, S.-O.; Chae, S.Y.; Oh, S.J.; Seo, M.; Lee, S.H.; Oh, J.S.; Ryu, J.-S.; Huh, J.-R.; et al. Clinicopathological Characteristics of Primary Central Nervous System Lymphoma with Low 18F-Fludeoxyglucose Uptake on Brain Positron Emission Tomography. Medicine 2020, 99, e20140. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Ono, T.; Takahashi, M.; Oda, M.; Shimizu, H. Differentiating between Primary Central Nervous System Lymphoma and Glioblastoma: The Diagnostic Value of Combining 18F-Fluorodeoxyglucose Positron Emission Tomography with Arterial Spin Labeling. Neurol. Med. Chir. 2021, 61, 367–375. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshiura, T.; Hiwatashi, A.; Togao, O.; Yoshimoto, K.; Suzuki, S.O.; Abe, K.; Kikuchi, K.; Maruoka, Y.; Mizoguchi, M.; et al. Differentiating Primary CNS Lymphoma from Glioblastoma Multiforme: Assessment Using Arterial Spin Labeling, Diffusion-Weighted Imaging, and 18F-Fluorodeoxyglucose Positron Emission Tomography. Neuroradiology 2013, 55, 135–143. [Google Scholar] [CrossRef]
- Makino, K.; Hirai, T.; Nakamura, H.; Murakami, R.; Kitajima, M.; Shigematsu, Y.; Nakashima, R.; Shiraishi, S.; Uetani, H.; Iwashita, K.; et al. Does Adding FDG-PET to MRI Improve the Differentiation between Primary Cerebral Lymphoma and Glioblastoma? Observer Performance Study. Ann. Nucl. Med. 2011, 25, 432–438. [Google Scholar] [CrossRef]
- Zhou, W.; Wen, J.; Hua, F.; Xu, W.; Lu, X.; Yin, B.; Geng, D.; Guan, Y. 18F-FDG PET/CT in Immunocompetent Patients with Primary Central Nervous System Lymphoma: Differentiation from Glioblastoma and Correlation with DWI. Eur. J. Radiol. 2018, 104, 26–32. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, K.; Li, X.; Zhao, X.; Chen, Q.; Zhang, W.; Ai, L. Differentiation of High-Grade Glioma and Primary Central Nervous System Lymphoma: Multiparametric Imaging of the Enhancing Tumor and Peritumoral Regions Based on Hybrid 18F-FDG PET/MRI. Eur. J. Radiol. 2022, 150, 110235. [Google Scholar] [CrossRef]
- Cassinelli Petersen, G.I.; Shatalov, J.; Verma, T.; Brim, W.R.; Subramanian, H.; Brackett, A.; Bahar, R.C.; Merkaj, S.; Zeevi, T.; Staib, L.H.; et al. Machine Learning in Differentiating Gliomas from Primary CNS Lymphomas: A Systematic Review, Reporting Quality, and Risk of Bias Assessment. AJNR Am. J. Neuroradiol. 2022, 43, 526–533. [Google Scholar] [CrossRef]
- Kong, Z.; Jiang, C.; Zhu, R.; Feng, S.; Wang, Y.; Li, J.; Chen, W.; Liu, P.; Zhao, D.; Ma, W.; et al. 18F-FDG-PET-Based Radiomics Features to Distinguish Primary Central Nervous System Lymphoma from Glioblastoma. NeuroImage Clin. 2019, 23, 101912. [Google Scholar] [CrossRef]
- O’Malley, J.P.; Ziessman, H.A.; Kumar, P.N.; Harkness, B.A.; Tall, J.G.; Pierce, P.F. Diagnosis of Intracranial Lymphoma in Patients with AIDS: Value of 201TI Single-Photon Emission Computed Tomography. AJR Am. J. Roentgenol. 1994, 163, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, J.; Bai, H.X.; Tao, Y.; Tang, X.; States, L.J.; Zhang, Z.; Zhou, J.; Farwell, M.D.; Zhang, P.; et al. Diagnostic Accuracy of SPECT, PET, and MRS for Primary Central Nervous System Lymphoma in HIV Patients: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e6676. