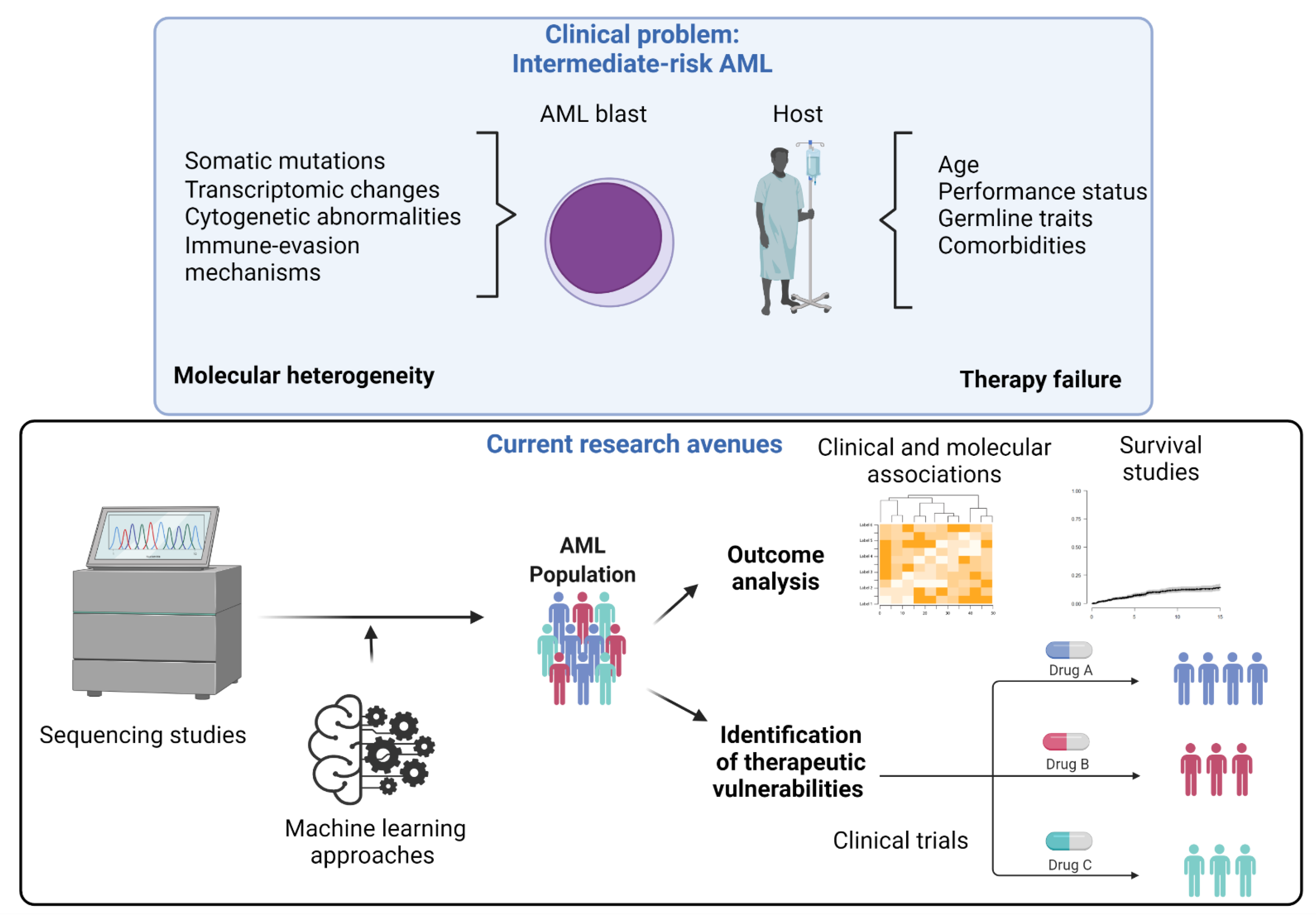
A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges
Abstract
:Simple Summary
Abstract
1. Introduction
2. Intermediate-Risk Definition and Prognosis
3. Current Therapeutic Approaches
4. Clinical Trials
5. Targeted Agents and Challenges in Intermediate-Risk AML
5.1. FLT3 Inhibitors
5.2. IDH1/2 Inhibitors
5.3. Others
5.4. Challenges
6. Immunotherapies in AML
6.1. The Role of Immune Checkpoint Inhibitors in AML
6.2. Monoclonal Antibodies and CAR-T Cell Therapy in AML
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Gurnari, C.; Pagliuca, S.; Visconte, V. Deciphering the Therapeutic Resistance in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2020, 21, 8505. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Meggendorfer, M.; Kerr, C.M.; Kuzmanovic, T.; Durrani, J.; Shreve, J.; Nagata, Y.; et al. Machine Learning Integrates Genomic Signatures for Subclassification Beyond Primary and Secondary Acute Myeloid Leukemia. Blood 2021, 138, 1885–1895. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood 2022, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Gönen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. Clinical implications of molecular markers in acute myeloid leukemia. Eur. J. Haematol. 2019, 102, 20–35. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J., Jr.; Laird, P.W.; Baty, J.D.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef] [Green Version]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef]
- Ley, T.J.; Ding, L.; Walter, M.J.; McLellan, M.D.; Lamprecht, T.; Larson, D.E.; Kandoth, C.; Payton, J.E.; Baty, J.; Welch, J.; et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 2010, 363, 2424–2433. [Google Scholar] [CrossRef]
- Herold, T.; Rothenberg-Thurley, M.; Grunwald, V.V.; Janke, H.; Goerlich, D.; Sauerland, M.C.; Konstandin, N.P.; Dufour, A.; Schneider, S.; Neusser, M.; et al. Validation and refinement of the revised 2017 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2020, 34, 3161–3172. [Google Scholar] [CrossRef]
- Buccisano, F.; Maurillo, L.; Schuurhuis, G.J.; Del Principe, M.I.; Di Veroli, A.; Gurnari, C.; Venditti, A. The emerging role of measurable residual disease detection in AML in morphologic remission. Semin. Hematol. 2019, 56, 125–130. [Google Scholar] [CrossRef]
- Del Principe, M.I.; De Bellis, E.; Gurnari, C.; Buzzati, E.; Savi, A.; Consalvo, M.A.I.; Venditti, A. Applications and efficiency of flow cytometry for leukemia diagnostics. Expert Rev. Mol. Diagn. 2019, 19, 1089–1097. [Google Scholar] [CrossRef]
- Eisfeld, A.K.; Kohlschmidt, J.; Mims, A.; Nicolet, D.; Walker, C.J.; Blachly, J.S.; Carroll, A.J.; Papaioannou, D.; Kolitz, J.E.; Powell, B.E.; et al. Additional gene mutations may refine the 2017 European LeukemiaNet classification in adult patients with de novo acute myeloid leukemia aged <60 years. Leukemia 2020, 34, 3215–3227. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Itzykson, R.; Fournier, E.; Berthon, C.; Röllig, C.; Braun, T.; Marceau-Renaut, A.; Pautas, C.; Nibourel, O.; Lemasle, E.; Micol, J.B.; et al. Genetic identification of patients with AML older than 60 years achieving long-term survival with intensive chemotherapy. Blood 2021, 138, 507–519. [Google Scholar] [CrossRef]
- Ossenkoppele, G.J.; Schuurhuis, G.J. MRD in AML: It is time to change the definition of remission. Best Pract. Res. Clin. Haematol. 2014, 27, 265–271. [Google Scholar] [CrossRef]
- Radakovich, N.; Cortese, M.; Nazha, A. Acute myeloid leukemia and artificial intelligence, algorithms and new scores. Best Pract. Res. Clin. Haematol. 2020, 33, 101192. [Google Scholar] [CrossRef]
