Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada
Abstract
:Simple Summary
Abstract
1. Introduction
2. Molecular Markers
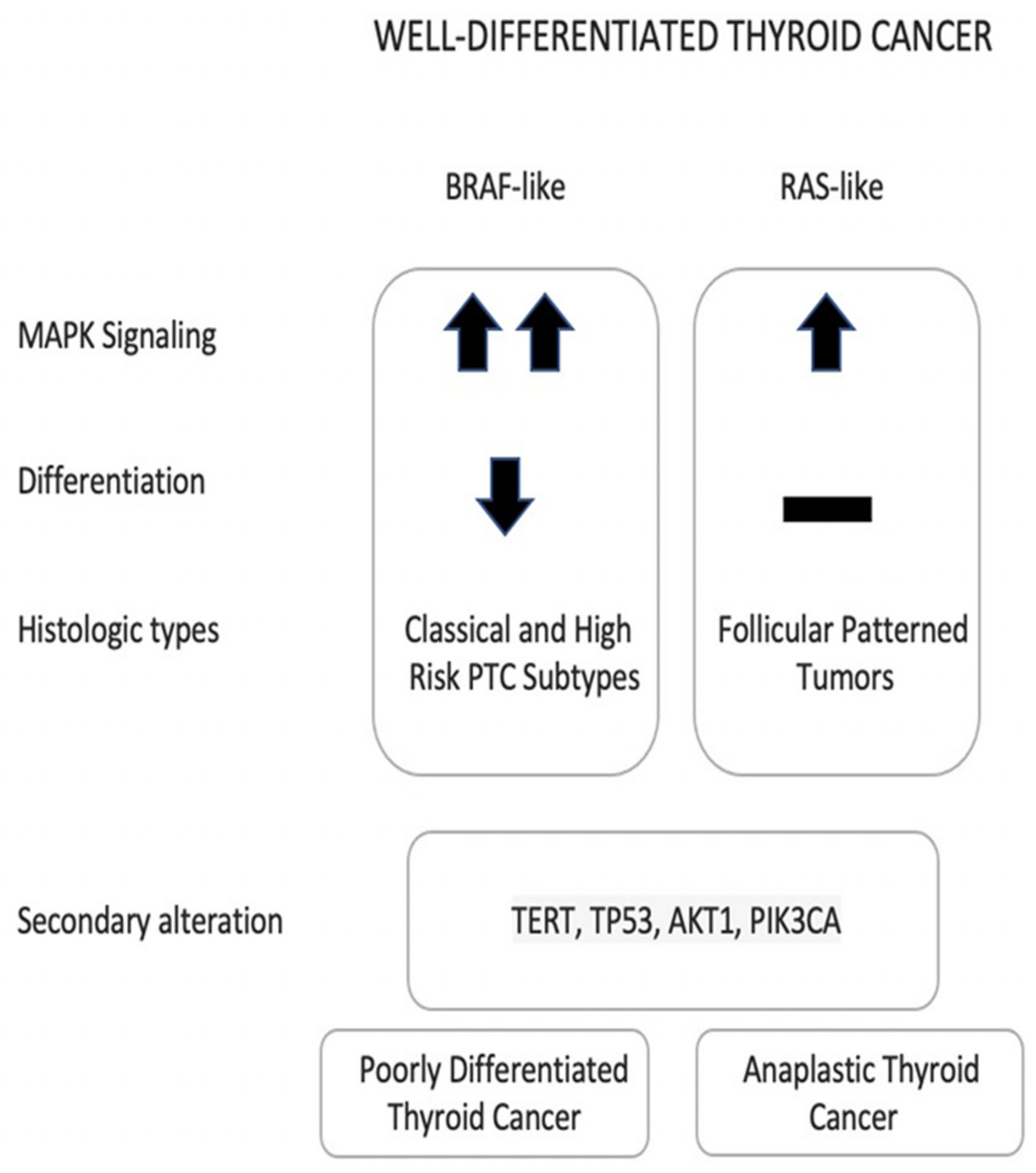
2.1. Papillary and Follicular Thyroid Cancers
2.2. Oncocytic Carcinoma of the Thyroid (OCT)
2.3. Advanced Differentiated Thyroid Cancer, PDTC and ATC
3. Molecular Tests
3.1. ThyroSeq v3
3.2. Afirma GSC/XA
3.3. ThyGenNEXT/ThyraMIR
4. Clinical Utility and Our Group’s Experience
4.1. Bethesda III and IV Nodules (Indeterminate Thyroid Nodules)
4.2. Bethesda V and VI Nodules
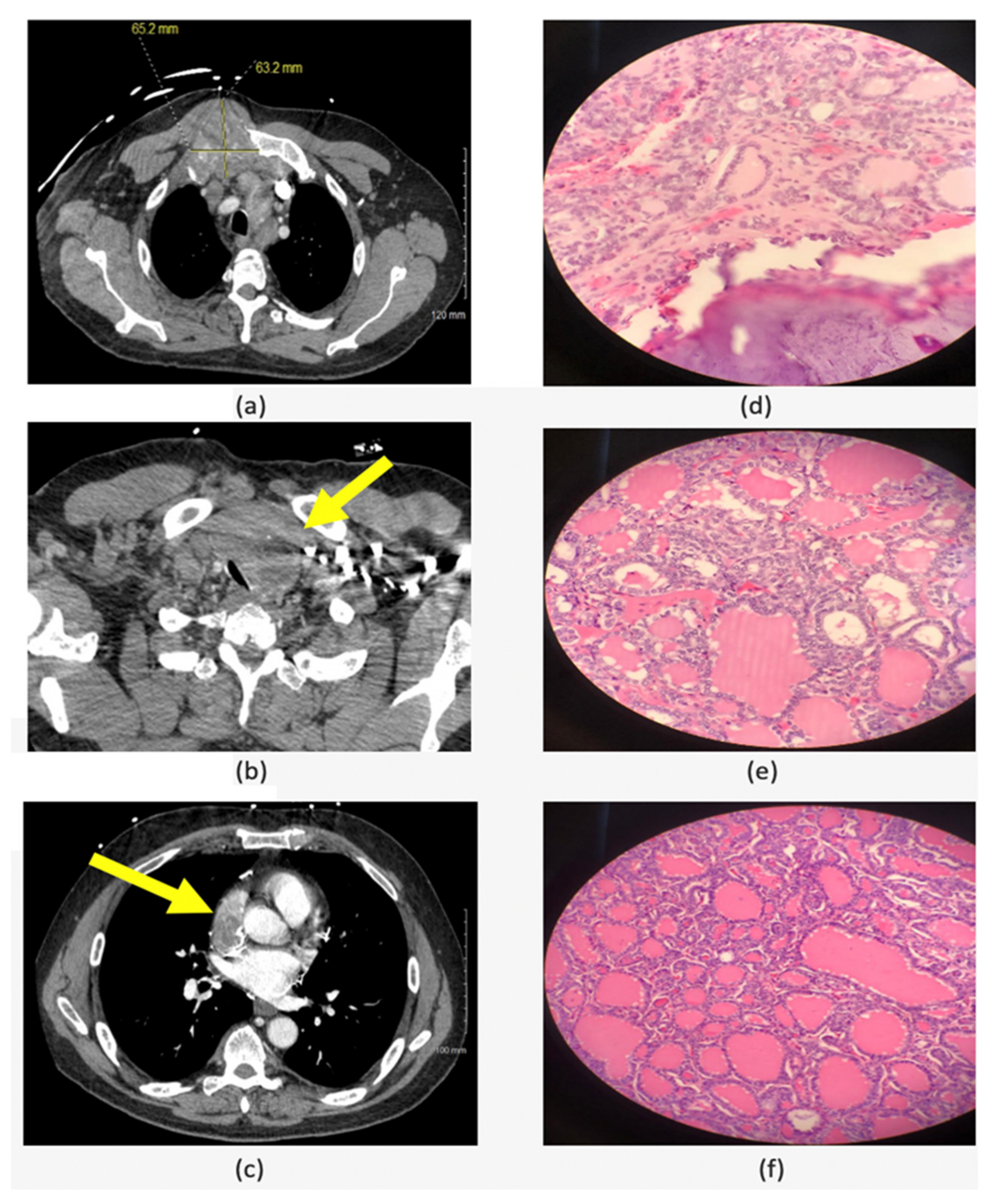
4.3. Advanced Thyroid Cancers
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.S.; Lawrence, L.; Bena, J.; Madhun, N.Z.; Berber, E.; Elsheikh, T.M.; E Nasr, C. Real-world Comparison of Afirma GEC and GSC for the Assessment of Cytologically Indeterminate Thyroid Nodules. J. Clin. Endocrinol. Metab. 2019, 105, e428–e435. [Google Scholar] [CrossRef] [PubMed]
- Merdad, M. A contemporary look at thyroid nodule management. Saudi Med. J. 2020, 41, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Oka, A.; Omoto, R.; Hirose, M. Ultrasound scanning of the thyroid gland as a new diagnostic approach. Ultrasonics 1967, 5, 177–180. [Google Scholar] [CrossRef]
- Jung, C.K.; Hong, S.; Bychkov, A.; Kakudo, K. The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology. J. Pathol. Transl. Med. 2017, 51, 571–578. [Google Scholar] [CrossRef]
- Grani, G.; Sponziello, M.; Pecce, V.; Ramundo, V.; Durante, C. Contemporary Thyroid Nodule Evaluation and Management. J. Clin. Endocrinol. Metab. 2020, 105, 2869–2883. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.-E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean Thyroid Imaging Reporting and Data System and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2021, 22, 2094. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules—2016 Update Appendix. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Vielh, P. Thyroid and Molecular Testing. Advances in Thyroid Molecular Cytopathology. J. Mol. Pathol. 2021, 2, 77–92. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Molecular analysis of thyroid tumors. Mod. Pathol. 2011, 24, S34–S43. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; De Souza, P.B.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Macerola, E.; Poma, A.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Santini, F.; Basolo, F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers 2021, 13, 1139. [Google Scholar] [CrossRef]
- Kakarmath, S.; Heller, H.T.; Alexander, C.A.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Lindeman, N.I.; Frates, M.C.; Benson, C.B.; Gawande, A.A.; et al. Clinical, Sonographic, and Pathological Characteristics of RAS-Positive Versus BRAF-Positive Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2016, 101, 4938–4944. [Google Scholar] [CrossRef]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef]
