A miRNA-Based Prognostic Model to Trace Thyroid Cancer Recurrence
Abstract
:Simple Summary
Abstract
1. Introduction
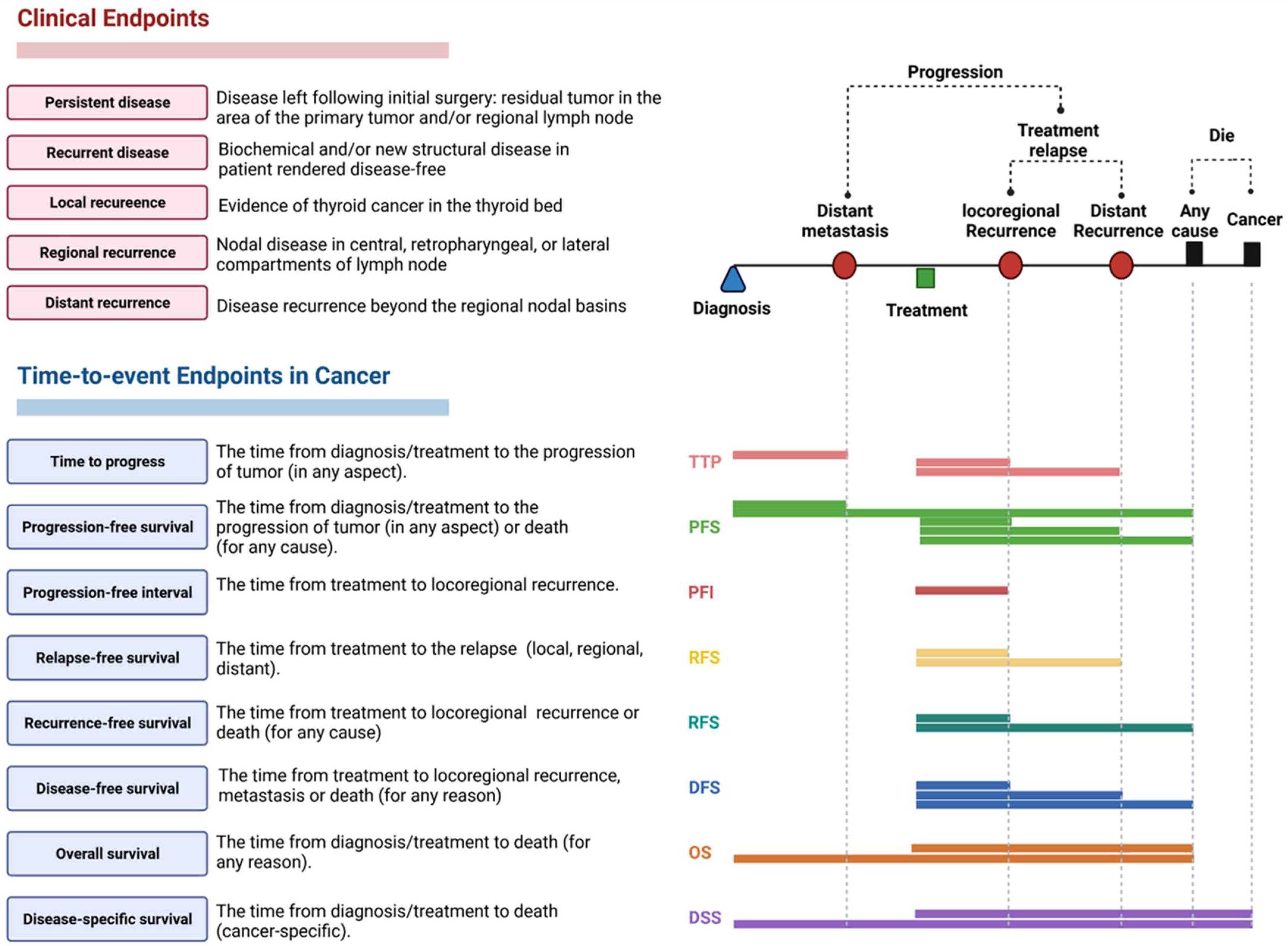
2. Methods
2.1. Identification of Recurrence-Specific Regulatory Network from TCGA Database
2.1.1. Data Source and Pre-Processing
2.1.2. Identification of Differentially Expressed Genes (DEG) and miRNAs (DEmiR) in Recurrence
2.1.3. Functional Enrichment Analysis
2.1.4. Predictive Performance of DEG and DEmiR
2.2. External Validation in GEO
2.2.1. Expression Pattern of miR-145 in Tissues of Cancer Patients
2.2.2. Expression Pattern of miR-145 in Liquid Biopsies of Cancer Patients
2.3. External Validation in Independent Cohorts
2.3.1. Ethical Statement
2.3.2. Human Specimens
2.3.3. Clinical Assessment and Outcomes
2.3.4. Histopathological Assessment
2.3.5. RNA Extraction and Quantification of miR-145-5p in Tissues and Liquid Biopsies
2.4. Systematic Review
2.5. Functional Role of miR-145
2.6. Statistical Analysis
3. Results
3.1. Network Discovery from TCGA Cohorts
3.1.1. Characteristics of TCGA Cohorts
3.1.2. Expression Signature for Recurrence
3.1.3. Functional Enrichment Analysis
3.1.4. Association of DEG and DEmiR with Clinicopathological Characteristics and Survival Analysis
3.1.5. Low miR-145 Level Is a Poor Prognostic Marker
3.2. External Validation in GEO
3.2.1. Expression Pattern of miR-145 in Tumor Tissues of Cancer Patients
3.2.2. Expression Pattern of miR-145 in Liquid Biopsies of Cancer Patients
3.3. Validation in Independent Cohorts
3.3.1. Expression Pattern of miR-145 in Tissues and Blood
3.3.2. Association with Clinicopathological Characteristics
3.3.3. Predictive Accuracy of miR-145
3.3.4. Survival Analysis and Predictors of Progression
3.4. Systemic Review on the Functional Role of miR-145 in Thyroid Cancer
3.5. Functional Role of miR-145 in Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Ferris, R.L. Clinical application of molecular testing of fine-needle aspiration specimens in thyroid nodules. Otolaryngol. Clin. N. Am. 2014, 47, 557–571. [Google Scholar] [CrossRef]
- Nierengarten, M.B. Greater price transparency needed for thyroid cancer treatment among US cancer centres. Lancet Oncol. 2021, 22, 914. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Grant, C.S. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. 2015, 4, 52–62. [Google Scholar] [CrossRef]
- Liu, F.H.; Kuo, S.F.; Hsueh, C.; Chao, T.C.; Lin, J.D. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J. Surg. Oncol. 2015, 112, 149–154. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Yuan, X.; Wang, J.; Sun, L.; Jiang, J.; Zhang, L.; Liu, M.; Zhou, Q. Lymph node metastasis of papillary thyroid carcinoma in the context of Hashimoto’s thyroiditis. BMC Endocr. Disord. 2022, 22, 12. [Google Scholar] [CrossRef]
- Tubiana, M.; Schlumberger, M.; Rougier, P.; Laplanche, A.; Benhamou, E.; Gardet, P.; Caillou, B.; Travagli, J.P.; Parmentier, C. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer 1985, 55, 794–804. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, R.M.; Leboeuf, R. Follow up Approaches in Thyroid Cancer: A Risk Adapted Paradigm. Endocrinol. Metab. Clin. N. Am. 2008, 37, 419–435. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Wang, L.; Wang, J.; Wang, D.; Xin, Z.; Liu, Y.; Zhao, Q. Diagnostic value of fine-needle aspiration combined with ultrasound for thyroid cancer. Oncol. Lett. 2019, 18, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Platt, J. Probabilistic Outputs for Support Vector Machines and Comparisons to Regularized Likelihood Methods. In Advances in Large Margin Classifiers; MIT Press: Cambridge, MA, USA, 1999; pp. 61–74. [Google Scholar]
- Szujo, S.; Bajnok, L.; Bodis, B.; Nagy, Z.; Nemes, O.; Rucz, K.; Mezosi, E. The Prognostic Role of Postablative Non-Stimulated Thyroglobulin in Differentiated Thyroid Cancer. Cancers 2021, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yin, M.; Li, G. Antithyroglobulin Antibody Variation During Follow-Up Has a Good Prognostic Value for Preoperative Antithyroglobulin Antibody-Positive Differentiated Thyroid Cancer Patients: A Retrospective Study in Southwest China. Front. Endocrinol. 2021, 12, 774275. [Google Scholar] [CrossRef]
- Netzel, B.C.; Grebe, S.K.; Carranza Leon, B.G.; Castro, M.R.; Clark, P.M.; Hoofnagle, A.N.; Spencer, C.A.; Turcu, A.F.; Algeciras-Schimnich, A. Thyroglobulin (Tg) Testing Revisited: Tg Assays, TgAb Assays, and Correlation of Results with Clinical Outcomes. J. Clin. Endocrinol. Metab. 2015, 100, E1074–E1083. [Google Scholar] [CrossRef]
- Spencer, C.; Petrovic, I.; Fatemi, S.; LoPresti, J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J. Clin. Endocrinol. Metab. 2014, 99, 4589–4599. [Google Scholar] [CrossRef]
- Azmat, U.; Porter, K.; Senter, L.; Ringel, M.D.; Nabhan, F. Thyroglobulin Liquid Chromatography-Tandem Mass Spectrometry Has a Low Sensitivity for Detecting Structural Disease in Patients with Antithyroglobulin Antibodies. Thyroid 2017, 27, 74–80. [Google Scholar] [CrossRef]
- Qin, K.; Jian, D.; Xue, Y.; Cheng, Y.; Zhang, P.; Wei, Y.; Zhang, J.; Xiong, H.; Zhang, Y.; Yuan, X. DDX41 regulates the expression and alternative splicing of genes involved in tumorigenesis and immune response. Oncol. Rep. 2021, 45, 1213–1225. [Google Scholar] [CrossRef]
- Spencer, C.; Fatemi, S. Thyroglobulin antibody (TgAb) methods—Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 701–712. [Google Scholar] [CrossRef]