- Fernandez, H.F.; Sun, Z.; Yao, X.; Litzow, M.R.; Luger, S.M.; Paietta, E.M.; Racevskis, J.; Dewald, G.W.; Ketterling, R.P.; Bennett, J.M.; et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, B.; Ossenkoppele, G.J.; van Putten, W.; Schouten, H.C.; Graux, C.; Ferrant, A.; Sonneveld, P.; Maertens, J.; Jongen-Lavrencic, M.; von Lilienfeld-Toal, M.; et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 2009, 361, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Joo, Y.D.; Kim, H.; Bae, S.H.; Kim, M.K.; Zang, D.Y.; Lee, J.L.; Lee, G.W.; Lee, J.H.; Park, J.H.; et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 2011, 118, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Kell, J.; Freeman, S.; Kjeldsen, L.; Hunter, A.E.; Yin, J.; Craddock, C.F.; Dufva, I.H.; et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 3924–3931. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Fournier, E.; Duployez, N.; Ducourneau, B.; Raffoux, E.; Turlure, P.; Caillot, D.; Thomas, X.; Marceau-Renaut, A.; Chantepie, S.; Malfuson, J.V.; et al. Mutational profile and benefit of gemtuzumab ozogamicin in acute myeloid leukemia. Blood 2020, 135, 542–546. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Bixby, D.; Perl, A.; Bhatt, V.R.; Altman, J.K.; Appelbaum, F.R.; de Lima, M.; Fathi, A.T.; Foran, J.M.; Gojo, I.; et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 16–27. [Google Scholar] [CrossRef]
- Heuser, M.; Ofran, Y.; Boissel, N.; Brunet Mauri, S.; Craddock, C.; Janssen, J.; Wierzbowska, A.; Buske, C. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 697–712. [Google Scholar] [CrossRef]
- Mayer, R.J.; Davis, R.B.; Schiffer, C.A.; Berg, D.T.; Powell, B.L.; Schulman, P.; Omura, G.A.; Moore, J.O.; McIntyre, O.R.; Frei, E. Intensive Postremission Chemotherapy in Adults with Acute Myeloid Leukemia. N. Engl. J. Med. 1994, 331, 896–903. [Google Scholar] [CrossRef]
- Bloomfield, C.D.; Lawrence, D.; Byrd, J.C.; Carroll, A.; Pettenati, M.J.; Tantravahi, R.; Patil, S.R.; Davey, F.R.; Berg, D.T.; Schiffer, C.A.; et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998, 58, 4173–4179. [Google Scholar]
- Burnett, A.K.; Wheatley, K.; Goldstone, A.H.; Stevens, R.F.; Hann, I.M.; Rees, J.H.; Harrison, G. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: Results of the UK MRC AML 10 trial. Br. J. Haematol. 2002, 118, 385–400. [Google Scholar] [CrossRef]
- Suciu, S.; Mandelli, F.; de Witte, T.; Zittoun, R.; Gallo, E.; Labar, B.; De Rosa, G.; Belhabri, A.; Giustolisi, R.; Delarue, R.; et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): An intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood 2003, 102, 1232–1240. [Google Scholar] [CrossRef]
- Röllig, C.; Bornhäuser, M.; Thiede, C.; Taube, F.; Kramer, M.; Mohr, B.; Aulitzky, W.; Bodenstein, H.; Tischler, H.-J.; Stuhlmann, R.; et al. Long-Term Prognosis of Acute Myeloid Leukemia According to the New Genetic Risk Classification of the European LeukemiaNet Recommendations: Evaluation of the Proposed Reporting System. J. Clin. Oncol. 2011, 29, 2758–2765. [Google Scholar] [CrossRef]
- Rücker, F.G.; Agrawal, M.; Corbacioglu, A.; Weber, D.; Kapp-Schwoerer, S.; Gaidzik, V.I.; Jahn, N.; Schroeder, T.; Wattad, M.; Lübbert, M.; et al. Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): Results from the AML Study Group. Blood 2019, 134, 1608–1618. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Tobal, K.; Newton, J.; Macheta, M.; Chang, J.; Morgenstern, G.; Evans, P.A.; Morgan, G.; Lucas, G.S.; Liu Yin, J.A. Molecular quantitation of minimal residual disease in acute myeloid leukemia with t(8;21) can identify patients in durable remission and predict clinical relapse. Blood 2000, 95, 815–819. [Google Scholar] [CrossRef]
- Nucifora, G.; Larson, R.A.; Rowley, J.D. Persistence of the 8;21 translocation in patients with acute myeloid leukemia type M2 in long-term remission. Blood 1993, 82, 712–715. [Google Scholar] [CrossRef]
- Satake, N.; Maseki, N.; Kozu, T.; Sakashita, A.; Kobayashi, H.; Sakurai, M.; Ohki, M.; Kaneko, Y. Disappearance of AML1-MTG8(ETO) fusion transcript in acute myeloid leukaemia patients with t(8;21) in long-term remission. Br. J. Haematol. 1995, 91, 892–898. [Google Scholar] [CrossRef]