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.A.; Johnson, M.D.; Maciunas, R.J.; Murray, M.J.; Allen, G.S.; Harbison, M.A.; Creasy, J.L.; Kessler, R.M. Evaluating Contrast-Enhancing Brain Lesions in Patients with AIDS by Using Positron Emission Tomography. Ann. Intern. Med. 1995, 123, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.E.; Hoffman, J.M.; Bartlett, J.A.; Waskin, H.A. Differentiation of Central Nervous System Lesions in AIDS Patients Using Positron Emission Tomography (PET). Int. J. STD AIDS 1996, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Villringer, K.; Jäger, H.; Dichgans, M.; Ziegler, S.; Poppinger, J.; Herz, M.; Kruschke, C.; Minoshima, S.; Pfister, H.W.; Schwaiger, M. Differential Diagnosis of CNS Lesions in AIDS Patients by FDG-PET. J. Comput. Assist. Tomogr. 1995, 19, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Waskin, H.A.; Schifter, T.; Hanson, M.W.; Gray, L.; Rosenfeld, S.; Coleman, R.E. FDG-PET in Differentiating Lymphoma from Nonmalignant Central Nervous System Lesions in Patients with AIDS. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1993, 34, 567–575. [Google Scholar]
- Marcus, C.; Feizi, P.; Hogg, J.; Summerfield, H.; Castellani, R.; Sriwastava, S.; Marano, G.D. Imaging in Differentiating Cerebral Toxoplasmosis and Primary CNS Lymphoma With Special Focus on FDG PET/CT. AJR Am. J. Roentgenol. 2021, 216, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Westwood, T.D.; Hogan, C.; Julyan, P.J.; Coutts, G.; Bonington, S.; Carrington, B.; Taylor, B.; Khoo, S.; Bonington, A. Utility of FDG-PETCT and Magnetic Resonance Spectroscopy in Differentiating between Cerebral Lymphoma and Non-Malignant CNS Lesions in HIV-Infected Patients. Eur. J. Radiol. 2013, 82, e374–e379. [Google Scholar] [CrossRef]
- Dibble, E.H.; Boxerman, J.L.; Baird, G.L.; Donahue, J.E.; Rogg, J.M. Toxoplasmosis versus Lymphoma: Cerebral Lesion Characterization Using DSC-MRI Revisited. Clin. Neurol. Neurosurg. 2017, 152, 84–89. [Google Scholar] [CrossRef]
- Bakshi, R. Neuroimaging of HIV and AIDS Related Illnesses: A Review. Front. Biosci. 2004, 9, 632. [Google Scholar] [CrossRef]
- Schroeder, P.C.; Post, M.J.D.; Oschatz, E.; Stadler, A.; Bruce-Gregorios, J.; Thurnher, M.M. Analysis of the Utility of Diffusion-Weighted MRI and Apparent Diffusion Coefficient Values in Distinguishing Central Nervous System Toxoplasmosis from Lymphoma. Neuroradiology 2006, 48, 715–720. [Google Scholar] [CrossRef]
- Floriano, V.H.; Torres, U.S.; Spotti, A.R.; Ferraz-Filho, J.R.L.; Tognola, W.A. The Role of Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging in Differentiating between Infectious and Neoplastic Focal Brain Lesions: Results from a Cohort of 100 Consecutive Patients. PLoS ONE 2013, 8, e81509. [Google Scholar] [CrossRef]