- Trinh, L.N.; Crawford, A.R.; Hussein, M.H.; Zerfaoui, M.; Toraih, E.A.; Randolph, G.W.; Kandil, E. Deciphering the Risk of Developing Second Primary Thyroid Cancer Following a Primary Malignancy—Who Is at the Greatest Risk? Cancers 2021, 13, 1402. [Google Scholar]
- Teng, C.J.; Hu, Y.W.; Chen, S.C.; Yeh, C.M.; Chiang, H.L.; Chen, T.J.; Liu, C.J. Use of Radioactive Iodine for Thyroid Cancer and Risk of Second Primary Malignancy: A Nationwide Population-Based Study. J. Natl. Cancer Inst. 2016, 108, djv314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teepen, J.C.; Curtis, R.E.; Dores, G.M.; Berrington de Gonzalez, A.; van den Heuvel-Eibrink, M.M.; Kremer, L.C.M.; Gilbert, E.S.; van Leeuwen, F.E.; Ronckers, C.M.; Morton, L.M. Risk of subsequent myeloid neoplasms after radiotherapy treatment for a solid cancer among adults in the United States, 2000–2014. Leukemia 2018, 32, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.J.; Sidana, S.; Radivoyevitch, T.; Advani, A.S.; Gerds, A.T.; Carraway, H.E.; Angelini, D.; Kalaycio, M.; Nazha, A.; Adelstein, D.J.; et al. Risk of Hematologic Malignancies After Radioiodine Treatment of Well-Differentiated Thyroid Cancer. J. Clin. Oncol. 2018, 36, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bi, X.; Pan, D.; Chen, Y.; Carling, T.; Ma, S.; Udelsman, R.; Zhang, Y. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 2013, 23, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Saeed, O.; Goldberg, A.S.; Farooq, S.; Fazelzad, R.; Goldstein, D.P.; Tsang, R.W.; Brierley, J.D.; Ezzat, S.; Thabane, L.; et al. A Systematic Review and Meta-Analysis of Subsequent Malignant Neoplasm Risk After Radioactive Iodine Treatment of Thyroid Cancer. Thyroid 2018, 28, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef]
- Subramanian, S.; Goldstein, D.P.; Parlea, L.; Thabane, L.; Ezzat, S.; Ibrahim-Zada, I.; Straus, S.; Brierley, J.D.; Tsang, R.W.; Gafni, A.; et al. Second primary malignancy risk in thyroid cancer survivors: A systematic review and meta-analysis. Thyroid 2007, 17, 1277–1288. [Google Scholar] [CrossRef]
- Sawka, A.M.; Thabane, L.; Parlea, L.; Ibrahim-Zada, I.; Tsang, R.W.; Brierley, J.D.; Straus, S.; Ezzat, S.; Goldstein, D.P. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid 2009, 19, 451–457. [Google Scholar] [CrossRef]
- Biondi, B.; Cooper, D.S. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 2010, 20, 135–146. [Google Scholar] [CrossRef]
- Do Cao, C.; Wémeau, J.L. Risk-benefit ratio for TSH- suppressive Levothyroxine therapy in differentiated thyroid cancer. Ann. D’endocrinol. 2015, 76, 1S47–1S52. [Google Scholar] [CrossRef]
- Kostopoulos, G.; Doundoulakis, I.; Antza, C.; Bouras, E.; Nirantharakumar, K.; Tsiachris, D.; Thomas, G.N.; Lip, G.Y.H.; Toulis, K.A. Incident atrial fibrillation in patients with differentiated thyroid cancer: A meta-analysis. Endocr. Relat. Cancer 2021, 28, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
- Toraih, E.A.; Mohammed, E.A.; Farrag, S.; Ramsis, N.; Hosny, S. Pilot Study of Serum MicroRNA-21 as a Diagnostic and Prognostic Biomarker in Egyptian Breast Cancer Patients. Mol. Diagn. Ther. 2015, 19, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C. The future of liquid biopsy. Nature 2020, 579, S9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Toraih, E.A.; Fawzy, M.S.; Mohammed, E.A.; Hussein, M.H.; El-Labban, M.M. MicroRNA-196a2 Biomarker and Targetome Network Analysis in Solid Tumors. Mol. Diagn. Ther. 2016, 20, 559–577. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Bang, S.; Jee, S.; Jang, K. MicroRNA-130b functions as an oncogene and is a predictive marker of poor prognosis in lung adenocarcinoma. Lab. Investig. 2021, 101, 155–164. [Google Scholar] [CrossRef]
- Stewart, L.A.; Kuo, J.H. Advancements in the treatment of differentiated thyroid cancer. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211000251. [Google Scholar] [CrossRef]
- Boufraqech, M.; Zhang, L.; Jain, M.; Patel, D.; Ellis, R.; Xiong, Y.; He, M.; Nilubol, N.; Merino, M.J.; Kebebew, E. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr. Relat. Cancer 2014, 21, 517–531. [Google Scholar] [CrossRef]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Li, D.; Luo, Q.; Wei, C.; Song, H.; Hua, K.; Song, J.; Luo, Y.; Li, X.; Fang, L. MicroRNA-145 inhibits human papillary cancer TPC1 cell proliferation by targeting DUSP6. Int. J. Clin. Exp. Med. 2015, 8, 8590–8598. [Google Scholar] [PubMed]
- Zhang, W.; Ji, W.; Li, T.; Liu, T.; Zhao, X. MiR-145 functions as a tumor suppressor in Papillary Thyroid Cancer by inhibiting RAB5C. Int. J. Med. Sci. 2020, 17, 1992–2001. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Hu, Y.; Yang, Z.; Zhang, L.; Luo, J. CircNUP214 sponges miR-145 to promote the expression of ZEB2 in thyroid cancer cells. Biochem. Biophys. Res. Commun. 2018, 507, 168–172. [Google Scholar] [CrossRef]
- Lei, H.; Gao, Y.; Xu, X. LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim. Biophys. Sin. 2017, 49, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, Q.; Yi, H.; Ma, S.; Li, D.; Xu, Y.; Wang, J.; Yin, S. A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis. 2019, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Zhao, W.; Li, Z.; Zhu, J.; Wei, T.; Song, H. Association between functional polymorphisms in the flanking region of miR-143/145 and risk of papillary thyroid carcinoma: A case-control study. Medicine 2020, 99, e23560. [Google Scholar] [CrossRef]