- Zhu, H.H.; Zhang, X.H.; Qin, Y.Z.; Liu, D.H.; Jiang, H.; Chen, H.; Jiang, Q.; Xu, L.P.; Lu, J.; Han, W.; et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: Results from the AML05 multicenter trial. Blood 2013, 121, 4056–4062. [Google Scholar] [CrossRef]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.C.; et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- Venditti, A.; Piciocchi, A.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; de Fabritiis, P.; Storti, G.; Salutari, P.; Lanza, F.; et al. GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 2019, 134, 935–945. [Google Scholar] [CrossRef]
- Buccisano, F.; Palmieri, R.; Piciocchi, A.; Arena, V.; Candoni, A.; Melillo, L.; Calafiore, V.; Cairoli, R.; De Fabritiis, P.; Storti, G.; et al. ELN2017 risk stratification improves outcome prediction when applied to the prospective GIMEMA AML1310 protocol. Blood Adv. 2021, 6, 2510–2516. [Google Scholar] [CrossRef]
- Huls, G.; Chitu, D.A.; Havelange, V.; Jongen-Lavrencic, M.; van de Loosdrecht, A.A.; Biemond, B.J.; Sinnige, H.; Hodossy, B.; Graux, C.; Kooy, R.v.M.; et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood 2019, 133, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Pautas, C.; Merabet, F.; Thomas, X.; Raffoux, E.; Gardin, C.; Corm, S.; Bourhis, J.H.; Reman, O.; Turlure, P.; Contentin, N.; et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: Results of the ALFA-9801 study. J. Clin. Oncol. 2010, 28, 808–814. [Google Scholar] [CrossRef]
- Castaigne, S.; Pautas, C.; Terré, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomised, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Burnett, A.K.; Russell, N.H.; Hills, R.K.; Hunter, A.E.; Kjeldsen, L.; Yin, J.; Gibson, B.E.; Wheatley, K.; Milligan, D. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J. Clin. Oncol. 2013, 31, 3360–3368. [Google Scholar] [CrossRef]
- Willemze, R.; Suciu, S.; Meloni, G.; Labar, B.; Marie, J.P.; Halkes, C.J.; Muus, P.; Mistrik, M.; Amadori, S.; Specchia, G.; et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: Results of the EORTC-GIMEMA AML-12 trial. J. Clin. Oncol. 2014, 32, 219–228. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C.; et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients with Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Ohanian, M.; Garcia-Manero, G.; Levis, M.; Jabbour, E.; Daver, N.; Borthakur, G.; Kadia, T.; Pierce, S.; Burger, J.; Richie, M.A.; et al. Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Heidel, F.H.; Hellmann, A.; Fiedler, W.; Smith, B.D.; Robak, T.; Montesinos, P.; Pollyea, D.A.; DesJardins, P.; Ottmann, O.; et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 2019, 33, 379–389. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Tallman, M.S.; de Botton, S.; Kantarjian, H.M.; Collins, R.; Stein, A.S.; Frattini, M.G.; Xu, Q.; Tosolini, A.; See, W.L.; et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia 2019, 33, 2575–2584. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Roboz, G.J.; DiNardo, C.D.; Stein, E.M.; de Botton, S.; Mims, A.S.; Prince, G.T.; Altman, J.K.; Arellano, M.L.; Donnellan, W.; Erba, H.P.; et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020, 135, 463–471. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.-P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Gurnari, C.; Voso, M.T.; Maciejewski, J.P.; Visconte, V. From Bench to Bedside and Beyond: Therapeutic Scenario in Acute Myeloid Leukemia. Cancers 2020, 12, 357. [Google Scholar] [CrossRef]
- Döhner, K.; Thiede, C.; Jahn, N.; Panina, E.; Gambietz, A.; Larson, R.A.; Prior, T.W.; Marcucci, G.; Jones, D.; Krauter, J.; et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood 2020, 135, 371–380. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Stein, E.M.; DiNardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.H.; de Botton, S.; Zeidan, A.M.; Fathi, A.T.; Kantarjian, H.M.; Bennett, J.M.; et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): A single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021, 22, 1597–1608. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Montesinos, P.; Schuh, A.C.; Papayannidis, C.; Vyas, P.; Wei, A.H.; Zeidan, A.M.; Bluemmert, I.; Yu, X.; Hasan, M.; et al. Outcomes for Patients with Late-Stage Mutant-IDH2 (m IDH2) Relapsed/Refractory Acute Myeloid Leukemia (R/R AML) Treated with Enasidenib Vs Other Lower-Intensity Therapies in the Randomized, Phase 3 IDHentify Trial. Blood 2021, 138, 1243. [Google Scholar] [CrossRef]