- Baldassari, L.E.; Wattjes, M.P.; Cortese, I.C.M.; Gass, A.; Metz, I.; Yousry, T.; Reich, D.S.; Richert, N. The Neuroradiology of Progressive Multifocal Leukoencephalopathy: A Clinical Trial Perspective. Brain 2022, 145, 426–440. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary Central Nervous System Lymphoma: The Memorial Sloan-Kettering Cancer Center Prognostic Model. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Blay, J.-Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic Scoring System for Primary CNS Lymphomas: The International Extranodal Lymphoma Study Group Experience. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, T.; Wang, S.; Chen, H.; Mao, Y.; Chen, T. Current and Emerging Therapies for Primary Central Nervous System Lymphoma. Biomark. Res. 2021, 9, 32. [Google Scholar] [CrossRef]
- Sasayama, T.; Nakamizo, S.; Nishihara, M.; Kawamura, A.; Tanaka, H.; Mizukawa, K.; Miyake, S.; Taniguchi, M.; Hosoda, K.; Kohmura, E. Cerebrospinal Fluid Interleukin-10 Is a Potentially Useful Biomarker in Immunocompetent Primary Central Nervous System Lymphoma (PCNSL). Neuro-Oncology 2012, 14, 368–380. [Google Scholar] [CrossRef]
- Yuan, X.-G.; Huang, Y.-R.; Yu, T.; Xu, Y.; Liang, Y.; Zhang, X.-H.; Sun, C.-R.; Zhao, X.-Y. Primary Central Nervous System Lymphoma in China: A Single-Center Retrospective Analysis of 167 Cases. Ann. Hematol. 2020, 99, 93–104. [Google Scholar] [CrossRef]
- Tabouret, E.; Houillier, C.; Martin-Duverneuil, N.; Blonski, M.; Soussain, C.; Ghesquières, H.; Houot, R.; Larrieu, D.; Soubeyran, P.; Gressin, R.; et al. Patterns of Response and Relapse in Primary CNS Lymphomas after First-Line Chemotherapy: Imaging Analysis of the ANOCEF-GOELAMS Prospective Randomized Trial. Neuro-Oncology 2017, 19, 422–429. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Valles, F.; Issa, S.; Behler, C.M.; Hwang, J.; McDermott, M.; Treseler, P.; O’Brien, J.; Shuman, M.A.; Cha, S.; et al. Immunochemotherapy with Intensive Consolidation for Primary CNS Lymphoma: A Pilot Study and Prognostic Assessment by Diffusion-Weighted MRI. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1146–1155. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Oh, J.H.; Buck, O.; Lin, X.; Lee, M.; Shukla-Dave, A.; Young, R.J.; Peck, K.K.; Vachha, B.; Holodny, A.I.; et al. Pretreatment Dynamic Contrast-Enhanced MRI Biomarkers Correlate with Progression-Free Survival in Primary Central Nervous System Lymphoma. J. Neurooncol. 2018, 140, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Zhen, H.-N.; Miyake, K.; Yamamaoto, Y.; Nishiyama, Y.; Tamiya, T. Prognostic Value of Pretreatment 18F-FDG PET in Patients with Primary Central Nervous System Lymphoma: SUV-Based Assessment. J. Neurooncol. 2010, 100, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kasenda, B.; Haug, V.; Schorb, E.; Fritsch, K.; Finke, J.; Mix, M.; Hader, C.; Weber, W.A.; Illerhaus, G.; Meyer, P.T. 18F-FDG PET Is an Independent Outcome Predictor in Primary Central Nervous System Lymphoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2013, 54, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Okuyucu, K.; Alagoz, E.; Ince, S.; Ozaydin, S.; Arslan, N. Can Metabolic Tumor Parameters on Primary Staging 18F-FDG PET/CT Aid in Risk Stratification of Primary Central Nervous System Lymphomas for Patient Management as a Prognostic Model? Rev. Espanola Med. Nucl. E Imagen Mol. 2018, 37, 9–14. [Google Scholar] [CrossRef]