- Gopal, R.K.; Kübler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef] [Green Version]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Chun, S.-M.; Kim, D.; Kwon, H.; Jang, E.K.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jang, S.J.; Song, D.E. Genomic Alterations of Anaplastic Thyroid Carcinoma Detected by Targeted Massive Parallel Sequencing in a BRAFV600E Mutation-Prevalent Area. Thyroid 2016, 26, 683–690. [Google Scholar] [CrossRef]
- Sykorova, V.; Dvorakova, S.; Vcelak, J.; Vaclavikova, E.; Halkova, T.; Kodetova, D.; Lastuvka, P.; Betka, J.; Vlcek, P.; Reboun, M.; et al. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res. 2015, 35, 2029–2036. [Google Scholar]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Liu, X.; Ren, X.; Zhang, H.; Wu, H.; Liang, Z. Mutation profiles of follicular thyroid tumors by targeted sequencing. Diagn. Pathol. 2019, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Lynch, R.A.; Biddinger, P.W.; Alexander, E.K.; Dorn, G.W.; Tallini, G.; Kroll, T.G.; Nikiforov, Y.E. RAS Point Mutations and PAX8-PPARγ Rearrangement in Thyroid Tumors: Evidence for Distinct Molecular Pathways in Thyroid Follicular Carcinoma. J. Clin. Endocrinol. Metab. 2003, 88, 2318–2326. [Google Scholar] [CrossRef]
- Vivanco, I.; Sawyers, C.L. The phosphatidylinositol 3-Kinase–AKT pathway in human cancer. Nat. Cancer 2002, 2, 489–501. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Ciampi, R.; Nikiforov, Y.E. RET/PTC Rearrangements and BRAF Mutations in Thyroid Tumorigenesis. Endocrinology 2007, 148, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Xing, M.; Mambo, E.; Guo, Z.; Wu, G.; Trink, B.; Beller, U.; Westra, W.H.; Ladenson, P.W.; Sidransky, D. BRAF Mutation in Papillary Thyroid Carcinoma. JNCI 2003, 95, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Yip, L.; Nikiforova, M.N.; Yoo, J.Y.; McCoy, K.L.; Stang, M.T.; Armstrong, M.J.; Nicholson, K.; Ohori, N.P.; Coyne, C.; Hodak, S.P.; et al. Tumor Genotype Determines Phenotype and Disease-related Outcomes in Thyroid Cancer. Ann. Surg. 2015, 262, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Ricarte-Filho, J.; Knauf, J.; Shaha, A.; Tuttle, M.; Fagin, J.A.; Ghossein, R.A. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod. Pathol. 2010, 23, 1191–1200. [Google Scholar] [CrossRef]
- Howitt, B.E.; Jia, Y.; Sholl, L.M.; Barletta, J.A. Molecular Alterations in Partially-Encapsulated or Well-Circumscribed Follicular Variant of Papillary Thyroid Carcinoma. Thyroid 2013, 23, 1256–1262. [Google Scholar] [CrossRef]
- Silver, J.A.; Bogatchenko, M.; Pusztaszeri, M.; Forest, V.-I.; Hier, M.P.; Yang, J.W.; Tamilia, M.; Payne, R.J. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas ≤ 1.5 cm. J. Otolaryngol. Head Neck Surg. 2021, 50, 63. [Google Scholar] [CrossRef]
