An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
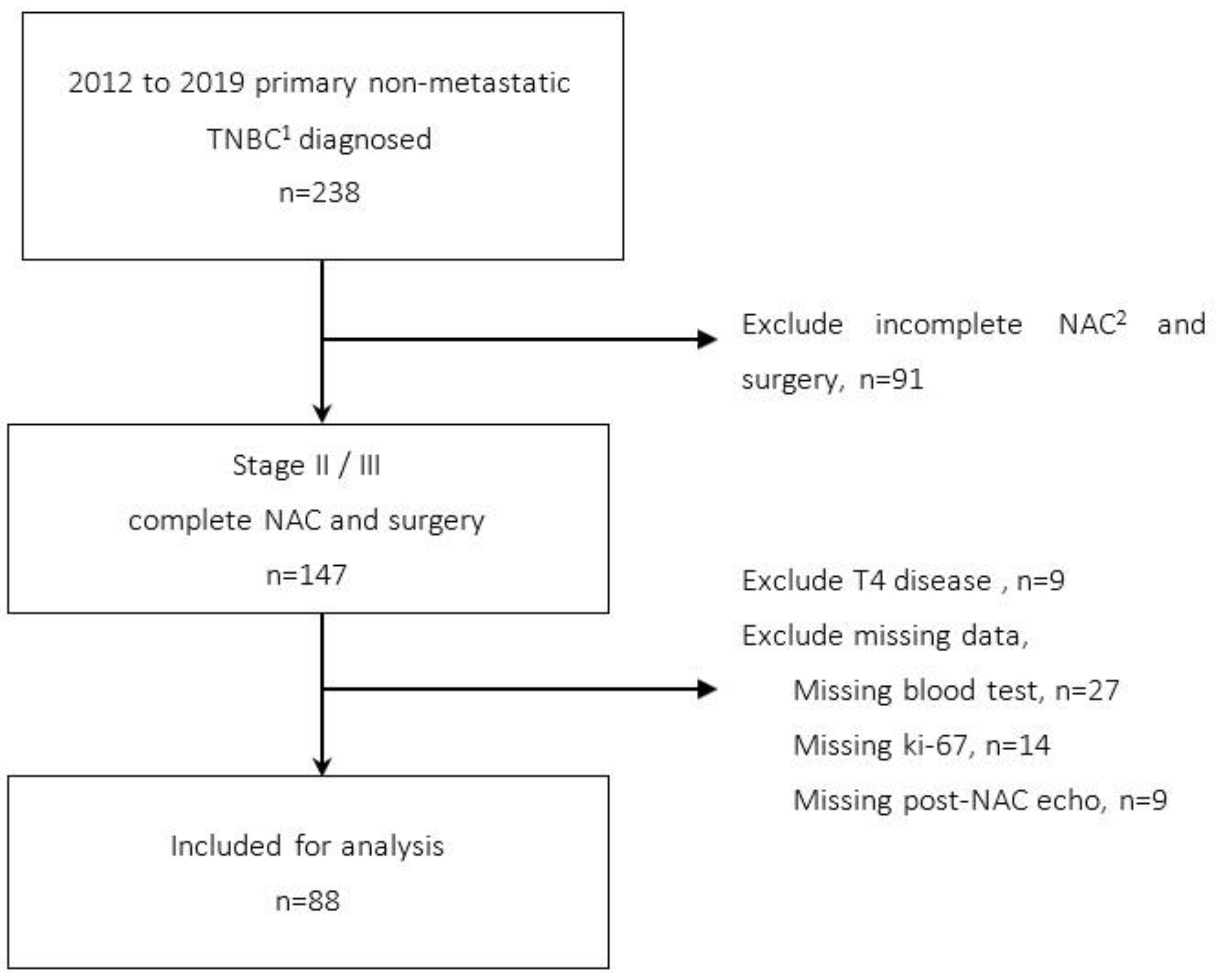
2.1. Patients
2.2. Parameters
2.3. Statistical Analyses
3. Results
3.1. Clinical Characteristics of Patients
3.2. Factor Predicting pCR for Patients Receiving NAC
3.3. Model for the Prediction of pCR in Patients Receiving NAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; Committee, E.G. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Ponde, N.F.; La Valle, G.; Del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Bear, H.D.; Anderson, S.; Smith, R.E.; Geyer, C.E., Jr.; Mamounas, E.P.; Fisher, B.; Brown, A.M.; Robidoux, A.; Margolese, R.; Kahlenberg, M.S.; et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2006, 24, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Van der Hage, J.A.; van de Velde, C.J.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef]
- Sharma, P.; Lopez-Tarruella, S.; Garcia-Saenz, J.A.; Ward, C.; Connor, C.S.; Gomez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; et al. Efficacy of Neoadjuvant Carboplatin plus Docetaxel in Triple-Negative Breast Cancer: Combined Analysis of Two Cohorts. Clin. Cancer Res. 2017, 23, 649–657. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017, 8, 112712–112719. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.V.; Goodwin, E.C.; Chen, J.; Obenauer, J.C.; Tannenbaum, S.H.; Brufsky, A.M. A Predictor of Pathological Complete Response to Neoadjuvant Chemotherapy Stratifies Triple Negative Breast Cancer Patients with High Risk of Recurrence. Sci. Rep. 2019, 9, 14863. [Google Scholar] [CrossRef]
- Ayers, M.; Symmans, W.F.; Stec, J.; Damokosh, A.I.; Clark, E.; Hess, K.; Lecocke, M.; Metivier, J.; Booser, D.; Ibrahim, N.; et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J. Clin. Oncol. 2004, 22, 2284–2293. [Google Scholar] [CrossRef]
- Hatzis, C.; Pusztai, L.; Valero, V.; Booser, D.J.; Esserman, L.; Lluch, A.; Vidaurre, T.; Holmes, F.; Souchon, E.; Wang, H.; et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 2011, 305, 1873–1881. [Google Scholar] [CrossRef]
- Harada, T.L.; Uematsu, T.; Nakashima, K.; Sugino, T.; Nishimura, S.; Takahashi, K.; Hayashi, T.; Tadokoro, Y.; Watanabe, J.; Nakamoto, S.; et al. Is the presence of edema and necrosis on T2WI pretreatment breast MRI the key to predict pCR of triple negative breast cancer? Eur. Radiol. 2020, 30, 3363–3370. [Google Scholar] [CrossRef]
- Pineda, B.; Diaz-Lagares, A.; Perez-Fidalgo, J.A.; Burgues, O.; Gonzalez-Barrallo, I.; Crujeiras, A.B.; Sandoval, J.; Esteller, M.; Lluch, A.; Eroles, P. A two-gene epigenetic signature for the prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer patients. Clin. Epigenetics 2019, 11, 33. [Google Scholar] [CrossRef]
- Brown, L.J.; Achinger-Kawecka, J.; Portman, N.; Clark, S.; Stirzaker, C.; Lim, E. Epigenetic Therapies and Biomarkers in Breast Cancer. Cancers 2022, 14, 474. [Google Scholar] [CrossRef]
