The Clinical Utility of Systemic Immune-Inflammation Index Supporting Charlson Comorbidity Index and CAPRA-S Score in Determining Survival after Radical Prostatectomy—A Single Centre Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
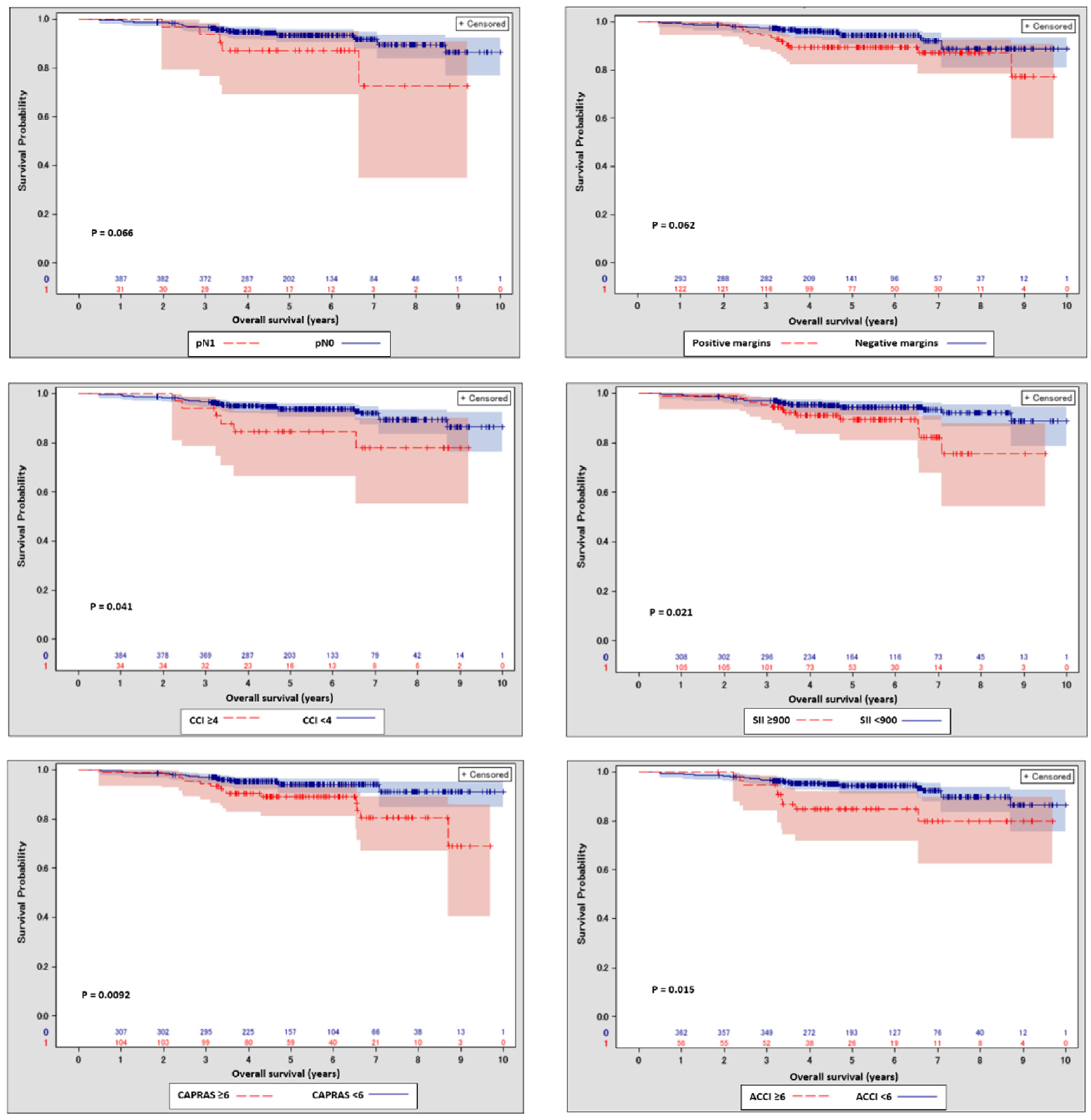
3. Results
3.1. Baseline Characteristics
3.2. Survival Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, G.; Dufmats, M.; Varenhorst, E. Long-Term Survival in a Swedish Population-Based Cohort of Men with Prostate Cancer. Urology 2000, 56, 442–447. [Google Scholar] [CrossRef]
- Albertsen, P.C.; Moore, D.F.; Shih, W.; Lin, Y.; Li, H.; Lu-Yao, G.L. Impact of Comorbidity on Survival among Men with Localized Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Boyle, H.J.; Alibhai, S.; Decoster, L.; Efstathiou, E.; Fizazi, K.; Mottet, N.; Oudard, S.; Payne, H.; Prentice, M.; Puts, M.; et al. Updated Recommendations of the International Society of Geriatric Oncology on Prostate Cancer Management in Older Patients. Eur. J. Cancer Oxf. Engl. 1990 2019, 116, 116–136. [Google Scholar] [CrossRef] [PubMed]
- van Walree, I.C.; Scheepers, E.R.M.; van den Bos, F.; van Huis-Tanja, L.H.; Emmelot-Vonk, M.H.; Hamaker, M.E. Clinical Judgment versus Geriatric Assessment for Frailty in Older Patients with Cancer. J. Geriatr. Oncol. 2020, 11, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Casas Duran, F.; Valduvieco, I.; Oses, G.; Cortés, K.S.; Barreto, T.D.; Muñoz-Guglielmetti, D.; Ferrer, F. Spanish Validation of Charlson Index Applied to Prostate Cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 1187–1192. [Google Scholar] [CrossRef]
- Daskivich, T.J.; Kwan, L.; Dash, A.; Greenfield, S.; Litwin, M.S. Weighted versus Unweighted Charlson Score to Predict Long-Term Other-Cause Mortality in Men with Early-Stage Prostate Cancer. Eur. Urol. 2014, 66, 1002–1009. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S Score: A Straightforward Tool for Improved Prediction of Outcomes after Radical Prostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef]
- Punnen, S.; Freedland, S.J.; Presti, J.C.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Carroll, P.R.; Cooperberg, M.R. Multi-Institutional Validation of the CAPRA-S Score to Predict Disease Recurrence and Mortality after Radical Prostatectomy. Eur. Urol. 2014, 65, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Zapała, Ł.; Ślusarczyk, A.; Garbas, K.; Mielczarek, Ł.; Ślusarczyk, C.; Zapała, P.; Wróbel, A.; Radziszewski, P. Complete Blood Count-Derived Inflammatory Markers and Survival in Patients with Localized Renal Cell Cancer Treated with Partial or Radical Nephrectomy: A Retrospective Single-Tertiary-Center Study. Front. Biosci. Sch. Ed. 2022, 14, 5. [Google Scholar] [CrossRef]