- Albano, D.; Bertoli, M.; Battistotti, M.; Rodella, C.; Statuto, M.; Giubbini, R.; Bertagna, F. Prognostic Role of Pretreatment 18F-FDG PET/CT in Primary Brain Lymphoma. Ann. Nucl. Med. 2018, 32, 532–541. [Google Scholar] [CrossRef]
- Krebs, S.; Mauguen, A.; Yildirim, O.; Hatzoglou, V.; Francis, J.H.; Schaff, L.R.; Mellinghoff, I.K.; Schöder, H.; Grommes, C. Prognostic Value of [18F]FDG PET/CT in Patients with CNS Lymphoma Receiving Ibrutinib-Based Therapies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3940–3950. [Google Scholar] [CrossRef]
- Abrey, L.E.; Batchelor, T.T.; Ferreri, A.J.M.; Gospodarowicz, M.; Pulczynski, E.J.; Zucca, E.; Smith, J.R.; Korfel, A.; Soussain, C.; DeAngelis, L.M.; et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5034–5043. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, H.S.; Park, J.E.; Jung, S.C.; Choi, C.G.; Kim, S.J. Primary Central Nervous System Lymphoma: Diagnostic Yield of Whole-Body CT and FDG PET/CT for Initial Systemic Imaging. Radiology 2019, 292, 440–446. [Google Scholar] [CrossRef]
- Bertaux, M.; Houillier, C.; Edeline, V.; Habert, M.-O.; Mokhtari, K.; Giron, A.; Bergeret, S.; Hoang-Xuan, K.; Cassoux, N.; Touitou, V.; et al. Use of FDG-PET/CT for Systemic Assessment of Suspected Primary Central Nervous System Lymphoma: A LOC Study. J. Neurooncol. 2020, 148, 343–352. [Google Scholar] [CrossRef]
- Malani, R.; Bhatia, A.; Wolfe, J.; Grommes, C. Staging Identifies Non-CNS Malignancies in a Large Cohort with Newly Diagnosed Lymphomatous Brain Lesions. Leuk. Lymphoma 2019, 60, 2278–2282. [Google Scholar] [CrossRef]
- Mohile, N.A.; DeAngelis, L.M.; Abrey, L.E. The Utility of Body FDG PET in Staging Primary Central Nervous System Lymphoma. Neuro-Oncol. 2008, 10, 223–228. [Google Scholar] [CrossRef]
- Park, H.Y.; Suh, C.H.; Huang, R.Y.; Guenette, J.P.; Kim, H.S. Diagnostic Yield of Body CT and Whole-Body FDG PET/CT for Initial Systemic Staging in Patients With Suspected Primary CNS Lymphoma: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2021, 216, 1172–1182. [Google Scholar] [CrossRef]
- Fox, C.P.; Phillips, E.H.; Smith, J.; Linton, K.; Gallop-Evans, E.; Hemmaway, C.; Auer, D.P.; Fuller, C.; Davies, A.J.; McKay, P.; et al. Guidelines for the Diagnosis and Management of Primary Central Nervous System Diffuse Large B-Cell Lymphoma. Br. J. Haematol. 2019, 184, 348–363. [Google Scholar] [CrossRef]
- Seidel, S.; Nilius-Eliliwi, V.; Kowalski, T.; Vangala, D.B.; Schlegel, U.; Schroers, R. High-Dose Chemotherapy with Autologous Hematopoietic Stem Cell Transplantation in Relapsed or Refractory Primary CNS Lymphoma: A Retrospective Monocentric Analysis of Long-Term Outcome, Prognostic Factors, and Toxicity. Cancers 2022, 14, 2100. [Google Scholar] [CrossRef]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef]
- Maza, S.; Buchert, R.; Brenner, W.; Munz, D.L.; Thiel, E.; Korfel, A.; Kiewe, P. Brain and Whole-Body FDG-PET in Diagnosis, Treatment Monitoring and Long-Term Follow-up of Primary CNS Lymphoma. Radiol. Oncol. 2013, 47, 103–110. [Google Scholar] [CrossRef]