- Semsar-Kazerooni, K.; Morand, G.B.; Payne, A.E.; da Silva, S.D.; Forest, V.-I.; Hier, M.P.; Pusztaszeri, M.P.; Tamilia, M.; Payne, R.J. Mutational status may supersede tumor size in predicting the presence of aggressive pathologic features in well differentiated thyroid cancer. J. Otolaryngol. Head Neck Surg. 2022, 51, 9. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Baloch, Z.W.; Hodak, S.P.; Giordano, T.J.; Lloyd, R.V.; Seethala, R.R.; Wenig, B.M. Change in Diagnostic Criteria for Noninvasive Follicular Thyroid Neoplasm with Papillarylike Nuclear Features. JAMA Oncol. 2018, 4, 1125–1126. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef]
- Limberg, J.; Ullmann, T.M.; Stefanova, D.; Buicko, J.L.; Finnerty, B.M.; Zarnegar, R.; Fahey, T.J., 3rd; Beninato, T. Does Aggressive Variant Histology Without Invasive Features Predict Overall Survival in Papillary Thyroid Cancer? A National Cancer Database Analysis. Ann. Surg. 2021, 274, e276–e281. [Google Scholar] [CrossRef]
- Nath, M.C.; Erickson, L.A. Aggressive Variants of Papillary Thyroid Carcinoma: Hobnail, Tall Cell, Columnar, and Solid. Adv. Anat. Pathol. 2018, 25, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chereau, N.; Giudicelli, X.; Pattou, F.; Lifante, J.-C.; Triponez, F.; Mirallié, E.; Goudet, P.; Brunaud, L.; Trésallet, C.; Tissier, F.; et al. Diffuse Sclerosing Variant of Papillary Thyroid Carcinoma Is Associated with Aggressive Histopathological Features and a Poor Outcome: Results of a Large Multicentric Study. J. Clin. Endocrinol. Metab. 2016, 101, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Ghuzlan, A.A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.J.; Nikiforov, Y.E. Molecular Genetics and Diagnostics of Thyroid Cancer. In The Thyroid and Its Diseases; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 549–561. [Google Scholar]
- Morariu, E.M.; McCoy, K.L.; Chiosea, S.I.; Nikitski, A.V.; Manroa, P.; Nikiforova, M.N.; Nikiforov, Y.E. Clinicopathologic Characteristics of Thyroid Nodules Positive for the THADA-IGF2BP3 Fusion on Preoperative Molecular Analysis. Thyroid 2021, 31, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, N.; Akahane, T.; Goto, T.; Kono, M.; Ichikawa, H.; Sawada, T.; Yamaguchi, T.; Nakano, H.; Kawase, Y.; Kato, Y.; et al. Targeted next-generation sequencing of cancer-related genes in thyroid carcinoma: A single institution’s experience. Oncol. Lett. 2018, 16, 7278–7286. [Google Scholar] [CrossRef]
- Xu, B.; Ghossein, R. Poorly differentiated thyroid carcinoma. Semin. Diagn. Pathol. 2020, 37, 243–247. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef]
- Chen, H.; Luthra, R.; Routbort, M.J.; Patel, K.P.; Cabanillas, M.E.; Broaddus, R.R.; Williams, M.D. Molecular Profile of Advanced Thyroid Carcinomas by Next-Generation Sequencing: Characterizing Tumors Beyond Diagnosis for Targeted Therapy. Mol. Cancer Ther. 2018, 17, 1575–1584. [Google Scholar] [CrossRef]