- Zapała, Ł.; Ślusarczyk, A.; Wolański, R.; Kurzyna, P.; Garbas, K.; Zapała, P.; Radziszewski, P. The Four-Feature Prognostic Models for Cancer-Specific and Overall Survival after Surgery for Localized Clear Cell Renal Cancer: Is There a Place for Inflammatory Markers? Biomedicines 2022, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Rajwa, P.; Życzkowski, M.; Paradysz, A.; Slabon-Turska, M.; Suliga, K.; Bujak, K.; Bryniarski, P. Novel Hematological Biomarkers Predict Survival in Renal Cell Carcinoma Patients Treated with Nephrectomy. Arch. Med. Sci. AMS 2020, 16, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Kool, R.; Marcq, G.; Shinde-Jadhav, S.; Mansure, J.J.; Saleh, R.; Rajan, R.; Aprikian, A.; Tanguay, S.; Cury, F.L.; Brimo, F.; et al. Role of Serum Lymphocyte-Derived Biomarkers in Nonmetastatic Muscle-Invasive Bladder Cancer Patients Treated with Trimodal Therapy. Eur. Urol. Open Sci. 2022, 36, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Sudoł, D.; Widz, D.; Mitura, P.; Płaza, P.; Godzisz, M.; Kuliniec, I.; Yadlos, A.; Cabanek, M.; Bar, M.; Bar, K. Neutrophil-to-Lymphocyte Ratio as a Predictor of Overall Survival and Cancer Advancement in Patients Undergoing Radical Cystectomy for Bladder Cancer. Cent. Eur. J. Urol. 2022, 75, 41–46. [Google Scholar] [CrossRef]
- Rajwa, P.; Schuettfort, V.M.; Quhal, F.; Mori, K.; Katayama, S.; Laukhtina, E.; Pradere, B.; Motlagh, R.S.; Mostafaei, H.; Grossmann, N.C.; et al. Role of Systemic Immune-Inflammation Index in Patients Treated with Salvage Radical Prostatectomy. World J. Urol. 2021, 39, 3771–3779. [Google Scholar] [CrossRef]
- Jiang, Z.-G.; Liao, S.-G. Baseline Neutrophil-Lymphocyte Ratio Is Associated with Outcomes in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel in South China. Medicine (Baltimore) 2021, 100, e27361. [Google Scholar] [CrossRef]
- Rajwa, P.; Schuettfort, V.M.; D’Andrea, D.; Quhal, F.; Mori, K.; Katayama, S.; Laukhtina, E.; Pradere, B.; Motlagh, R.S.; Mostafaei, H.; et al. Impact of Systemic Immune-Inflammation Index on Oncologic Outcomes in Patients Treated with Radical Prostatectomy for Clinically Nonmetastatic Prostate Cancer. Urol. Oncol. 2021, 39, 785.e19–785.e27. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Yu, Z.; Du, P.; Sheng, X.; Cao, Y.; Yan, X.; Ma, J.; Yang, Y. The Values of Systemic Immune-Inflammation Index and Neutrophil–Lymphocyte Ratio in Predicting Biochemical Recurrence in Patients with Localized Prostate Cancer After Radical Prostatectomy. Front. Oncol. 