- Giri, B.; Gupta, V.K.; Yaffe, B.; Modi, S.; Roy, P.; Sethi, V.; Lavania, S.P.; Vickers, S.M.; Dudeja, V.; Banerjee, S.; et al. Preclinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J. Transl. Med. 2019, 17, 163. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. Advances in targeted therapy for acute myeloid leukaemia. Br. J. Haematol. 2018, 180, 484–500. [Google Scholar] [CrossRef]
- Perl, A.E. The role of targeted therapy in the management of patients with AML. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 54–65. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: Integrating Morphological, Clinical, and Genomic Data. Blood 2022. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Huang, J.; Tan, J.; Chen, Y.; Huang, S.; Xu, L.; Zhang, Y.; Lu, Y.; Yu, Z.; Chen, S.; Li, Y. A skewed distribution and increased PD-1+Vβ+CD4+/CD8+ T cells in patients with acute myeloid leukemia. J. Leukoc. Biol. 2019, 106, 725–732. [Google Scholar] [CrossRef]
- Chen, C.; Liang, C.; Wang, S.; Chio, C.L.; Zhang, Y.; Zeng, C.; Chen, S.; Wang, C.; Li, Y. Expression patterns of immune checkpoints in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 28. [Google Scholar] [CrossRef]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; Thompson, P.A.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, cytarabine, and nivolumab in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: A single-arm, phase 2 study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A randomized phase 2 trial of azacitidine with or without durvalumab as first-line therapy for older patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.B.; McSweeney, P.; et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar] [CrossRef]
- Davids, M.S.; Kim, H.T.; Costello, C.; Herrera, A.F.; Locke, F.L.; Maegawa, R.O.; Savell, A.; Mazzeo, M.; Anderson, A.; Boardman, A.P.; et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood 2020, 135, 2182–2191. [Google Scholar] [CrossRef]
- Isidori, A.; Cerchione, C.; Daver, N.; DiNardo, C.; Garcia-Manero, G.; Konopleva, M.; Jabbour, E.; Ravandi, F.; Kadia, T.; Burguera, A.F.; et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front. Oncol. 2021, 11, 656218. [Google Scholar] [CrossRef]
- Abedin, S.; Guru Murthy, G.S.; Runaas, L.; Michaelis, L.C.; Atallah, E.L.; Hamadani, M.; Harrington, A.M.; Carlson, K. Lintuzumab Ac-225 in Combination with CLAG-M Chemotherapy in Relapsed/Refractory AML: Interim Results of a Phase I Study. Blood 2019, 134, 2605. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef]
- Gill, S.I. How close are we to CAR T-cell therapy for AML? Best Pract. Res. Clin. Haematol. 2019, 32, 101104. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Ball, E.D.; Varki, A. Myeloid precursors and acute myeloid leukemia cells express multiple CD33-related Siglecs. Exp. Hematol. 2006, 34, 728–735. [Google Scholar] [CrossRef]
- Riether, C.; Pabst, T.; Höpner, S.; Bacher, U.; Hinterbrandner, M.; Banz, Y.; Müller, R.; Manz, M.G.; Gharib, W.H.; Francisco, D.; et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat. Med. 2020, 26, 1459–1467. [Google Scholar] [CrossRef]
- Sauer, T.; Parikh, K.; Sharma, S.; Omer, B.; Sedloev, D.; Chen, Q.; Angenendt, L.; Schliemann, C.; Schmitt, M.; Müller-Tidow, C.; et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood 2021, 138, 318–330. [Google Scholar] [CrossRef]
| Intermediate-risk category | Mutated NPM1 and FLT3-ITDhigh † |
| Wild-type NPM1 without FLT3-ITD or with FLT3-ITDlow † (without adverse-risk genetic lesions) | |
| t(9;11)(p21.3;q23.3); MLLT3-KMT2A ‡ | |
| Cytogenetic abnormalities not classified as favorable or adverse |