- Shonka, D.C.; Ho, A.; Chintakuntlawar, A.V.; Geiger, J.L.; Park, J.C.; Seetharamu, N.; Jasim, S.; Ahmed, A.H.A.; Bible, K.C.; Brose, M.S.; et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: Defining advanced thyroid cancer and its targeted treatment. Head Neck 2022, 44, 1277–1300. [Google Scholar] [CrossRef]
- Silaghi, C.A.; Lozovanu, V.; Georgescu, C.E.; Georgescu, R.D.; Susman, S.; Năsui, B.A.; Dobrean, A.; Silaghi, H. Thyroseq v3, Afirma GSC, and microRNA Panels Versus Previous Molecular Tests in the Preoperative Diagnosis of Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 649522. [Google Scholar] [CrossRef]
- Vargas-Salas, S.; Martínez, J.R.; Urra, S.; Domínguez, J.M.; Mena, N.; Uslar, T. Genetic testing for indeterminate thyroid cytology: Review and meta-analysis. In Endocrine-Related Cancer; BioScientifica Ltd.: Bristol, UK, 2018; Volume 25, pp. R163–R177. [Google Scholar]
- Nikiforov, Y.E.; Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar]
- Krane, J.F.; Cibas, E.S.; Endo, M.; Marqusee, E.; Hu, M.I.; Nasr, C.E.; Waguespack, S.G.; Wirth, L.J.; Kloos, R.T.; Krane, J.F. The Afirma Xpression Atlas for thyroid nodules and thyroid cancer metastases: Insights to inform clinical decision-making from a fine-needle aspiration sample. Cancer Cytopathol. 2020, 128, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Lupo, M.A.; Walts, A.E.; Sistrunk, J.W.; Giordano, T.J.; Sadow, P.M.; Massoll, N.; Campbell, R.; Jackson, S.A.; Toney, N.; Narick, C.M.; et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagn. Cytopathol. 2020, 48, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.-Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef]
- Angell, T.E.; Wirth, L.J.; Cabanillas, M.E.; Shindo, M.L.; Cibas, E.S.; Babiarz, J.E.; Hao, Y.; Kim, S.Y.; Walsh, P.S.; Huang, J.; et al. Analytical and Clinical Validation of Expressed Variants and Fusions from the Whole Transcriptome of Thyroid FNA Samples. Front. Endocrinol. 2019, 10, 612. [Google Scholar] [CrossRef]
- Hier, J.; Avior, G.; Pusztaszeri, M.; Krasner, J.R.; Alyouha, N.; Forest, V.-I.; Hier, M.P.; Mlynarek, A.; Richardson, K.; Sadeghi, N.; et al. Molecular testing for cytologically suspicious and malignant (Bethesda V and VI) thyroid nodules to optimize the extent of surgical intervention: A retrospective chart review. J. Otolaryngol. Head Neck Surg. 2021, 50, 29. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; De Moura, M.B.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef]
- Mayson, S.E.; Haugen, B.R. Molecular Diagnostic Evaluation of Thyroid Nodules. Endocrinol. Metab. Clin. N. Am. 2018, 48, 85–97. [Google Scholar] [CrossRef]