2022, 12, 907625. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Koh, D.H.; Jang, W.S.; Lee, J.Y.; Cho, K.S.; Ham, W.S.; Rha, K.H.; Jung, W.H.; Hong, S.J.; Choi, Y.D. Age-Adjusted Charlson Comorbidity Index as a Prognostic Factor for Radical Prostatectomy Outcomes of Very High-Risk Prostate Cancer Patients. PLoS ONE 2018, 13, e0199365. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, B.E.; Dorn, P.L.; Martinez, C.; Arif, F.; Cloutier, D.; Antic, T.; Golden, D.W.; Karrison, T.; Pitroda, S.P.; Szmulewitz, R.Z.; et al. A Prospective Clinical and Transcriptomic Feasibility Study of Oral-Only Hormonal Therapy with Radiation for Unfavorable Prostate Cancer in Men 70 Years of Age and Older or with Comorbidity. Cancer 2021, 127, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical Prostatectomy versus Observation for Localized Prostate Cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. The European Randomized Study of Screening for Prostate Cancer—Prostate Cancer Mortality at 13 Years of Follow-Up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Ferro, M.; Crocetto, F.; Bruzzese, D.; Imbriaco, M.; Fusco, F.; Longo, N.; Napolitano, L.; La Civita, E.; Cennamo, M.; Liotti, A.; et al. Prostate Health Index and Multiparametric MRI: Partners in Crime Fighting Overdiagnosis and Overtreatment in Prostate Cancer. Cancers 2021, 13, 4723. [Google Scholar] [CrossRef]
- Wenzel, M.; Würnschimmel, C.; Nocera, L.; Collà Ruvolo, C.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Graefen, M.; Becker, A.; et al. Salvage Radical Prostatectomy: Baseline Prostate Cancer Characteristics and Survival Across SEER Registries. Clin. Genitourin. Cancer 2021, 19, e255–e263. [Google Scholar] [CrossRef]
- Matsumoto, K.; Niwa, N.; Hagiwara, M.; Kosaka, T.; Tanaka, N.; Takeda, T.; Morita, S.; Mizuno, R.; Shinojima, T.; Hara, S.; et al. Type of Patients in Whom Biochemical Recurrence after Radical Prostatectomy Can Be Observed without Salvage Therapy. World J. Urol. 2020, 38, 1749–1756. [Google Scholar] [CrossRef]
- Marshall, C.H.; Chen, Y.; Kuo, C.; Cullen, J.; Jiang, J.; Rosner, I.; Markowski, M.; McLeod, D.G.; Trock, B.J.; Eisenberger, M.A. Timing of Androgen Deprivation Treatment for Men with Biochemical Recurrent Prostate Cancer in the Context of Novel Therapies. J. Urol. 2021, 206, 623–629. [Google Scholar] [CrossRef]
- EAU Guidelines on Prostate Cancer—Uroweb. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 30 May 2022).