| Intermediate-risk category | Mutated NPM1 †‡ with FLT3-ITD |
| Wild-type NPM1 with FLT3-ITD | |
| t(9;11)(p21.3;q23.3)/MLLT3::KMT2A *† | |
| Cytogenetic and/or molecular abnormalities not classified as favorable or adverse |
| Drug/Regimen | Trial/Year | AML-Specific FDA Approval | Design/Setting | Study Population | Experimental Arm | Comparison Regimen | Age Group and Characteristics | Risk Group | Pertinent Finding | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| Newly Diagnosed AML-Induction-Eligible | ||||||||||
| 7 + 3 Regimen | Fernandez [23]/2009 | Daunorubicin: remission induction in AML (myelogenous, monocytic, erythroid) in adults. | Multi-institutional, randomized, open-label trial | De novo or secondary AML. | Induction: daunorubicin 60 mg/m2 IV days 1–3 with Ara-C 100 mg/m2 continuous IV infusion days 1–7. | Induction: daunorubicin 45 mg/m2 IV days 1–3 with Ara-C 100 mg/m2 continuous IV infusion days 1–7. | 17–60 years | No risk groups excluded | HR for death in the high-dose daunorubicin group 0.74 (p < 0.05). Improved OS (HR 0.8, p = 0.02) in intermediate-risk with high-dose daunorubicin. | Risk classification was based on the 2000 SWOG/ECOG classification. |
| 7 + 3 Regimen | Pautas [45]/2010 | Idarubicin: indicated for the treatment of AML in adults. | Multi-institutional, randomized, open-label trial | de novo AML | Induction daunorubicin 80 mg/m2 IV days 1–3 with Ara-C 200 mg/m2 IV continuous infusion days 1–7. | Induction Idarubicin 12 mg/m2 IV days 1–3 or 1–4 with Ara-C 200 mg/m2 continuous IV infusion days 1–7. | 50–70 years | No risk groups excluded | CR rate 83% with idarubicin Days 1–3, 78% with idarubicin Days 1–4, and 70% with daunorubicin. No difference in OS, EFS or relapse incidence. | |
| 7 + 3 +GO Regimen | Castaigne [46]/2012 | Gemtuzumab ozogamicin: newly diagnosed AML, CD33+. | Multi-institutional, randomized, open-label trial | De novo AML, CD33+. | Induction daunorubicin and Ara-C with gemtuzumab ozogamicin 3 mg/m2 days 1, 4, 7. Similar regimen in consolidation. | Induction daunorubicin and Ara-C. | 50–70 years | No risk groups excluded | Two-year HR of EFS was 0.56 (p < 0.01), and HR for OS was 0.58 (p < 0.05) for 7 + 3 + GO. Combined favorable and intermediate cytogenetic groups showed improved outcomes with gemtuzumab (HR 0.5, p = 0.08). | In follow-up study, 7 + 3 + GO improved EFS (HR: 0.66, p < 0.05) but not OS (0.81, p = 0.16) [27]. Risk classification was based on ISCN. |
| FLAG-Ida Regimen | Burnett [47]/2013 | Fludarabine: NA | Multi-institutional, randomized, open-label trial | De novo or secondary AML. | Fludarabine 30 mg/m2 IV days 2–6, Ara-C 2 g/m2 days 2–6, G-CSF SC daily days 1–7, idarubicin 8 mg/m2 IV days 4–6. | Induction daunorubicin plus Ara-C with or without etoposide/gemtuzumab ozogamicin. Variables doses and schedules were used. | No age restriction | No risk groups excluded | CR rate 81% in ADE vs. 84% in FLAG-Ida (p = 0.2). No difference in 30- or 60-day mortality. Intermediate-risk cytogenetics had a lower relapse rate (OR 0.79, CI: 0.63–0.98) with FLAG-IDA. | Risk classification was based on MRC AML 10 Trial (15). |
| ADE Regimen | Willemze [48]/2013 | Cytarabine Injection in combination with other approved drugs is indicated for remission induction in AML in adults. | Multi-institutional, randomized, open-label trial | De novo or secondary AML. | Daunorubicin 50 mg/m2 IV days 1, 3, 5 plus etoposide 50 mg/m2 days 1–5 plus Ara-C 3000 mg/m2 every 12 h IV infusion days 1, 3, 5, 7. | Daunorubicin 50 mg/m2 IV on days 1, 3, 5 plus etoposide 50 mg/m2 days 1–5 plus 10 days of Ara-C 100 mg/m2 as continuous IV infusion. | 15–60 years | No risk groups excluded | 6-year OS in high dose Ara-C, and the standard dose was 42.5% and 38.7% (p = 0.06). Subgroup analysis showed improved OS with high dose Ara-C in intermediate-risk (HR: 0.88, CI: 0.64–1.21). | In patients < 46 years, high-dose Ara-C was associated with improved 6-year OS (51.9% vs. 43.3%, p < 0.05). Intermediate-risk was defined as a normal karyotype or absence of low- and high-risk cytogenetics and of FLT3-ITD. |