- Chen, G.; Gao, Y.; Wang, G.; Dai, G.; Tong, L. MiR-145 inhibits the migration and invasion of papillary thyroid carcinoma cells through NF-κB pathway regulation. J. Cell. Biochem. 2020, 121, 3325–3332. [Google Scholar] [CrossRef]
- Sachdeva, M.; Mo, Y.Y. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010, 70, 378–387. [Google Scholar] [CrossRef]
- Climent, M.; Quintavalle, M.; Miragoli, M.; Chen, J.; Condorelli, G.; Elia, L. TGFβ Triggers miR-143/145 Transfer From Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilization. Circ. Res. 2015, 116, 1753–1764. [Google Scholar] [CrossRef]
- Huang, Y.; Du, K.L.; Guo, P.Y.; Zhao, R.M.; Wang, B.; Zhao, X.L.; Zhang, C.Q. IL-16 regulates macrophage polarization as a target gene of mir-145-3p. Mol. Immunol. 2019, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Papagiannakopoulos, T.; Pan, G.; Thomson, J.A.; Kosik, K.S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009, 137, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Q.; Li, P.; Wang, Y.; Zheng, C.; Lei, X.; Li, S.; Gong, W.; Yin, B.; Luo, C.; et al. MicroRNA-145 promotes the epithelial-mesenchymal transition in peritoneal dialysis-associated fibrosis by suppressing fibroblast growth factor 10. J. Biol. Chem. 2019, 294, 15052–15067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, H.; Thayanithy, V.; Subramanian, S.; Oberg, A.L.; Cunningham, J.M.; Cerhan, J.R.; Steer, C.J.; Thibodeau, S.N. Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res. 2009, 69, 9490–9497. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef]
- Hamidi, A.A.; Taghehchian, N.; Basirat, Z.; Zangouei, A.S.; Moghbeli, M. MicroRNAs as the critical regulators of cell migration and invasion in thyroid cancer. Biomark. Res. 2022, 10, 40. [Google Scholar] [CrossRef]
- Tao, S.; Ju, X.; Zhou, H.; Zeng, Q. Circulating microRNA-145 as a diagnostic biomarker for non-small-cell lung cancer: A systemic review and meta-analysis. Int. J. Biol. Markers 2020, 35, 51–60. [Google Scholar] [CrossRef]
- Kadkhoda, S.; Ghafouri-Fard, S. Function of miRNA-145-5p in the pathogenesis of human disorders. Pathol. Res. Pract. 2022, 231, 153780. [Google Scholar] [CrossRef]
- Spizzo, R.; Nicoloso, M.; Lupini, L.; Lu, Y.; Fogarty, J.; Rossi, S.; Zagatti, B.; Fabbri, M.; Veronese, A.; Liu, X. miR-145 participates with TP53 in a death-promoting regulatory loop and targets estrogen receptor-α in human breast cancer cells. Cell Death Differ. 2010, 17, 246–254. [Google Scholar] [CrossRef]
- Gao, P.; Xing, A.; Zhou, G.; Zhang, T.; Zhang, J.; Gao, C.; Li, H.; Shi, D. The molecular mechanism of microRNA-145 to suppress invasion–metastasis cascade in gastric cancer. Oncogene 2013, 32, 491–501. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Enokida, H.; Tatarano, S.; Kawahara, K.; Uchida, Y.; Nishiyama, K.; Fujimura, L.; Kikkawa, N.; Seki, N.; Nakagawa, M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer 2010, 102, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yan, G.; Yin, L.; Liu, T.; Li, C.; Wang, L. Prognostic roles of microRNA 143 and microRNA 145 in colorectal cancer: A meta-analysis. Int. J. Biol. Markers 2019, 34, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Hong, W.; Huang, L.; Shao, J.; Yu, W.; Xu, X. Examination of the effects of microRNA-145-5p and phosphoserine aminotransferase 1 in colon cancer. Bioengineered 2022, 13, 12794–12806. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Xie, C.; Yin, X.; Liu, Y.; Cao, Y.; Fang, Y.; Lin, X.; Xu, Y.; Xu, W. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. Int. J. Cancer 2014, 135, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Dan, H.C.; Park, S.; Yang, L.; Liu, Q.; Kaneko, S.; Ning, J.; He, L.; Yang, H.; Sun, M.; et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front. Biosci. 2005, 10, 975–987. [Google Scholar] [CrossRef]
- Scott, C.L.; Omilusik, K.D. ZEBs: Novel Players in Immune Cell Development and Function. Trends Immunol. 2019, 40, 431–446. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X. Analysis of the Role and Mechanism of ZEB1 in Regulating Cervical Carcinoma Progression via Modulating PD-1/PD-L1 Checkpoint. Comput. Math. Methods Med. 2022, 2022, 1565094. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, X.; Chen, Y.; Rendon, B.; Mitchell, R.A.; Cuatrecasas, M.; Cortés, M.; Postigo, A.; Liu, Y.; Dean, D.C. Zeb1 induces immune checkpoints to form an immunosuppressive envelope around invading cancer cells. Sci. Adv. 2021, 7, eabd7455. [Google Scholar] [CrossRef]
- Li, L.Y.; Yang, J.F.; Rong, F.; Luo, Z.P.; Hu, S.; Fang, H.; Wu, Y.; Yao, R.; Kong, W.H.; Feng, X.W.; et al. ZEB1 serves an oncogenic role in the tumourigenesis of HCC by promoting cell proliferation, migration, and inhibiting apoptosis via Wnt/β-catenin signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1676–1689. [Google Scholar] [CrossRef]