- Lin, Y.; Fu, F.; Lin, S.; Qiu, W.; Zhou, W.; Lv, J.; Wang, C. A nomogram prediction for the survival of patients with triple negative breast cancer. Oncotarget 2018, 9, 32108–32118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, M.; Zhou, H.; Chen, K.; Jin, J.; Wu, Y.; Ying, L.; Ding, X.; Su, D.; Zou, D. A Nomogram to Predict the Pathologic Complete Response of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Based on Simple Laboratory Indicators. Ann. Surg. Oncol. 2019, 26, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Chiang, K.C.; Kuo, W.L.; Shen, S.C.; Lo, Y.F.; Chen, S.C. Low re-excision rate for positive margins in patients treated with ultrasound-guided breast-conserving surgery. Breast 2013, 22, 698–702. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Houssami, N.; Macaskill, P.; von Minckwitz, G.; Blohmer, J.U.; Irwig, L. Accuracy of ultrasound for predicting pathologic response during neoadjuvant therapy for breast cancer. Int. J. Cancer 2015, 136, 2730–2737. [Google Scholar] [CrossRef]
- Graeser, M.; Harbeck, N.; Gluz, O.; Wurstlein, R.; Zu Eulenburg, C.; Schumacher, C.; Grischke, E.M.; Forstbauer, H.; Dimpfl, M.; Braun, M.; et al. The use of breast ultrasound for prediction of pathologic complete response in different subtypes of early breast cancer within the WSG-ADAPT subtrials. Breast 2021, 59, 58–66. [Google Scholar] [CrossRef]
- Durmus, T.; Stöckel, J.; Slowinski, T.; Thomas, A.; Fischer, T. The hyperechoic zone around breast lesions—An indirect parameter of malignancy. Ultraschall Med. 2014, 35, 547–553. [Google Scholar] [CrossRef]
- Wojcinski, S.; Soliman, A.A.; Schmidt, J.; Makowski, L.; Degenhardt, F.; Hillemanns, P. Sonographic features of triple-negative and non-triple-negative breast cancer. J. Ultrasound Med. 2012, 31, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Lee, B.H.; Kim, H.A.; Noh, W.C.; Kim, M.S.; Lee, S.A. Triple-negative breast cancer: Correlation between imaging and pathological findings. Eur. Radiol. 2010, 20, 1111–1117. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Cho, U.; Park, H.S.; Im, S.Y.; Yoo, C.Y.; Jung, J.H.; Suh, Y.J.; Choi, H.J. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE 2018, 13, e0200936. [Google Scholar] [CrossRef]
- Noh, H.; Eomm, M.; Han, A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J. Breast Cancer 2013, 16, 55–59. [Google Scholar] [CrossRef]
- el-Hag, A.; Clark, R.A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J. Immunol. 1987, 139, 2406–2413. [Google Scholar]