| Azacitidine | Dombret [49]/2015 | NA | Multi-institutional, randomized, open-label trial | De novo or secondary AML from MDS with >30% BM blasts who are not considered eligible for hematopoietic stem cell transplantation. | Azacitidine 75 mg/m2 SC daily for 7 consecutive days per 28-day treatment cycle | Investigators chose protocol-designated conventional care regimens (best supportive care, low-dose ara-c, or standard induction chemotherapy). | ≥65 years | Intermediate- or poor-risk cytogenetics | Median OS 10.4 mos in azacitidine arm was vs. 6.5 mos in comparison arm (p = 0.1). | Outcomes with intermediate-risk cytogenetics were not statistically significant (HR: 0.9, p = 0.4). Risk classification was based on 2009 NCCN guidelines. |
| 7 + 3 + Midostaurin Regimen | Stone [50]/2017 | Midostaurin: newly diagnosed AML with FLT3 mutation in combination with Ara-C and daunorubicin induction and Ara-C consolidation. | Multi-institutional, randomized, double-blind placebo-controlled trial | FLT3-ITD and TKD mutated. Not therapy-related. | Induction daunorubicin 60 mg/m2 IV days 1,2,3 with Ara-C 200 mg/m2 IV continuous infusion days 1–7 with midostaurin 50 mg orally twice daily, days 8–21. | Same but with placebo instead of midostaurin. | 18–59 years | No risk groups excluded | HR for death in midostaurin group was 0.78 (p < 0.05). Subgroup analysis not statistically significant. | The trial was stratified to high (>0.7) vs. low (0.05–0.7) ITD or TKD allelic ratio. |
| Newly Diagnosed AML-Induction-Ineligible | ||||||||||
| GO | Amadori [51]/2016 | Gemtuzumab ozogamicin: newly diagnosed CD33-positive AML. | Multi-institutional, randomized, open-label trial | CD33+. | Gemtuzumab ozogamicin 6 mg/m2, Day 1, 3 mg/m2 Day? | Best supportive care. | >75 years or ≤75 years with WHO PS > 2 | No risk groups excluded | HR for OS was 0.69 (p < 0.05). Subgroup analysis of combined favorable and intermediate cytogenetics showed improved outcomes with gemtuzumab (HR 0.52, p < 0.05). | Improvement in OS only seen with >80% CD33+ blasts. |
| Decitabine | Welch [52]/2016 | NA | Single-institution, prospective, single-arm | Newly diagnosed or relapsed AML and MDS. | Decitabine 20 mg/m2 days 1–10 of 28-day cycles. | - | ≥60 years | No risk groups excluded | ORR 46%. Median OS of favorable/intermediate-risk 10 mos. | Intermediate-risk cytogenetics in 5% of TP53 mutated, 69% of TP53 wild-type and 65% of TP53 untested. |
| HMA + Sorafenib | Ohanian [53]/2018 | Not approved. | Phase II, multi-institutional, open-label trial | Untreated patients with FLT3 mutated AML unfit for standard chemotherapy. | Azacitidine 75 mg/m2 daily × 7 days and sorafenib 400 mg twice daily. | NA | ≥60 years | No risk groups excluded | ORR 78%. Median OS 8.3 mos (range: 1–63). | 63% of patients had a normal karyotype, 7% had a complex karyotype, and 15% had other karyotypic changes. |
| Low-dose Ara-C + Glasdegib | Cortes [54]/2019 | Glasdegib: indicated in combination with low-dose Ara-C to treat newly diagnosed AML in adult patients ≥ 75 years old or with comorbidities that preclude the use of intensive induction chemotherapy. | Phase II, multi-institutional, randomized, open-label trial | Previously untreated AML or high-risk MDS unfit for intensive chemotherapy. | Glasdegib 100 mg orally QD continuously in 28-day cycles plus Ara-C 20 mg SC BID for 10 of 28 days. | Ara-C 20 mg SC BID for 10 per 28 days. | ≥55 years | No risk groups excluded | Median OS 8.8 months in glasdegib group vs. 4.9 months in comparison group (p < 0.05). | Benefits mainly seen in good/intermediate groups combined (12.2 vs. 4.8 months, p < 0.05) but not in high-risk group (4.7 vs. 4.9, p = 0.06). |
| Enasidenib | Pollyea [55]/2019 | Not FDA-approved. | Phase I, multi-institutional, open-label trial | Previously untreated IDH2-mutated AML unfit for standard AML treatments. | Enasedinib 100 mg orally once daily. | NA | ≥18 years | No risk groups excluded | ORR 30.8%. Median OS 11.3 mos (CI: 5.7–15.1). | 49% had intermediate-risk cytogenetics. |