- Nurwidya, F.; Takahashi, F.; Winardi, W.; Tajima, K.; Mitsuishi, Y.; Murakami, A.; Kobayashi, I.; Nara, T.; Hashimoto, M.; Kato, M.; et al. Zinc-finger E-box-binding homeobox 1 (ZEB1) plays a crucial role in the maintenance of lung cancer stem cells resistant to gefitinib. Thorac. Cancer 2021, 12, 1536–1548. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, G.; Zheng, W.; Xue, Q.; Wei, D.; Zheng, Y.; Yuan, J. MiR-454-3p and miR-374b-5p suppress migration and invasion of bladder cancer cells through targetting ZEB2. Biosci. Rep. 2018, 38, BSR20181436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhong, Y.; Li, L. miR-124 and miR-203 synergistically inactivate EMT pathway via coregulation of ZEB2 in clear cell renal cell carcinoma (ccRCC). J. Transl. Med. 2020, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Shen, W.; Hu, H.; Li, C.; Han, G. MicroRNA-140 Represses Esophageal Cancer Progression via Targeting ZEB2 to Regulate Wnt/β-Catenin Pathway. J. Surg. Res. 2021, 257, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kempers, L.; Wakayama, Y.; van der Bijl, I.; Furumaya, C.; De Cuyper, I.M.; Jongejan, A.; Kat, M.; van Stalborch, A.D.; van Boxtel, A.L.; Hubert, M.; et al. The endosomal RIN2/Rab5C machinery prevents VEGFR2 degradation to control gene expression and tip cell identity during angiogenesis. Angiogenesis 2021, 24, 695–714. [Google Scholar] [CrossRef]
- Arriagada, C.; Silva, P.; Millet, M.; Solano, L.; Moraga, C.; Torres, V.A. Focal adhesion kinase-dependent activation of the early endocytic protein Rab5 is associated with cell migration. J. Biol. Chem. 2019, 294, 12836–12845. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, A.R.; Landemberger, M.C.; Dias, M.V.S.; Giudice, F.S.; Rodrigues, B.R.; da Silva, P.; Cassinela, E.K.; Lacerda, T.C.; Marchi, F.A.; Leme, A.F.P.; et al. Rab5C enhances resistance to ionizing radiation in rectal cancer. J. Mol. Med. 2019, 97, 855–869. [Google Scholar] [CrossRef]
- Degl’Innocenti, D.; Romeo, P.; Tarantino, E.; Sensi, M.; Cassinelli, G.; Catalano, V.; Lanzi, C.; Perrone, F.; Pilotti, S.; Seregni, E.; et al. DUSP6/MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr. Relat. Cancer 2013, 20, 23–37. [Google Scholar] [CrossRef]
- Skjefstad, K.; Johannessen, C.; Grindstad, T. A gender specific improved survival related to stromal miR-143 and miR-145 expression in non-small cell lung cancer. Sci. Rep. 2018, 8, 8549. [Google Scholar] [CrossRef]
- Anindo, M.I.K.; Yaqinuddin, A. Insights into the potential use of microRNAs as biomarker in cancer. Int. J. Surg. 2012, 10, 443–449. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, M.; Liu, Q.; Han, Z.; Zhao, Y.; Ji, S. miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncol. Lett. 2018, 16, 6355–6360. [Google Scholar] [CrossRef]
- Ashirbekov, Y.; Abaildayev, A.; Omarbayeva, N.; Botbayev, D.; Belkozhayev, A.; Askandirova, A.; Neupokoyeva, A.; Utegenova, G.; Sharipov, K.; Aitkhozhina, N. Combination of circulating miR-145-5p/miR-191-5p as biomarker for breast cancer detection. PeerJ 2020, 8, e10494. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yu, G.; Peng, K.; Liu, S. MicroRNA-145-5p suppresses fascin to inhibit the invasion and migration of cervical carcinoma cells. Mol. Med. Rep. 2020, 22, 5282–5292. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Chen, P.; Li, S. miR-145-5p inhibits the proliferation, migration, and invasion of esophageal carcinoma cells by targeting ABRACL. BioMed Res. Int. 2021, 2021, 6692544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Li, Y.; Han, J.; Gao, X.; Li, S.; Wang, F. c-Myb facilitates immune escape of esophageal adenocarcinoma cells through the miR-145-5p/SPOP/PD-L1 axis. Clin. Transl. Med. 2021, 11, e464. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Zhang, Y.; Yu, S.; Zhao, G.; Xu, J. CircDUSP16 promotes the tumorigenesis and invasion of gastric cancer by sponging miR-145-5p. Gastric Cancer 2020, 23, 437–448. [Google Scholar] [CrossRef]
- Jiang, S.-B.; He, X.-J.; Xia, Y.-J.; Hu, W.-J.; Luo, J.-G.; Zhang, J.; Tao, H.-Q. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial–mesenchymal transition. OncoTargets Ther. 2016, 9, 2305. [Google Scholar]
- Zhang, Y.; Ta, W.-W.; Sun, P.-F.; Meng, Y.-F.; Zhao, C.-Z. Diagnostic and prognostic significance of serum miR-145-5p expression in glioblastoma. Int. J. Clin. Exp. Pathol. 2019, 12, 2536. [Google Scholar] [PubMed]
- Ding, B.; Fan, W.; Lou, W. hsa_circ_0001955 enhances in vitro proliferation, migration, and invasion of HCC cells through miR-145-5p/NRAS axis. Mol. Ther.-Nucleic Acids 2020, 22, 445–455. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Gu, P. microRNA-145-5p inhibits migration, invasion, and metastasis in hepatocellular carcinoma by inhibiting ARF6. Cancer Manag. Res. 2021, 13, 3473. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, J.; Ran, Y.; Pan, H.; Jia, J.; Zhao, Y.; Zhao, X.; Li, W.; Song, S.; Yu, X. Circular RNA hsa_circ_101555 promotes hepatocellular carcinoma cell proliferation and migration by sponging miR-145-5p and regulating CDCA3 expression. Cell Death Dis. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, C.; Li, W.; Li, H.; Sang, J.; Zhao, Q.; Bo, Y.; Luo, H.; Zheng, X.; Lu, Y. Promoter methylation-regulated miR-145-5p inhibits laryngeal squamous cell carcinoma progression by targeting FSCN1. Mol. Ther. 2019, 27, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; Feng, Y.; Sang, Z.; Yang, Y.; Zhu, Y.; Huang, Q.; Xi, X. Downregulation of miR-145-5p in cancer cells and their derived exosomes may contribute to the development of ovarian cancer by targeting CT. Int. J. Mol. Med. 2019, 43, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhen, M.; Zhou, J. LncRNA BRE-AS1 interacts with miR-145-5p to regulate cancer cell proliferation and apoptosis in prostate carcinoma and has early diagnostic values. Biosci. Rep. 2019, 39, BSR20182097. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Zhang, H.; Chen, Y.; Zhu, S.; Shu, J.; Pan, H. Propofol modulates the proliferation, invasion and migration of bladder cancer cells through the miR-145-5p/TOP2A axis. Mol. Med. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Liu, L.; Shi, Z.; Zhang, L.; Yang, Y. Ropivacaine inhibits cell proliferation, migration and invasion, whereas induces oxidative stress and cell apoptosis by circSCAF11/miR-145-5p axis in glioma. Cancer Manag. Res. 2020, 12, 11145. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.K.; Kim, J. Apoptosis-related microRNA-145-5p enhances the effects of pheophorbide a-based photodynamic therapy in oral cancer. Oncotarget 2017, 8, 35184. [Google Scholar] [CrossRef]
- Ju, C.; Zhou, J.; Miao, H.; Chen, X.; Zhang, Q. Bupivacaine suppresses the progression of gastric cancer through regulating circ_0000376/miR-145-5p axis. BMC Anesthesiol. 2020, 20, 275. [Google Scholar] [CrossRef]
- Iscaife, A.; Reis, S.T.; Morais, D.R.; Viana, N.I.; da Silva, I.A.; Pimenta, R.; Bordini, A.; Dip, N.; Srougi, M.; Leite, K.R.M. Treating metastatic prostate cancer with microRNA-145. Apoptosis 2018, 23, 388–395. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Weirauch, U.; Thomas, M.; Grünweller, A.; Hartmann, R.K.; Aigner, A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011, 71, 5214–5224. [Google Scholar] [CrossRef]
- Wang, W.; Ding, B.; Lou, W.; Lin, S. Promoter hypomethylation and miR-145-5p downregulation-mediated HDAC11 overexpression promotes sorafenib resistance and metastasis of hepatocellular carcinoma cells. Front. Cell Dev. Biol. 2020, 8, 724. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, X.; Shen, J.; Xu, R.; Liu, Y.; Yu, X. Circular RNA hsa_circ_0000073 contributes to osteosarcoma cell proliferation, migration, invasion and methotrexate resistance by sponging miR-145-5p and miR-151-3p and upregulating NRAS. Aging 2020, 12, 14157. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Lu, H.; Xu, H.Y.; Zhou, C.X.; He, X.Z. Retracted: Long noncoding RNA MALAT 1 enhances the docetaxel resistance of prostate cancer cells via miR-145-5p-mediated regulation of AKAP 12. J. Cell. Mol. Med. 2018, 22, 3223–3237. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hua, Y.; Deng, F.; Wang, D.; Wu, Y.; Zhang, W.; Tang, J. MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci. 2020, 111, 3122–3131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lian, Y.; Ge, C. MiR-145 changes sensitivity of non-small cell lung cancer to gefitinib through targeting ADAM19. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5831–5839. [Google Scholar]
- Zheng, T.L.; Li, D.P.; He, Z.F.; Zhao, S. miR-145 sensitizes esophageal squamous cell carcinoma to cisplatin through directly inhibiting PI3K/AKT signaling pathway. Cancer Cell Int. 2019, 19, 250. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Becker, S.A.; Hurst, K.; Nogueira, L.M.; Findlay, V.J.; Camp, E.R. miR-145 antagonizes SNAI1-mediated stemness and radiation resistance in colorectal cancer. Mol. Ther. 2018, 26, 744–754. [Google Scholar] [CrossRef]
- Liu, R.-L.; Dong, Y.; Deng, Y.-Z.; Wang, W.-J.; Li, W.-D. Tumor suppressor miR-145 reverses drug resistance by directly targeting DNA damage-related gene RAD18 in colorectal cancer. Tumor Biol. 2015, 36, 5011–5019. [Google Scholar] [CrossRef]
- Qian, C.; Wang, B.; Zou, Y.; Zhang, Y.; Hu, X.; Sun, W.; Xiao, H.; Liu, H.; Shi, L. MicroRNA 145 enhances chemosensitivity of glioblastoma stem cells to demethoxycurcumin. Cancer Manag. Res. 2019, 11, 6829. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, J.; Wu, Z.; Zhao, J.; Ma, D. miR-145 Regulates the sensitivity of esophageal squamous cell carcinoma cells to 5-FU via targeting REV3L. Pathol.-Res. Pract. 2019, 215, 152427. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Yang, Q.; Xin, C.; Du, J.; Sun, F.; Zhou, L. MIR145-3p promotes autophagy and enhances bortezomib sensitivity in multiple myeloma by targeting HDAC4. Autophagy 2020, 16, 683–697. [Google Scholar] [CrossRef]
- Zhi, X.; Tao, J.; Xiang, G.; Cao, H.; Liu, Z.; Yang, K.; Lv, C.; Ni, S. APRIL induces cisplatin resistance in gastric cancer cells via activation of the NF-κB pathway. Cell. Physiol. Biochem. 2015, 35, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Zhang, Y.; Wang, Z.; Ding, J.; Song, Y.; Zhao, W. Cisplatin-mediated down-regulation of miR-145 contributes to up-regulation of PD-L1 via the c-Myc transcription factor in cisplatin-resistant ovarian carcinoma cells. Clin. Exp. Immunol. 2020, 200, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Zhao, X.; Wang, H.; Chen, W.; Xu, S.; Wang, W.; Shen, H.; Huang, S.; Wang, J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumor Biol. 2016, 37, 10553–10562. [Google Scholar] [CrossRef]
- Zhang, X.; Hua, R.; Wang, X.; Huang, M.; Gan, L.; Wu, Z.; Zhang, J.; Wang, H.; Cheng, Y.; Li, J. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget 2016, 7, 9815. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Chan, L.; Ip, J.; Lo, C.; Yip, T.; Ngan, R.; Wong, R.; Lo, K.; Ng, W.; Lee, A. Therapeutic targeting of CBP/beta-catenin signaling reduces cancer stem-like population and synergistically suppresses growth of EBV-positive nasopharyngeal carcinoma cells with cisplatin. Sci. Rep. 2015, 5, 9979. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Xu, W.; Li, K.; Liao, C. Silencing long intergenic non-coding RNA 00707 enhances cisplatin sensitivity in cisplatin-resistant non-small-cell lung cancer cells by sponging miR-145. Oncol. Lett. 2019, 18, 6261–6268. [Google Scholar] [CrossRef]