- Rimando, J.; Campbell, J.; Kim, J.H.; Tang, S.C.; Kim, S. The Pretreatment Neutrophil/Lymphocyte Ratio Is Associated with All-Cause Mortality in Black and White Patients with Non-metastatic Breast Cancer. Front. Oncol. 2016, 6, 81. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, K.; Xiao, X.; Nie, Y.; Qu, S.; Gong, C.; Su, F.; Song, E. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: A retrospective study. BMC Cancer 2016, 16, 320. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Platelet-derived growth factor: Mechanism of action and possible in vivo function. Cell Regul. 1990, 1, 555–566. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Growth factors: Mechanism of action and relation to oncogenes. Cell 1984, 37, 9–20. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, T.H.; Yoon, H.K.; Lee, A. The Role of Neutrophil-lymphocyte Ratio and Platelet-lymphocyte Ratio in Predicting Neoadjuvant Chemotherapy Response in Breast Cancer. J. Breast Cancer 2019, 22, 425–438. [Google Scholar] [CrossRef]
- Seretis, C.; Seretis, F.; Lagoudianakis, E.; Politou, M.; Gemenetzis, G.; Salemis, N.S. Enhancing the accuracy of platelet to lymphocyte ratio after adjustment for large platelet count: A pilot study in breast cancer patients. Int. J. Surg. Oncol. 2012, 2012, 653608. [Google Scholar] [CrossRef]
- Graziano, V.; Grassadonia, A.; Iezzi, L.; Vici, P.; Pizzuti, L.; Barba, M.; Quinzii, A.; Camplese, A.; Di Marino, P.; Peri, M.; et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019, 44, 33–38. [Google Scholar] [CrossRef]
- Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-stage Breast Cancer. Available online: https://www.federalregister.gov/documents/2014/10/07/201423845/pathological-complete-response-in-neoadjuvant-treatment-of-high-risk-early-stage-breast-cancer-use (accessed on 14 August 2022).
- Mieog, J.S.; van der Hage, J.A.; van de Velde, C.J. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst. Rev. 2007, 2, CD005002. [Google Scholar] [CrossRef]
- Wolmark, N.; Wang, J.; Mamounas, E.; Bryant, J.; Fisher, B. Preoperative chemotherapy in patients with operable breast cancer: Nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001, 30, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Cristofanilli, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Lluch, A.; Barrios, C.H.; Torrecillas, L.; Ruiz-Borrego, M.; Bines, J.; Segalla, J.; Guerrero-Zotano, A.; Garcia-Saenz, J.A.; Torres, R.; de la Haba, J.; et al. Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01). J. Clin. Oncol. 2020, 38, 203–213. [Google Scholar] [CrossRef]



| Characteristic | Non-pCR (n = 61) | pCR (n = 27) |
|---|---|---|
| Age | ||
| Mean (SD) 1 Median [min.,max.] | 50.9 (10.4) | 50.6 (10.0) |
| 51 [21.0,79.0] | 51.0 [33.0,68.0] | |
| cT stage | ||
| T1 T2 T3 | 3 (4.9%) 43 (70.5%) 15 (24.6%) | 1 (3.7%) 24 (88.9%) 2 (7.4%) |
| Ki-67 (%) | ||
| ≤34 >34 | 26 (42.6%) | 5 (18.5%) |
| 35 (57.4%) | 22 (81.5%) | |
| Grade | ||
| 1&2 3 | 22 (36.1%) | 3 (11.1%) |
| 39 (63.9%) | 24 (88.9%) | |
| NLR 2 | ||
| ≤1.909 >1.909 | 16 (26.2%) 45 (73.8%) | 13 (48.1%) 14 (51.9%) |