- Carty, S.E.; Ohori, N.P.; Hilko, D.A.; McCoy, K.L.; French, E.K.; Manroa, P.; Morariu, E.; Sridharan, S.; Seethala, R.R.; Yip, L. The Clinical Utility of Molecular Testing in the Management of Thyroid Follicular Neoplasms (Bethesda IV Nodules). Ann. Surg. 2020, 272, 621–627. [Google Scholar] [CrossRef]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J.; et al. Executive Summary of the American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann. Surg. 2020, 271, 399–410. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.Y.; Ehya, H.; et al. Thyroid Carcinoma, Version 2.2022, NCCN Clinical Pracrice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef] [PubMed]
- Kay-Rivest, E.; Tibbo, J.; Bouhabel, S.; Tamilia, M.; Leboeuf, R.; Forest, V.-I.; Hier, M.P.; Savoury, L.; Payne, R.J. The first Canadian experience with the Afirma® gene expression classifier test. J. Otolaryngol. Head Neck Surg. 2017, 46, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Gilfix, B.M.; Rivera, J.A.; Sadeghi, N.; Richardson, K.; Hier, M.P.; Forest, V.-I.; Fishman, D.; Caglar, D.; Pusztaszeri, M.; et al. The Role of the ThyroSeq v3 Molecular Test in the Surgical Management of Thyroid Nodules in the Canadian Public Health Care Setting. Thyroid 2020, 30, 1280–1287. [Google Scholar] [CrossRef]
- Morris, L.G.T. Molecular Profiling of Thyroid Nodules—Are These Findings Meaningful, or Merely Measurable? JAMA Otolaryngol. Neck Surg. 2020, 146, 845. [Google Scholar] [CrossRef] [PubMed]
- Dublin, J.C.; Papazian, M.; Zan, E.; Oweity, T.; Sun, W.; Jacobson, A.; Patel, K.; Brandler, T.C.; Givi, B. Predictive Value of a Genomic Classifier in Indeterminate Thyroid Nodules Based on Nodule Size. JAMA Otolaryngol. Neck Surg. 2022, 148, 53. [Google Scholar] [CrossRef] [PubMed]
- Turkdogan, S.; Pusztaszeri, M.; Forest, V.-I.; Hier, M.P.; Payne, R.J. Are Bethesda III Thyroid Nodules More Aggressive than Bethesda IV Thyroid Nodules When Found to Be Malignant? Cancers 2020, 12, 2563. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, N.R.; Mayall, E.S.; Wyllie, F.S.; Williams, E.D.; Goyns, M.; Stringer, B.; Wynford-Thomas, D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989, 4, 159–164. [Google Scholar]
- Patel, S.G.; Carty, S.E.; McCoy, K.L.; Ohori, N.P.; LeBeau, S.O.; Seethala, R.R.; Nikiforova, M.N.; Nikiforov, Y.E.; Yip, L. Preoperative detection of RAS mutation may guide extent of thyroidectomy. Surgery 2016, 161, 168–175. [Google Scholar] [CrossRef]
- Mascarella, M.A.; Peeva, M.; Forest, V.I.; Pusztaszeri, M.P.; Avior, G.; Tamilia, M.; Mlynarek, A.M.; Hier, M.P.; Payne, R.J. Association of Bethesda category and molecular mutation in patients undergoing thyroidectomy. Clin. Otolaryngol. 2022, 47, 75–80. [Google Scholar] [CrossRef]
- Tessler, I.; Shochat, I.; Cohen, O.; Payne, R.J.; Avior, G. Positive correlation of thyroid nodule cytology with molecular profiling—Analysis of over 4000 nodules from multicenter study and systematic literature review. Presented at European Congress of Endocrinology 2022, Milan, Italy. Endocr. Abstr. 2022, 81, OC11.5. [Google Scholar] [CrossRef]