- Bar, J.; Gorn-Hondermann, I.; Moretto, P.; Perkins, T.J.; Niknejad, N.; Stewart, D.J.; Goss, G.D.; Dimitroulakos, J. miR profiling identifies cyclin-dependent kinase 6 downregulation as a potential mechanism of acquired cisplatin resistance in non–small-cell lung carcinoma. Clin. Lung Cancer 2015, 16, e121–e129. [Google Scholar] [CrossRef]
- Cui, Y.; Li, G.; Zhang, X.; Dai, F.; Zhang, R. Increased MALAT1 expression contributes to cisplatin resistance in non-small cell lung cancer. Oncol. Lett. 2018, 16, 4821–4828. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.; Tao, L.; Zhang, K.; Wang, R.; Chu, X.; Chen, L. Long noncoding RNA ROR regulates chemoresistance in docetaxel-resistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget 2017, 8, 33144. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Gao, M.; Miao, L.; Liu, M.; Li, C.; Yu, C.; Yan, H.; Yin, Y.; Wang, Y.; Qi, X.; Ren, J. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget 2016, 7, 59714. [Google Scholar] [CrossRef] [PubMed]
- Amri, J.; Molaee, N.; Baazm, M.; Karami, H. Targeting epidermal growth factor receptor by MiRNA-145 inhibits cell growth and sensitizes NSCLC cells to Erlotinib. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2781. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Shen, L.; Yang, L.; Huang, X.; Lu, Q.; Cui, Y.; Zheng, X.; Zhao, X.; Zhang, D.; Huang, R. TGFβ1 promotes gemcitabine resistance through regulating the LncRNA-LET/NF90/miR-145 signaling axis in bladder cancer. Theranostics 2017, 7, 3053. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ge, X.; Wen, Y.; Shi, Z.-M.; Chen, Q.-D.; Wang, M.; Liu, L.-Z.; Jiang, B.-H.; Lu, Y. MiRNA-145 increases therapeutic sensibility to gemcitabine treatment of pancreatic adenocarcinoma cells. Oncotarget 2016, 7, 70857. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Qiu, Y.; Li, Y.; Zhang, H.; Wang, W. TGF-β1 elevates P-gp and BCRP in hepatocellular carcinoma through HOTAIR/miR-145 axis. Biopharm. Drug Dispos. 2019, 40, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cheng, J.; Zhang, J.; Zhang, Y.; Chen, X.; Xie, J.; Luo, S. miR-145 inhibits drug resistance to Oxaliplatin in colorectal cancer cells through regulating G protein coupled receptor 98. Zhonghua Wei Chang. Wai Ke Za Zhi = Chin. J. Gastrointest. Surg. 2017, 20, 566–570. [Google Scholar]
- Gao, R.; Fang, C.; Xu, J.; Tan, H.; Li, P.; Ma, L. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch. Biochem. Biophys. 2019, 663, 183–191. [Google Scholar] [CrossRef]
- Ye, C.; Sun, N.-x.; Ma, Y.; Zhao, Q.; Zhang, Q.; Xu, C.; Wang, S.-b.; Sun, S.-h.; Wang, F.; Li, W. MicroRNA-145 contributes to enhancing radiosensitivity of cervical cancer cells. FEBS Lett. 2015, 589, 702–709. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; He, X.; Zhang, J.; Zhu, Z.; Zhang, M.; Li, X. lncRNA CCAT2 promotes radiotherapy resistance for human esophageal carcinoma cells via the miR-145/p70S6K1 and p53 pathway. Int. J. Oncol. 2020, 56, 327–336. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Z.; Zhi, Y.; Zhou, H.; Zhang, K.; Wang, T.; Feng, B.; Chen, Y.; Song, H.; Wang, R. Long non-coding RNA ROR promotes radioresistance in hepatocelluar carcinoma cells by acting as a ceRNA for microRNA-145 to regulate RAD18 expression. Arch. Biochem. Biophys. 2018, 645, 117–125. [Google Scholar] [CrossRef]








| Characteristics | Levels | Total | Disease-Free | Recurred/Progressed | p-Value |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, years | Median (IQR) | 46.0 (34.0–57.0) | 46.0 (34.0–56.0) | 51.5 (32.7–63.0) | 0.21 |
| <55 y | 321 (68.7) | 297 (70.2) | 24 (54.5) | 0.040 | |
| ≥55 y | 146 (31.3) | 126 (29.8) | 20 (45.5) | ||
| Sex | Female | 342 (73.2) | 313 (74) | 29 (65.9) | 0.28 |
| Male | 125 (26.8) | 110 (26) | 15 (34.1) | ||
| Ethnicity | Not Hispanic or Latino | 328 (70.2) | 292 (69) | 36 (81.8) | 0.17 |
| Hispanic or Latino | 36 (7.7) | 33 (7.8) | 3 (6.8) | ||
| Missing | 103 (22.1) | 98 (23.2) | 5 (11.4) | ||
| Race | White | 312 (66.8) | 279 (66) | 33 (75) | 0.018 |
| Asian | 48 (10.3) | 44 (10.4) | 4 (9.1) | ||
| Black | 16 (3.4) | 15 (3.5) | 1 (2.3) | ||
| American Indian or Alaska Native | 1 (0.2) | 0 (0) | 1 (2.3) | ||
| Missing | 90 (19.3) | 85 (20.1) | 5 (11.4) | ||
| Pathological assessment | |||||
| Primary tumor laterality | Right lobe | 201 (43) | 183 (43.3) | 18 (40.9) | 0.87 |
| Left lobe | 161 (34.5) | 145 (34.3) | 16 (36.4) | ||
| Bilateral | 81 (17.3) | 72 (17) | 9 (20.5) | ||
| Isthmus | 20 (4.3) | 19 (4.5) | 1 (2.3) | ||
| Missing | 4 (0.9) | 4 (0.9) | 0 (0) | ||
| Focality | Unifocal | 247 (52.9) | 223 (52.7) | 24 (54.5) | 0.72 |
| Multifocal | 214 (45.8) | 194 (45.9) | 20 (45.5) | ||
| Missing | 6 (1.3) | 6 (1.4) | 0 (0) | ||
| Histopathology type | Papillary | 363 (77.7) | 325 (76.8) | 38 (86.4) | 0.18 |
| Follicular | 104 (22.3) | 98 (23.2) | 6 (13.6) | ||
| Pathology Stage | Stage I | 271 (58) | 252 (59.6) | 19 (43.2) | 0.051 |
| Stage II | 48 (10.3) | 45 (10.6) | 3 (6.8) | ||
| Stage III | 99 (21.2) | 86 (20.3) | 13 (29.5) | ||