| PLR 3 | ||
| ≤148.14 | 40 (65.6%) | 10 (37.0%) |
| >148.14 | 21 (34.4%) | 17 (63.0%) |
| NLR2 percentage change | ||
| ≤−0.165 >−0.165 | 16 (26.2%) 45 (73.8%) | 12 (44.4%) 15 (55.6%) |
| PLR3 percentage change | ||
| ≤0.038 | 22 (36.1%) | 19 (70.4%) |
| >0.038 | 39 (63.9%) | 8 (29.6%) |
| Initial echo lesion boundary | ||
| Echogenic halo | 20 (32.8%) | 20 (74.1%) |
| Others | 41 (67.2%) | 7 (25.9%) |
| Initial echo posterior features | ||
| Enhancement | 31 (50.8%) | 16 (59.3%) |
| Others | 30 (49.2%) | 11 (40.7%) |
| Initial Echo H/W 4 ratio | ||
| ≤1.221 | 41 (67.2%) | 12 (44.4%) |
| >1.221 | 20 (32.8%) | 15 (55.6%) |
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value * | |
| Age | 0.997 | 0.95–1.04 | 0.884 | |||
| Ki-67 (%) | ||||||
| ≤34 | 1 | |||||
| >34 | 3.269 | 1.16–10.97 | 0.034 | |||
| cT stage | ||||||
| T1 | 1 | 0.2–34.87 | 0.663 | |||
| T2 | 1.674 | 0.03–10.22 | 0.506 | |||
| T3 | 0.4 | |||||
| Grade | ||||||
| 1&2 | 1 | 1 | ||||
| 3 | 4.513 | 1.37–20.51 | 0.024 | 4.013 | 0.81–29.64 | 0.119 |
| NLR 1 | ||||||
| ≤1.482 | 1 | 1 | ||||
| >1.482 | 0.40 | 0.16–1.00 | 0.051 | 8.188 | 0.02–0.46 | 0.01 |
| PLR 2 | ||||||
| ≤149.546 | 1 | 1 | ||||
| >149.546 | 1.46 | 0.74–2.89 | 0.278 | 0.102 | 1.94–45.73 | 0.01 |
| NLR1 percentage change | ||||||
| ≤−0.165 | 1 | |||||
| >−0.165 | 0.444 | 0.17–1.15 | 0.094 | |||
| PLR2 percentage change | ||||||
| ≤0.038 | 1 | 1 | ||||
| >0.038 | 0.238 | 0.09–0.61 | 0.004 | 0.189 | 0.04–0.7 | 0.02 |
| Initial echo lesion boundary | ||||||
| Echogenic halo | 1 | 1 | ||||
| Others | 0.171 | 0.06–0.45 | 0.001 | 0.131 | 0.03–0.45 | 0.002 |
| Initial echo posterior features | ||||||
| Enhancement | 1 | |||||
| Others | 0.71 | 0.28–1.77 | 0.465 | |||
| Initial Echo H/W 3 ratio | ||||||
| ≤1.221 | 1 | 1 | ||||
| >1.221 | 2.562 | 1.02–6.61 | 0.047 | 4.524 | 1.28–18.89 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.-S.; Chen, S.-C.; Ko, T.-M.; Lin, Y.-C.; Lin, S.-H.; Lo, Y.-F.; Tseng, S.-C.; Yu, C.-C. An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancers 2022, 14, 4170. https://doi.org/10.3390/cancers14174170
Chung W-S, Chen S-C, Ko T-M, Lin Y-C, Lin S-H, Lo Y-F, Tseng S-C, Yu C-C. An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancers. 2022; 14(17):4170. https://doi.org/10.3390/cancers14174170
Chicago/Turabian StyleChung, Wai-Shan, Shin-Cheh Chen, Tai-Ming Ko, Yung-Chang Lin, Sheng-Hsuan Lin, Yung-Feng Lo, Shu-Chi Tseng, and Chi-Chang Yu. 2022. "An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy" Cancers 14, no. 17: 4170. https://doi.org/10.3390/cancers14174170
APA StyleChung, W.-S., Chen, S.-C., Ko, T.-M., Lin, Y.-C., Lin, S.-H., Lo, Y.-F., Tseng, S.-C., & Yu, C.-C. (2022). An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancers, 14(17), 4170. https://doi.org/10.3390/cancers14174170