- Lee, J.Y.; Kang, H.W.; Rha, K.H.; Cho, N.H.; Choi, Y.D.; Hong, S.J.; Cho, K.S. Age-Adjusted Charlson Comorbidity Index Is a Significant Prognostic Factor for Long-Term Survival of Patients with High-Risk Prostate Cancer after Radical Prostatectomy: A Bayesian Model Averaging Approach. J. Cancer Res. Clin. Oncol. 2016, 142, 849–858. [Google Scholar] [CrossRef]
- Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E.; et al. Systemic Immune-Inflammation Index Predicts the Clinical Outcome in Patients with MCRPC Treated with Abiraterone. Front. Pharmacol. 2016, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, R.; Chi, C.; Cai, W.; Zhang, Y.; Qian, H.; Shao, X.; Wang, Y.; Xu, F.; Pan, J.; et al. Systemic Immune-Inflammation Index Predicts the Combined Clinical Outcome after Sequential Therapy with Abiraterone and Docetaxel for Metastatic Castration-Resistant Prostate Cancer Patients. Prostate 2018, 78, 250–256. [Google Scholar] [CrossRef]
- Stangl-Kremser, J.; Mari, A.; Suarez-Ibarrola, R.; D’Andrea, D.; Korn, S.M.; Pones, M.; Kramer, G.; Karakiewicz, P.; Enikeev, D.V.; Glybochko, P.V.; et al. Development of a Prognostic Model for Survival Time Prediction in Castration-Resistant Prostate Cancer Patients. Urol. Oncol. 2020, 38, 600.e9–600.e15. [Google Scholar] [CrossRef] [PubMed]
- Rajwa, P.; Huebner, N.A.; Hostermann, D.I.; Grossmann, N.C.; Schuettfort, V.M.; Korn, S.; Quhal, F.; König, F.; Mostafaei, H.; Laukhtina, E.; et al. Evaluation of the Predictive Role of Blood-Based Biomarkers in the Context of Suspicious Prostate MRI in Patients Undergoing Prostate Biopsy. J. Pers. Med. 2021, 11, 1231. [Google Scholar] [CrossRef]
- Bravi, C.A.; Rosiello, G.; Fallara, G.; Vertosick, E.; Tin, A.; Sjoberg, D.; Bianchi, M.; Mazzone, E.; Martini, A.; Dell’oglio, P.; et al. Predictive Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Localized Prostate Cancer: Results from a Surgical Series at a High-Volume Institution. Minerva Urol. Nephrol. 2021, 73, 481–488. [Google Scholar] [CrossRef] [PubMed]


| Variable | Overall | SII < 900 | SII ≥ 900 | p | |
|---|---|---|---|---|---|
| Clinical baseline characteristics | |||||
| PSA (ng/mL, median, IQR) | 7.40 (6.7) | 7.5 (6.7) | 7 (5.5) | 0.44 | |
| cT (n%) | cT1 | 149 149 (37.44%) | 101 (34.95%) | 45 (43.27%) | 0.17 |
| cT2 | 244 (61.31%) | 183 (63.32%) | 59 (56.73%) | ||
| ≥cT3 | 5 (1.26%) | 5 (1.73%) | 0 | ||
| Biopsy grade group (n,%) | 1 | 158 (38.73%) | 115 (38.33%) | 40 (38.83%) | 0.73 |
| 2 | 128 (31.37%) | 90 (30%) | 37 (35.92%) | ||
| 3 | 54 (13.24%) | 41 (13.67%) | 13 (12.62%) | ||
| 4 | 47 (11.52%) | 37 (12.33%) | 9 (8.74%) | ||
| 5 | 21 (5.15%) | 17 (5.67%) | 4 (3.88%) | ||
| CCI (n,%) | 2 | 308 (73.33%) | 224 72.49%) | 79 (74.53%) | 0.37 |
| 3 | 78 (18.57%) | 56 (18.12%) | 22 (20.75%) | ||
| 4 | 27 (6.43%) | 24 (7.77%) | 3 (2.83%) | ||
| 5 | 4 (0.95%) | 3 (0.97%) | 1 (0.94%) | ||