- Van der Meulen, M.; Postma, A.A.; Smits, M.; Bakunina, K.; Minnema, M.C.; Seute, T.; Cull, G.; Enting, R.H.; van der Poel, M.; Stevens, W.B.C.; et al. Extent of Radiological Response Does Not Reflect Survival in Primary Central Nervous System Lymphoma. Neuro-Oncology Adv. 2021, 3, vdab007. [Google Scholar] [CrossRef]
- Jo, J.-C.; Yoon, D.H.; Kim, S.; Lee, K.; Kang, E.H.; Park, J.S.; Ryu, J.-S.; Huh, J.; Park, C.-S.; Kim, J.H.; et al. Interim 18F-FGD PET/CT May Not Predict the Outcome in Primary Central Nervous System Lymphoma Patients Treated with Sequential Treatment with Methotrexate and Cytarabine. Ann. Hematol. 2017, 96, 1509–1515. [Google Scholar] [CrossRef]
- Birsen, R.; Blanc, E.; Willems, L.; Burroni, B.; Legoff, M.; Le Ray, E.; Pilorge, S.; Salah, S.; Quentin, A.; Deau, B.; et al. Prognostic Value of Early 18F-FDG PET Scanning Evaluation in Immunocompetent Primary CNS Lymphoma Patients. Oncotarget 2018, 9, 16822–16831. [Google Scholar] [CrossRef]
- Palmedo, H.; Urbach, H.; Bender, H.; Schlegel, U.; Schmidt-Wolf, I.G.H.; Matthies, A.; Linnebank, M.; Joe, A.; Bucerius, J.; Biersack, H.-J.; et al. FDG-PET in Immunocompetent Patients with Primary Central Nervous System Lymphoma: Correlation with MRI and Clinical Follow-Up. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 164–168. [Google Scholar] [CrossRef]
- Mercadal, S.; Cortés-Romera, M.; Vélez, P.; Climent, F.; Gámez, C.; González-Barca, E. Positron emission tomography combined with computed tomography in the initial evaluation and response assessment in primary central nervous system lymphoma. Med. Clin. 2015, 144, 503–506. [Google Scholar] [CrossRef]
- Kawai, N.; Miyake, K.; Yamamoto, Y.; Nishiyama, Y.; Tamiya, T. 18F-FDG PET in the Diagnosis and Treatment of Primary Central Nervous System Lymphoma. BioMed Res. Int. 2013, 2013, 247152. [Google Scholar] [CrossRef]
- Tun, H.W.; Johnston, P.B.; DeAngelis, L.M.; Atherton, P.J.; Pederson, L.D.; Koenig, P.A.; Reeder, C.B.; Omuro, A.M.P.; Schiff, D.; O’Neill, B.; et al. Phase 1 Study of Pomalidomide and Dexamethasone for Relapsed/Refractory Primary CNS or Vitreoretinal Lymphoma. Blood 2018, 132, 2240–2248. [Google Scholar] [CrossRef]
- Jang, S.J.; Lee, K.-H.; Lee, J.Y.; Choi, J.Y.; Kim, B.-T.; Kim, S.J.; Kim, W.S. (11)C-Methionine PET/CT and MRI of Primary Central Nervous System Diffuse Large B-Cell Lymphoma before and after High-Dose Methotrexate. Clin. Nucl. Med. 2012, 37, e241–e244. [Google Scholar] [CrossRef]
- Kawase, Y.; Yamamoto, Y.; Kameyama, R.; Kawai, N.; Kudomi, N.; Nishiyama, Y. Comparison of 11C-Methionine PET and 18F-FDG PET in Patients with Primary Central Nervous System Lymphoma. Mol. Imaging Biol. 2011, 13, 1284–1289. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Kwon, S.Y.; Jung, S.-H.; Ahn, J.-S.; Yoo, S.W.; Min, J.-J.; Bom, H.-S.; Ki, S.Y.; Kim, H.-J.; Lee, J.-J.; et al. Prognostic Significance of Interim 11C-Methionine PET/CT in Primary Central Nervous System Lymphoma. Clin. Nucl. Med. 2018, 43, e259–e264. [Google Scholar] [CrossRef]
- Inoue, A.; Ohnishi, T.; Kohno, S.; Matsumoto, S.; Nishikawa, M.; Ohue, S.; Ozaki, S.; Suehiro, S.; Kurata, M.; Fukushima, M.; et al. Prognostic Significance of Immunohistochemical Subtypes Based on the Stage of B-Cell Differentiation in Primary CNS Lymphoma. Int. J. Clin. Exp. Pathol. 2019, 12, 1457–1467. [Google Scholar] [CrossRef]