| Stage IV | 47 (10.1) | 38 (9) | 9 (20.5) | ||
| Missing | 2 (0.4) | 2 (0.5) | 0 (0) | ||
| T stage | T1a | 18 (3.9) | 17 (4) | 1 (2.3) | 0.009 |
| T1b | 120 (25.7) | 117 (27.7) | 3 (6.8) | ||
| T2 | 155 (33.2) | 141 (33.3) | 14 (31.8) | ||
| T3 | 155 (33.2) | 133 (31.4) | 22 (50) | ||
| T4a | 17 (3.6) | 13 (3.1) | 4 (9.1) | ||
| Missing | 2 (0.4) | 2 (0.5) | 0 (0) | ||
| N stage | N0 | 215 (46) | 201 (47.5) | 14 (31.8) | 0.053 |
| N1a | 86 (18.4) | 78 (18.4) | 8 (18.2) | ||
| N1b | 123 (26.3) | 104 (24.6) | 19 (43.2) | ||
| Nx | 43 (9.2) | 40 (9.5) | 3 (6.8) | ||
| M stage | M0 | 257 (55) | 237 (56) | 20 (45.5) | <0.001 |
| M1 | 8 (1.7) | 4 (0.9) | 4 (9.1) | ||
| Mx | 202 (43.3) | 182 (43) | 20 (45.5) | ||
| Extrathyroidal extension | Negative | 318 (68.1) | 295 (69.7) | 23 (52.3) | 0.06 |
| Minimal | 122 (26.1) | 103 (24.3) | 19 (43.2) | ||
| Advanced | 13 (2.8) | 12 (2.8) | 1 (2.3) | ||
| Missing | 14 (3) | 13 (3.1) | 1 (2.3) | ||
| Oncologic assessment | |||||
| BRAF mutation | Wild type | 87 (18.6) | 80 (18.9) | 7 (15.9) | 0.17 |
| Mutant | 225 (48.2) | 198 (46.8) | 27 (61.4) | ||
| Missing | 155 (33.2) | 145 (34.3) | 10 (22.7) | ||
| TERT mutation | Wild type | 329 (70.4) | 305 (72.1) | 24 (54.5) | 0.011 |
| Mutant | 31 (6.6) | 24 (5.7) | 7 (15.9) | ||
| Missing | 107 (22.9) | 94 (22.2) | 13 (29.5) | ||
| Mutation density | Median (IQR) | 0.51 (0.31–51.9) | 0.51 (0.31–0.70) | 0.64 (0.35–0.88) | 0.07 |
| ATA risk group | Low | 127 (27.2) | 121 (28.6) | 6 (13.6) | 0.002 |
| Intermediate | 233 (49.9) | 214 (50.6) | 19 (43.2) | ||
| High | 107 (22.9) | 88 (20.8) | 19 (43.2) | ||
| Intervention | |||||
| Radioactive iodine | Negative | 191 (40.9) | 171 (40.4) | 20 (45.5) | 0.36 |
| Positive | 17 (3.6) | 17 (4) | 0 (0) | ||
| Radiation treatment | Negative | 68 (14.6) | 65 (15.4) | 3 (6.8) | 0.20 |
| Positive | 140 (30) | 123 (29.1) | 17 (38.6) | ||
| Follow-up | |||||
| Mortality | Survived | 465 (99.6) | 423 (100) | 42 (95.5) | 0.009 |
| Died | 2 (0.4) | 0 (0) | 2 (4.5) | ||
| Overall survival, months | Median (IQR) | 31.0 (17.4–51.9) | 30.9 (16.8–50.2) | 42.1 (22.9–71.9) | 0.006 |
| Cancer Type | Source ID | Log Fold Change | Expression Status | Design |
| Biliary tract cancer | GSE59856 | −0.21 | DOWN | blood |
| Brain cancer | SRP262521 | −2.09 | DOWN | blood |
| GSE113740 | 1.74 | UP | blood | |
| GSE113486 | 2.06 | UP | blood | |
| GSE112264 | 2.52 | UP | blood | |
| GSE139031 | 2.82 | UP | blood | |
| Breast cancer | GSE113486 | 1.43 | UP | blood |
| GSE106817 | 1.51 | UP | blood | |
| Colon cancer | GSE39845 | 1.5 | UP | blood |
| Colorectal cancer | GSE106817 | 1.96 | UP | blood |
| Esophageal cancer | GSE59856 | −0.36 | DOWN | blood |
| GSE112840 | −0.04 | DOWN | blood | |
| GSE113486 | 1.23 | UP | blood | |
| GSE122497 | 1.82 | UP | blood | |
| GSE106817 | 2.3 | UP | blood | |
| Gastric cancer | GSE113740 | 1.5 | UP | blood |
| GSE106817 | 1.55 | UP | blood | |
| GSE113486 | 1.72 | UP | blood | |
| Head and neck cancer | SRP078325 | 1.6 | UP | exosomes |
| Hepatocellular carcinoma | GSE113740 | 0.97 | UP | blood |
| GSE106817 | 1.34 | UP | blood | |
| Leukemia | E_MTAB_1454 | −0.64 | DOWN | blood |
| Liver cancer | GSE59856 | −0.48 | DOWN | blood |
| Lung cancer | GSE113486 | 1.47 | UP | blood |
| GSE112264 | 1.59 | UP | blood | |
| GSE106817 | 2.42 | UP | blood | |
| Lymphoma | GSE139031 | 1.85 | UP | blood |
| Melanoma | SRP262521 | −0.84 | DOWN | blood |
| GSE31568 | 1.6 | UP | blood | |
| Ovarian cancer | GSE113740 | 1.6 | UP | blood |
| GSE113486 | 1.96 | UP | blood | |
| GSE106817 | 1.73 | UP | blood | |
| Pancreatic cancer | GSE113486 | 1.56 | UP | blood |
| GSE113740 | 1.66 | UP | blood | |
| GSE112264 | 1.71 | UP | blood | |
| GSE106817 | 1.89 | UP | blood | |
| Prostate cancer | GSE31568 | 1.26 | UP | blood |
| Sarcoma | GSE65071 | −1.22 | DOWN | blood |
| GSE106817 | 1.1 | UP | blood |
| Characteristics | Levels | FFPE Samples (n = 178) | p-Value | Frozen and Blood Samples (n = 64) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Non-Recurrence (n = 138) | Recurrence (n = 40) | Non-Recurrence (n = 56) | Recurrence (n = 8) | ||||
| Demographic data | |||||||
| Age, years | Median (IQR) | 45 (33.7–56.2) | 51.5 (33.5–62.7) | 0.19 | 41 (34–52) | 49.5 (32.0–60.7) | 0.59 |
| <55 y | 96 (69.6) | 21 (52.5) | 0.06 | 43 (76.8) | 4 (50) | 0.19 | |
| ≥55 y | 42 (30.4) | 19 (47.5) | 13 (23.2) | 4 (50) | |||
| Sex | Female | 95 (68.8) | 25 (62.5) | 0.45 | 48 (85.7) | 6 (75) | 0.60 |
| Male | 43 (31.2) | 15 (37.5) | 8 (14.3) | 2 (25) | |||
| Ethnicity | Not Hispanic or Latino | 138 (100) | 40 (100) | NA | 41 (73.2) | 7 (87.5) | 0.66 |
| Hispanic or Latino | -- | -- | 15 (26.8) | 1 (12.5) | |||
| Race | White | 138 (100) | 40 (100) | NA | 12 (21.4) | 2 (25) | 0.66 |
| Black | -- | -- | 14 (25) | 3 (37.5) | |||
| Asian | -- | -- | 30 (53.6) | 3 (37.5) | |||
| Hashimoto disease | Positive | 42 (30.4) | 13 (32.5) | 0.84 | 15 (26.8) | 1 (12.5) | 0.67 |
| Pathological assessment | |||||||
| Focality | Unifocal | 82 (59.4) | 22 (55) | 0.71 | 35 (62.5) | 4 (50) | 0.70 |
| Multifocal | 56 (40.6) | 18 (45) | 21 (37.5) | 4 (50) | |||
| Histopathology type | Follicular | 24 (17.4) | 5 (12.5) | 0.62 | 8 (14.3) | 2 (25) | 0.60 |
| Papillary | 114 (82.6) | 35 (87.5) | 48 (85.7) | 6 (75) | |||
| Pathology stage | Stage I | 80 (58) | 14 (35) | 0.001 | 40 (71.4) | 4 (50) | 0.08 |