- Gargano, S.M.; Badjatia, N.; Nikolaus, Y.; Peiper, S.C.; Wang, Z.X. Characterization and Clinical Significance of EIF1AX Mutations and Co-Mutations in Cytologically Indeterminate Thyroid Nodules: A 5-Year Retrospective Analysis. Acta Med. Acad. 2021, 50, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Toraldo, G.; Cerda, S.R.; Godley, F.A.; Rao, S.R.; McAneny, D.; Doherty, G.M.; Braverman, L.E.; Lee, S.L. Utilities of RAS Mutations in Preoperative Fine Needle Biopsies for Decision Making for Thyroid Nodule Management: Results from a Single-Center Prospective Cohort. Thyroid 2020, 30, 536–547. [Google Scholar] [CrossRef]
- Vuong, H.G.; Duong, U.N.; Altibi, A.M.; Ngo, H.T.; Pham, T.Q.; Tran, H.M.; Gandolfi, G.; Hassell, L. A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr. Connect. 2017, 6, R8–R17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotta, V.; Bifulco, M.; Vitale, M. Significance of RAS Mutations in Thyroid Benign Nodules and Non-Medullary Thyroid Cancer. Cancers 2021, 13, 3785. [Google Scholar] [CrossRef] [PubMed]
- Bongers, P.J.; Kluijfhout, W.P.; Verzijl, R.; Lustgarten, M.; Vermeer, M.; Goldstein, D.P.; Devon, K.; Rotstein, L.E.; Asa, S.; Brierley, J.D.; et al. Papillary Thyroid Cancers with Focal Tall Cell Change are as Aggressive as Tall Cell Variants and Should Not be Considered as Low-Risk Disease. Ann. Surg. Oncol. 2019, 26, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, F.; Shen, X.; Zhu, G.; Liu, R.; Viola, D.; Elisei, R.; Puxeddu, E.; Fugazzola, L.; Colombo, C.; et al. BRAF V600E Status Sharply Differentiates Lymph Node Metastasis-associated Mortality Risk in Papillary Thyroid Cancer. J. Clin. Endocrinol. Metab. 2021, 106, 3228–3238. [Google Scholar] [CrossRef]
- Danilovic, D.L.; Castroneves, L.A.; Suemoto, C.K.; Elias, L.O.; Soares, I.C.; Camargo, R.Y.; Correa, F.D.A.; Hoff, A.O.; Marui, S. Is There a Difference Between Minimal and Gross Extension into the Strap Muscles for the Risk of Recurrence in Papillary Thyroid Carcinomas? Thyroid 2020, 30, 1008–1016. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Feurer, I.D.; Griffin, M.R.; Phay, J.E. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008, 144, 1070–1078. [Google Scholar] [CrossRef]
- Suman, P.; Razdan, S.N.; Wang, C.-H.E.; Tulchinsky, M.; Ahmed, L.; Prinz, R.A.; Winchester, D.J. Thyroid Lobectomy for T1b-T2 Papillary Thyroid Cancer with High-Risk Features. J. Am. Coll. Surg. 2019, 230, 136–144. [Google Scholar] [CrossRef]
- Krasner, J.R.; Alyouha, N.; Pusztaszeri, M.; Forest, V.I.; Hier, M.P.; Avior, G. Molecular mutations as a possible factor for determining extent of thyroid surgery. J. Otolaryngol. Head Neck Surg. 2019, 48, 51. [Google Scholar] [CrossRef]