- Nomura, Y.; Asano, Y.; Shinoda, J.; Yano, H.; Ikegame, Y.; Kawasaki, T.; Nakayama, N.; Maruyama, T.; Muragaki, Y.; Iwama, T. Characteristics of Time-Activity Curves Obtained from Dynamic 11C-Methionine PET in Common Primary Brain Tumors. J. Neurooncol. 2018, 138, 649–658. [Google Scholar] [CrossRef]
- Starzer, A.M.; Berghoff, A.S.; Traub-Weidinger, T.; Haug, A.R.; Widhalm, G.; Hacker, M.; Rausch, I.; Preusser, M.; Mayerhoefer, M.E. Assessment of Central Nervous System Lymphoma Based on CXCR4 Expression In Vivo Using 68Ga-Pentixafor PET/MRI. Clin. Nucl. Med. 2021, 46, 16–20. [Google Scholar] [CrossRef]
- Herhaus, P.; Lipkova, J.; Lammer, F.; Yakushev, I.; Vag, T.; Slotta-Huspenina, J.; Habringer, S.; Lapa, C.; Pukrop, T.; Hellwig, D.; et al. CXCR4-Targeted PET Imaging of Central Nervous System B-Cell Lymphoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1765–1771. [Google Scholar] [CrossRef]
- Hovhannisyan, N.; Fillesoye, F.; Guillouet, S.; Ibazizene, M.; Toutain, J.; Gourand, F.; Valable, S.; Plancoulaine, B.; Barré, L. [18F]Fludarabine-PET as a Promising Tool for Differentiating CNS Lymphoma and Glioblastoma: Comparative Analysis with [18F]FDG in Human Xenograft Models. Theranostics 2018, 8, 4563–4573. [Google Scholar] [CrossRef] [PubMed]
- Postnov, A.; Toutain, J.; Pronin, I.; Valable, S.; Gourand, F.; Kalaeva, D.; Vikhrova, N.; Pyzhik, E.; Guillouet, S.; Kobyakov, G.; et al. First-in-Man Noninvasive Initial Diagnostic Approach of Primary CNS Lymphoma Versus Glioblastoma Using PET With 18F-Fludarabine and l-[Methyl-11C]Methionine. Clin. Nucl. Med. 2022, 47, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Barré, L.; Hovhannisyan, N.; Bodet-Milin, C.; Kraeber-Bodéré, F.; Damaj, G. [18F]-Fludarabine for Hematological Malignancies. Front. Med. 2019, 6, 77. [Google Scholar] [CrossRef] [PubMed]







| Biomarkers | Threshold | Analysis | HR | 95% CI | p | REF |
|---|---|---|---|---|---|---|
| SUVmax | ≥12 | Univariate | <0.05 | Kawai [62] | ||
| MILAS | >3 i.e. | Multivariate | 1.46 | 1.10–1.94 | 0.010 | Kasenda [63] |
| MTV | ≥9.8 cm3 | Mutlivariate | 5.35 | 1.89–12.8 | 0.037 | Albano [65] |
| TLG | ≥94 | Multivariate | 4.54 | 1.37–11.6 | 0.045 | Albano [65] |
| >227 | Univariate | 0.02 | Okoyucu [64] | |||
| Sum SUVmax | - | Multivariate | 1.09 | 1.04–1.14 | <0.001 | Krebs [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozenblum, L.; Houillier, C.; Soussain, C.; Bertaux, M.; Choquet, S.; Galanaud, D.; Hoang-Xuan, K.; Kas, A. Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma. Cancers 2022, 14, 4071. https://doi.org/10.3390/cancers14174071
Rozenblum L, Houillier C, Soussain C, Bertaux M, Choquet S, Galanaud D, Hoang-Xuan K, Kas A. Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma. Cancers. 2022; 14(17):4071. https://doi.org/10.3390/cancers14174071
Chicago/Turabian StyleRozenblum, Laura, Caroline Houillier, Carole Soussain, Marc Bertaux, Sylvain Choquet, Damien Galanaud, Khê Hoang-Xuan, and Aurélie Kas. 2022. "Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma" Cancers 14, no. 17: 4071. https://doi.org/10.3390/cancers14174071