| Azacitidne + Venetoclax | DiNardo [56]/2020 | Venetoclax: it is approved in combination with azacitidine or decitabine, or low-dose cytarabine for the treatment of newly-diagnosed AML in adults 75 years or older or with comorbidities that preclude intensive induction chemotherapy. Accelerated approval. | Multi-institutional randomized, double-blind placebo-controlled trial | Ineligible for standard induction therapy due to coexisting conditions or age 75 years. Excluded patients with previous MPN or MDS treated with a hypomethylating agent. | Azacitidine 75 mg/m2 SC or IV days 1–7 every 28-days plus venetoclax with a target dose of 400 mg daily. | Azacitidine 75 mg/m2 SC or IV days 1–7 every 28-day cycle plus placebo. | ≥18 years | Excluded patients with favorable-risk cytogenetics | Median OS in venetoclax group was 14.7 vs. 9.6 mos in the comparison (p < 0.05). | Statistically significant benefit in patients with intermediate-risk cytogenetics (HR 0.57, CI: 0.41–0.79) but not in high-risk group (HR: 0.78, CIL 0.54–1.12). Cytogenetics risk classification was based on the 2016 NCCN classification. |
| Ivosidenib | Roboz [57]/2020 | Adult patients with newly-diagnosed AML ≥ 75 years old or with comorbidities that preclude intensive induction chemotherapy. | Phase I, multi-institutional, open-label trial | IDH1-mutated AML. | Ivosidenib 500 mg daily. | NA | ≥18 years | No risk groups excluded | ORR 42.4%. Median OS 12.6 mos (CI: 4.5–25.7). | 71% had intermediate-risk cytogenetics. |
| Ivosidenib + Azacitidine | Montesinos [58] | Adult patients with newly diagnosed IDH1-mutated uneligible for intensive treatment. | Phase 3, multi-institutional, double-blind, randomized trial | IDH1-mutated AML. | Ivosidenib (500 mg once daily) plus Azacitidine 75 mg/m2 daily × 28-day cycle. | Azacitidine 75 mg/m2 daily × 28-day cycle. | ≥18 years | No risk groups excluded | Median OS was 24.0 months with experimental combination and 7.9 months with placebo and azacitidine (p = 0.001). | Similar toxicity profiles if expecting differentiation syndrome, higher in the experimental arm. |
| Clinical Trial Identifier | Name of Study | Design/Phase | Age Eligibility (Years) | Disease Characteristics | Study Start Date |
|---|---|---|---|---|---|
| NCT02152956 | Flotetuzumab in Primary Induction Failure (PIF) or Early Relapse (ER) AML | Multicenter, phase ½, open-label | >18 | R/R AML | June 2014 |
| NCT02397720 | Nivolumab and Azacitidine with or without Ipilimumab in Treating Patients with R/R or Newly Diagnosed AML | Phase 2, open-label study | >18 | R/R AML | April 2015 |
| NCT03190278 | Study Evaluating Safety and Efficacy of UCART123 in Patients with R/R AML (AMELI-01) | Phase 1, open-label | 18–65 | R/R AML with >5% bone marrow blasts, CD123+ | June 2017 |
| NCT03067571 | Daratumumab in Treating Patients with R/R AML or High-Risk MDS | Phase 2, open-label study | >18 | R/R AML | October 2017 |
| NCT03390296 | OX40, Venetoclax, Avelumab, Glasdegib, Gemtuzumab Ozogamicin, and Azacitidine in Treating Patients with R/R AML | Phase 1b/2, open-label multi-arm study | >18 | R/R AML | January 2018 |
| NCT03504410 | Study Evaluating Efficacy and Safety of CPI-613 in Combination with HD Cytarabine and Mitoxantrone Compared to HD Cytarabine and Mitoxantrone and Control Sub-groups: MEC and FLAG in Older Patients With R/R AML | Multicenter, phase 3, open-label, randomized study | >50 | R/R AML | April 2018 |
| NCT03672539 | Liposome-encapsulated Daunorubicin-Cytarabine and Gemtuzumab Ozogamicin in Treating Patients with R/R AML or High-Risk MDS | Phase 2, open-label study | >18 | CD33+ (≥3%), R/R AML | December 2018 |
| NCT03839446 | Phase II Study of the Combination of Mitoxantrone, Etoposide and Gemtuzumab Ozogamicin (MEGO) for Patients with AML refractory to Initial Standard Induction Therapy | Phase 2, open-label, single-arm study | 18–75 | R/R AML with CD33 expression in ≥30% of leukemic blasts on the bone marrow | February 2019 |