- Chen, Y.; Sadow, P.M.; Suh, H.; Lee, K.E.; Choi, J.Y.; Suh, Y.J. BRAFV600E is correlated with recurrence of papillary thyroid microcarcinoma: A systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid 2016, 26, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Catford, S.R.; Lee, K.T.; Pace, M.D.; Marasco, S.F.; Longano, A.; Topliss, D.J. Cardiac metastasis from thyroid carcinoma. Thyroid 2011, 21, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Ohori, N.P.; Landau, M.; Carty, S.E.; Yip, L.; Lebeau, S.O.; Manroa, P.; Seethala, R.R.; Schoedel, K.E.; Nikiforova, M.N.; Nikiforov, Y.E. Benign call rate and molecular test result distribution of ThyroSeq v3. Cancer Cytopathol. 2018, 127, 161–168. [Google Scholar] [CrossRef]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Cibas, E.S.; Chudova, D.; Diggans, J.; Friedman, L.; Kloos, R.T.; Livolsi, V.A.; Mandel, S.J.; et al. Preoperative Diagnosis of Benign Thyroid Nodules with Indeterminate Cytology. N. Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef]
- Ferris, R.L.; Baloch, Z.; Bernet, V.; Chen, A.; Fahey, T.J., 3rd; Ganly, I.; Hodak, S.P.; Kebebew, E.; Patel, K.N.; Shaha, A.; et al. American Thyroid Association Surgical Affairs Committee. American Thyroid Association Statement on Surgical Application of Molecular Profiling for Thyroid Nodules: Current Impact on Perioperative Decision Making. Thyroid 2015, 25, 760–768. [Google Scholar] [CrossRef]
- DiMarco, A.N.; Wong, M.S.; Jayasekara, J.; Cole-Clark, D.; Aniss, A.; Glover, A.; Delbridge, L.W.; Sywak, M.S.; Sidhu, S.B. Risk of needing completion thyroidectomy for low-risk papillary thyroid cancers treated by lobectomy. BJS Open 2018, 3, 299–304. [Google Scholar] [CrossRef]
- Dhir, M.; McCoy, K.L.; Ohori, N.P.; Adkisson, C.D.; LeBeau, S.O.; Carty, S.E.; Yip, L. Correct extent of thyroidectomy is poorly predicted preoperatively by the guidelines of the American Thyroid Association for low and intermediate risk thyroid cancers. Surgery 2018, 163, 81–87. [Google Scholar] [CrossRef]
- Kluijfhout, W.P.; Pasternak, J.D.; Lim, J.; Kwon, J.S.; Vriens, M.R.; Clark, O.H.; Shen, W.T.; Gosnell, J.E.; Suh, I.; Duh, Q.-Y. Frequency of High-Risk Characteristics Requiring Total Thyroidectomy for 1–4 cm Well-Differentiated Thyroid Cancer. Thyroid 2016, 26, 820–824. [Google Scholar] [CrossRef]
- Craig, S.J.; Bysice, A.M.; Nakoneshny, S.C.; Pasieka, J.L.; Chandarana, S.P. The Identification of Intraoperative Risk Factors Can Reduce, but Not Exclude, the Need for Completion Thyroidectomy in Low-Risk Papillary Thyroid Cancer Patients. Thyroid 2020, 30, 222–228. [Google Scholar] [CrossRef]