| Stage II | 19 (13.8) | 2 (5) | 3 (5.4) | 0 (0) | |||
| Stage III | 32 (23.2) | 16 (40) | 9 (16.1) | 1 (12.5) | |||
| Stage IV | 7 (5.1) | 8 (20) | 4 (7.1) | 3 (37.5) | |||
| T stage | T1 | 39 (28.3) | 2 (5) | <0.001 | 14 (25) | 1 (12.5) | 0.40 |
| T2 | 55 (39.9) | 12 (30) | 18 (32.1) | 1 (12.5) | |||
| T3 | 42 (30.4) | 23 (57.5) | 20 (35.7) | 5 (62.5) | |||
| T4 | 2 (1.4) | 3 (7.5) | 4 (7.1) | 1 (12.5) | |||
| N stage | N0 | 82 (59.4) | 14 (35) | 0.007 | 25 (44.6) | 4 (50) | 0.77 |
| N1 | 56 (40.6) | 26 (65) | 31 (55.4) | 4 (50) | |||
| M stage | M0 | 132 (95.7) | 35 (87.5) | 0.07 | 55 (98.2) | 6 (75) | 0.039 |
| M1 | 6 (4.3) | 5 (12.5) | 1 (1.8) | 2 (25) | |||
| Extrathyroidal extension | Negative | 100 (72.5) | 19 (47.5) | 0.004 | 32 (57.1) | 5 (62.5) | 0.77 |
| Positive | 38 (27.5) | 21 (52.5) | 24 (42.9) | 3 (37.5) | |||
| Oncologic assessment | |||||||
| BRAF mutation | Wild type | 73 (52.9) | 16 (40) | 0.15 | 20 (35.7) | 3 (37.5) | 0.92 |
| Mutant | 65 (47.1) | 24 (60) | 36 (64.3) | 5 (62.5) | |||
| TERT mutation | Wild type | 66 (47.8) | 0 (0) | <0.001 | 48 (85.7) | 7 (87.5) | 0.89 |
| Mutant | 70 (50.7) | 34 (85) | 8 (14.3) | 1 (12.5) | |||
| Intervention | |||||||
| Radioactive iodine | Positive | 1 (0.7) | 0 (0) | 0.59 | 3 (5.4) | 1 (12.5) | 0.42 |
| Radiation treatment | Positive | 31 (22.5) | 13 (32.5) | 0.21 | 18 (32.1) | 1 (12.5) | 0.25 |
| Follow-up | |||||||
| Mortality | Survived | 138 (100) | 39 (97.5) | 0.23 | 55 (98.2) | 8 (100) | 0.70 |
| Died | 0 (0) | 1 (2.5) | 1 (1.8) | 0 (0) | |||
| Overall survival, months | Median (IQR) | 37.9 (21.6–63.3) | 35.6 (24.0–63.7) | 0.88 | 31.8 (14.3–58.4) | 43.1 (32.8–50.0) | 0.22 |
| Disease-free survival, months | Median (IQR) | 37.9 (21.6–63.4) | 13.4 (7.1–25.2) | <0.001 | 31.8 (14.2–58.1) | 21.4 (7.0–43.6) | 0.33 |
| Cancer Type | Sample | Size (Cancer/Control) | Grade | T Stage | N Stage | TNM | Survival | Reference |
|---|---|---|---|---|---|---|---|---|
| Bladder | Tissues | 22/22 | ● | [81] | ||||
| Breast | Blood | 35/33 | ● | [82] | ||||
| Cervical | Tissue | 40/40 | ● | ● | [83] | |||
| Esophagus | Tissue | 30/30 | ● | [84] | ||||
| Gall bladder | Tissue | 40/8 | ● | [85] | ||||
| Gastric | Tissue | 289/0 | ● | [86] | ||||
| Gastric | Tissue | 60/60 | ● | ● | [87] | |||
| Glioblastoma | Blood | 117/0 | ● | [88] | ||||
| Liver | Tissues | 60/60 | ● | [89] | ||||
| Liver | Tissues | 150/150 | ● | ● | ● | ● | ● | [90] |
| Liver | Tissues | 10/10 | ● | [91] | ||||
| Larynx | Tissue | 188/0 | ● | ● | ● | ● | ● | [92] |
| Melanoma | Tissue | 83/83 | ● | ● | [93] | |||
| Ovarian | Tissue | 414/0 | ● | ● | [93] | |||
| Prostate | Tissue | 64/64 | ● | [94] | ||||
| Prostate | Blood | 64/55 | ● | [94] |
| Therapy | Cancer Type | Downstream Targets | Reference |
|---|---|---|---|
| 5-FU | Colorectal cancer | RAD18 | [108] |
| Esophageal carcinoma | REV3L | [110] | |
| Bortezomib | Multiple myeloma | HDAC4 | [111] |
| Cisplatin | Gastric cancer | APRIL | [112] |
| Ovarian cancer | c-Myc | [113] | |
| Gallbladder cancer | MRP1 | [114,115] | |
| Nasopharyngeal carcinoma | SOX2 | [116] | |
| Non-small cell lung cancer | MRP1 and P-gp | [117] | |
| CDK6 | [118] | ||
| KLF4 | [119] | ||
| Esophageal carcinoma | MRP1 and P-gp | [106] | |
| Docetaxel | Lung adenocarcinoma | FSCN1 | [120] |
| Prostate cancer | AKAP12 | [103] | |
| Doxorubicin | Hepatocellular carcinoma | SMAD3 | [121] |
| Breast cancer | MRP1 | [122] | |
| Erlotinib | Non-small cell lung cancer | EGFR | [123] |
| Gefitinib | Non-small cell lung cancer | ADAM19 | [105] |
| Gemcitabine | Bladder cancer | HMGA2 and KLF4 | [124] |
| Pancreatic adenocarcinoma | P70S6K1 | [125] | |
| Imatinib | Hepatocellular carcinoma | P-gp and BCRP | [126] |
| Oxaliplatin | Colorectal cancer | GPR98 | [127] |
| MRP1 | [128] | ||
| Paclitaxel | Ovarian cancer | Sp1 and CDK6 | [65] |
| Radiation | Cervical cancer | HLTF | [129] |
| Colorectal cancer | KLF4 and c-Myc | [107] | |
| Esophageal carcinoma | P70S6K1 | [130] | |
| Prostate cancer | RAD51, Mcl1, Par-4, and PARP1 | [127] | |
| Hepatocellular carcinoma | RAD18 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toraih, E.A.; Fawzy, M.S.; Ning, B.; Zerfaoui, M.; Errami, Y.; Ruiz, E.M.; Hussein, M.H.; Haidari, M.; Bratton, M.; Tortelote, G.G.; et al. A miRNA-Based Prognostic Model to Trace Thyroid Cancer Recurrence. Cancers 2022, 14, 4128. https://doi.org/10.3390/cancers14174128
Toraih EA, Fawzy MS, Ning B, Zerfaoui M, Errami Y, Ruiz EM, Hussein MH, Haidari M, Bratton M, Tortelote GG, et al. A miRNA-Based Prognostic Model to Trace Thyroid Cancer Recurrence. Cancers. 2022; 14(17):4128. https://doi.org/10.3390/cancers14174128
Chicago/Turabian StyleToraih, Eman A., Manal S. Fawzy, Bo Ning, Mourad Zerfaoui, Youssef Errami, Emmanuelle M. Ruiz, Mohammad H. Hussein, Muhib Haidari, Melyssa Bratton, Giovane G. Tortelote, and et al. 2022. "A miRNA-Based Prognostic Model to Trace Thyroid Cancer Recurrence" Cancers 14, no. 17: 4128. https://doi.org/10.3390/cancers14174128
APA StyleToraih, E. A., Fawzy, M. S., Ning, B., Zerfaoui, M., Errami, Y., Ruiz, E. M., Hussein, M. H., Haidari, M., Bratton, M., Tortelote, G. G., Hilliard, S., Nilubol, N., Russell, J. O., Shama, M. A., El-Dahr, S. S., Moroz, K., Hu, T., & Kandil, E. (2022). A miRNA-Based Prognostic Model to Trace Thyroid Cancer Recurrence. Cancers, 14(17), 4128. https://doi.org/10.3390/cancers14174128