| 6 | 3 (0.71%) | 2 (0.65%) | 1 (0.94%) | ||
| Age (years, median, IQR) | 65 (8) | 65 (8) | 64 (8) | 0.57 | |
| Postprostatectomy specimen | |||||
| Prostatectomy grade group (n,%) | 1 | 58 (13.94%) | 46 (14.98%) | 10 (9.62%) | 0.30 |
| 2 | 165 (39.66%) | 116 (37.79%) | 47 (45.19%) | ||
| 3 | 89 (21.39%) | 62 (20.20%) | 26 (25%) | ||
| 4 | 62 (14.90%) | 50 (16.29%) | 12 (11.54%) | ||
| 5 | 42 (10.10%) | 33 (10.75%) | 9 (8.65%) | ||
| pT (n,%) | pT2 | 241 (57.93%) | 171 (55.70%) | 65 (62.50%) | 0.41 |
| pT3 | 173 (41.59%) | 134 (43.65%) | 39 (37.50%) | ||
| pT4 | 2 (0.48%) | 2 (0.65%) | 0 | ||
| pN (n,%) | pN0 | 181 (43.20%) | 134 (43.51%) | 45 42.45%) | 0.20 |
| pN1+ | 31 (7.40%) | 27 (8.77%) | 4 (3.77%) | ||
| pNx | 207 (49.40%) | 147 (47.73%) | 57 (53.77%) | ||
| EPE (n,%) | 175 (42.37%) | 136 (44.59%) | 39 (37.86%) | 0.25 | |
| SVI (n,%) | 61 (14.52%) | 48 (15.53%) | 13 (12.26%) | 0.52 | |
| PSM (n,%) | 123 (29.50%) | 91 (29.55%) | 32 (30.77%) | 0.81 | |
| SII Cut-Off | Cut-Off Percentile | 8-Year Survival Probability Difference | Log-Rank p-Value |
|---|---|---|---|
| 600 | 50 | 7.28% | 0.1525 |
| 700 | 60 | 9.93% | 0.1259 |
| 800 | 68 | 12.75% | 0.0789 |
| 900 | 75 | 16.58% | 0.0206 |
| 1000 | 80 | 16.72% | 0.056 |
| Variable | HR (95% CI) | p |
|---|---|---|
| multivariate model 1 (c index = 0.67) | ||
| CAPRA-S ≥ 6 | 2.67 (1.33–5.35) | 0.006 |
| CCI ≥ 4 | 2.79 (1.14–6.84) | 0.025 |
| SII ≥ 900 | 2.59 (1.26–5.31) | 0.009 |
| multivariate model 2 (c index = 0.67) | ||
| CAPRA-S ≥ 6 | 2.65 (1.32–5.31) | 0.006 |
| ACCI ≥ 6 | 2.75 (1.27–5.95) | 0.01 |
| SII ≥ 900 | 2.54 (1.24–5.21) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapała, P.; Garbas, K.; Lewandowski, Z.; Zapała, Ł.; Ślusarczyk, A.; Ślusarczyk, C.; Mielczarek, Ł.; Radziszewski, P. The Clinical Utility of Systemic Immune-Inflammation Index Supporting Charlson Comorbidity Index and CAPRA-S Score in Determining Survival after Radical Prostatectomy—A Single Centre Study. Cancers 2022, 14, 4135. https://doi.org/10.3390/cancers14174135
Zapała P, Garbas K, Lewandowski Z, Zapała Ł, Ślusarczyk A, Ślusarczyk C, Mielczarek Ł, Radziszewski P. The Clinical Utility of Systemic Immune-Inflammation Index Supporting Charlson Comorbidity Index and CAPRA-S Score in Determining Survival after Radical Prostatectomy—A Single Centre Study. Cancers. 2022; 14(17):4135. https://doi.org/10.3390/cancers14174135
Chicago/Turabian StyleZapała, Piotr, Karolina Garbas, Zbigniew Lewandowski, Łukasz Zapała, Aleksander Ślusarczyk, Cezary Ślusarczyk, Łukasz Mielczarek, and Piotr Radziszewski. 2022. "The Clinical Utility of Systemic Immune-Inflammation Index Supporting Charlson Comorbidity Index and CAPRA-S Score in Determining Survival after Radical Prostatectomy—A Single Centre Study" Cancers 14, no. 17: 4135. https://doi.org/10.3390/cancers14174135