- Skaugen, J.M.; Taneja, C.; Liu, J.B.; Wald, A.I.; Nikitski, A.V.; Chiosea, S.I.; Seethala, R.; Ohori, N.P.; Karslioglu-French, E.; Carty, S.E.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Suspicious for Malignancy Cytology. Thyroid 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shimura, H.; Matsumoto, Y.; Murakami, T.; Fukunari, N.; Kitaoka, M.; Suzuki, S. Diagnostic Strategies for Thyroid Nodules Based on Ultrasonographic Findings in Japan. Cancers 2021, 13, 4629. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Yamashita, H.; Kakudo, K. Thyroid Cytology: The Japanese System and Experience at Yamashita Thyroid Hospital. J. Pathol. Transl. Med. 2017, 51, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.Y.; Nga, M.E. Atypia of undetermined significance/follicular lesion of undetermined significance: Asian vs. non-Asian practice, and the Singapore experience. Gland Surg. 2020, 9, 1764–1787. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.G.; Ngo, H.T.T.; Bychkov, A.; Jung, C.K.; Vu, T.H.; Lu, K.B.; Kakudo, K.; Kondo, T. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: A systematic review and meta-analysis. Cancer Cytopathol. 2019, 128, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jing, W.; Chang, Q.; Zhang, Z.; Liu, Z.; Cao, J.; Zhao, L.; Sun, Y.; Wang, C.; Zhao, H.; et al. Risk stratification of indeterminate thyroid nodules by novel multigene testing: A study of Asians with a high risk of malignancy. Mol. Oncol. 2022, 16, 1680–1693. [Google Scholar] [CrossRef]
- Ito, Y.; Onoda, N.; Okamoto, T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr. J. 2020, 67, 669–717. [Google Scholar] [CrossRef]
- Sugitani, I.; Ito, Y.; Takeuchi, D.; Nakayama, H.; Masaki, C.; Shindo, H.; Teshima, M.; Horiguchi, K.; Yoshida, Y.; Kanai, T.; et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021, 31, 183–192. [Google Scholar] [CrossRef]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyroid J. 2021, 10, 185–197. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Nilubol, N.; Tufano, R.P.; Kandil, E. Wolf in Sheep’s Clothing: Papillary Thyroid Microcarcinoma in the US. J. Am. Coll. Surg. 2020, 230, 484–491. [Google Scholar] [CrossRef]

|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajab, M.; Payne, R.J.; Forest, V.-I.; Pusztaszeri, M. Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada. Cancers 2022, 14, 4140. https://doi.org/10.3390/cancers14174140
Rajab M, Payne RJ, Forest V-I, Pusztaszeri M. Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada. Cancers. 2022; 14(17):4140. https://doi.org/10.3390/cancers14174140
Chicago/Turabian StyleRajab, Mohannad, Richard J. Payne, Véronique-Isabelle Forest, and Marc Pusztaszeri. 2022. "Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada" Cancers 14, no. 17: 4140. https://doi.org/10.3390/cancers14174140
APA StyleRajab, M., Payne, R. J., Forest, V.-I., & Pusztaszeri, M. (2022). Molecular Testing for Thyroid Nodules: The Experience at McGill University Teaching Hospitals in Canada. Cancers, 14(17), 4140. https://doi.org/10.3390/cancers14174140