| NCT03760523 | Dose Escalation Study of Minnelide in R/R AML | Phase 1, dose-escalation study | >18 | R/R AML ineligible for intensive chemotherapy | April 2019 |
| NCT04219163 | Chimeric Antigen Receptor T-cells for The Treatment of AML Expressing CLL-1 Antigen | Phase 1, open-label | ≤75 | R/R AML, at least 30% CLL-1+ blasts | July 2020 |
| NCT04207190 | Talazoparib and Gemtuzumab Ozogamicin for the Treatment of CD33 Positive R/R AML | Phase 1, open-label study | >18 | CD33+ R/R AML with evidence of ≥5% myeloblasts in the bone marrow, peripheral blood, or in an extramedullary site by pathology | July 2020 |
| NCT04278768 | Dose Escalation/Expansion Trial of CA-4948 as Monotherapy and in Combination with Azacitidine or Venetoclax in Patients with AML or MDS | Phase 1/2, open-label | >18 | AML (primary or secondary, including treatment-related) after failing at least 1 standard treatment | July 2020 |
| NCT04435691 | Magrolimab, Azacitidine, and Venetoclax for the Treatment of AML | Phase 1b/2, open-label study | >18 | R/R AML | July 2020 |
| NCT04659616 | Pemigatinib after Chemotherapy for the Treatment of Newly Diagnosed AML | Multicenter, phase 1, open-label study | >18 | Adverse- or intermediate-risk newly diagnosed AML | January 2021 |
| NCT04666649 | Pegcrisantaspase in Combination with Venetoclax for Treatment of R/R AML | Phase 1, open-label | >18 | R/R AML | March 2021 |
| NCT04669067 | TL-895 and KRT-232 Study in AML | Multicenter, phase 1b/2, open-label | >18 | FLT3-ITD or TKD mutation, TP53 wild-type, R/R AML, at least one prior therapy, including a FLT-3 inhibitor | March 2021 |
| NCT04752163 | DS-1594b with or without Azacitidine, Venetoclax, or Mini-HCVD for the Treatment of R/R AML or ALL | Phase 1b/2, open-label multi-arm study | >18 | R/R AML or R/R ALL subjects with an MLLr or NPM1m | March 2021 |
| NCT04582864 | Flotetuzumab for relapsed AML and MDS Following Allo-HCT | Phase 2, open-label | >18 | Relapsed AML | May 2021 |
| NCT04789408 | Study Evaluating the Safety of KITE-222 in Participants with R/R AML | Multicenter, phase 1, open-label | >18 | R/R AML | July 2021 |
| NCT05010122 | ASTX727, Venetoclax, and Gilteritinib for the Treatment of Newly Diagnosed, R/R FLT3-Mutated AML or High-Risk MDS | Phase 1/2, open-label | >18 | Newly diagnosed or R/R FLT3-mutated AML | July 2021 |
| NCT04956042 | Study of Fosciclopirox in Patients with R/R AML | Phase 1, open-label study | >18 | R/R AML | August 2021 |
| NCT03441048 | Lintuzumab-Ac225 in Combination with Cladribine + Cytarabine + Filgastrim + Mitoxantrone (CLAG-M) for R/R AML | Single center, non-randomized, open-label phase 1 | >18 | R/R AML with >25% of blasts must be CD33 positive | May 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awada, H.; Mustafa Ali, M.K.; Thapa, B.; Awada, H.; Seymour, L.; Liu, L.; Gurnari, C.; Kishtagari, A.; Wang, E.; Baer, M.R. A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges. Cancers 2022, 14, 4166. https://doi.org/10.3390/cancers14174166
Awada H, Mustafa Ali MK, Thapa B, Awada H, Seymour L, Liu L, Gurnari C, Kishtagari A, Wang E, Baer MR. A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges. Cancers. 2022; 14(17):4166. https://doi.org/10.3390/cancers14174166
Chicago/Turabian StyleAwada, Hassan, Moaath K. Mustafa Ali, Bicky Thapa, Hussein Awada, Leroy Seymour, Louisa Liu, Carmelo Gurnari, Ashwin Kishtagari, Eunice Wang, and Maria R. Baer. 2022. "A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges" Cancers 14, no. 17: 4166. https://doi.org/10.3390/cancers14174166
APA StyleAwada, H., Mustafa Ali, M. K., Thapa, B., Awada, H., Seymour, L., Liu, L., Gurnari, C., Kishtagari, A., Wang, E., & Baer, M. R. (2022). A Focus on Intermediate-Risk Acute Myeloid Leukemia: Sub-Classification Updates and Therapeutic Challenges. Cancers, 14(17), 4166. https://doi.org/10.3390/cancers14174166






