Clinical Safety and Effectiveness of Robotic-Assisted Surgery in Patients with Rectal Cancer: Real-World Experience over 8 Years of Multiple Institutions with High-Volume Robotic-Assisted Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Management
Confidentiality and Quality Control
2.3. Study Monitoring and Ethical Consideration
2.3.1. Monitoring and Inspecting
2.3.2. Ethical Consideration
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Perioperative Outcomes
3.2. Intraoperative Safety and Clinical Outcomes
3.3. Pathological Outcomes and Oncological Outcomes
3.4. Postoperative Complications
4. Discussion
4.1. Baseline Characteristics
4.2. Operation Time
4.3. Conversion
4.4. Circumferential Resection Margin Positivity
4.5. Harvested Lymph Node
4.6. Complication Rates
4.7. Reoperation and Readmission
4.8. Recurrence and Death
4.9. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar]
- Ministry of Health and Welfare, the Executive Yuan, Republic of China. Health and Vital Statistics. Available online: https://dep.mohw.gov.tw/dos/mp-113.html (accessed on 25 August 2021).
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef]
- Bosset, J.F.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Briffaux, A.; Collette, L. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results—EORTC 22921. J. Clin. Oncol. 2005, 23, 5620–5627. [Google Scholar] [CrossRef]
- Gérard, J.P.; Conroy, T.; Bonnetain, F.; Bouché, O.; Chapet, O.; Closon-Dejardin, M.T.; Untereiner, M.; Leduc, B.; Francois, E.; Maurel, J.; et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J. Clin. Oncol. 2006, 24, 4620–4625. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, J.W.; Nam, B.H.; Kim, S.; Kang, S.B.; Lim, S.B.; Choi, H.S.; Kim, D.W.; Chang, H.J.; Kim, D.Y.; et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 767–774. [Google Scholar] [CrossRef]
- Chen, C.F.; Lin, Y.C.; Tsai, H.L.; Huang, C.W.; Yeh, Y.S.; Ma, C.J.; Lu, C.Y.; Hu, H.M.; Shih, H.Y.; Shih, Y.L.; et al. Short- and long-term outcomes of laparoscopic-assisted surgery, mini-laparotomy and conventional laparotomy in patients with Stage I-III colorectal cancer. J. Minim. Access Surg. 2018, 14, 321–334. [Google Scholar]
- Baek, J.H.; Pastor, C.; Pigazzi, A. Robotic and laparoscopic total mesorectal excision for rectal cancer: A case-matched study. Surg. Endosc. 2010, 25, 521–525. [Google Scholar] [CrossRef]
- Chen, Y.T.; Huang, C.W.; Ma, C.J.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Chai, C.Y.; Wang, J.Y. An observational study of patho-oncological outcomes of various surgical methods in total mesorectal excision for rectal cancer: A single center analysis. BMC Surg. 2020, 20, 23. [Google Scholar] [CrossRef]
- Hellan, M.; Ouellette, J.; Lagares-Garcia, J.A.; Rauh, S.M.; Kennedy, H.L.; Nicholson, J.D.; Nesbitt, D.; Johnson, C.S.; Pigazzi, A. Robotic Rectal Cancer Resection: A Retrospective Multicenter Analysis. Ann. Surg. Oncol. 2015, 22, 2151–2158. [Google Scholar] [CrossRef]
- Han, C.; Yan, P.; Jing, W.; Li, M.; Du, B.; Si, M.; Yang, J.; Yang, K.; Cai, H.; Guo, T. Clinical, pathological, and oncologic outcomes of robotic-assisted versus laparoscopic proctectomy for rectal cancer: A meta-analysis of randomized controlled studies. Asian J. Surg. 2020, 43, 880–890. [Google Scholar] [CrossRef]
- Chen, T.C.; Liang, J.T. Robotic versus laparoscopic surgery for rectal cancer after neoadjuvant chemoradiotherapy: A propensity-score matching analysis. J. Formos. Med. Assoc. 2022, 121, 1532–1540. [Google Scholar] [CrossRef]
- Tong, G.; Zhang, G.; Zheng, Z. Robotic and robotic-assisted vs Laparoscopic rectal cancer surgery: A meta-analysis of short-term and long-term results. Asian J. Surg. 2021, 44, 1549. [Google Scholar] [CrossRef]
- Huang, C.W.; Yeh, Y.S.; Su, W.C.; Tsai, H.L.; Choy, T.K.; Huang, M.Y.; Huang, C.M.; Wu, I.C.; Hu, H.M.; Hsu, W.H.; et al. Robotic surgery with high dissection and low ligation technique for consecutive patients with rectal cancer following preoperative concurrent chemoradiotherapy. Int. J. Colorectal Dis. 2016, 31, 1169–1177. [Google Scholar] [CrossRef]
- Huang, C.W.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Huang, M.Y.; Huang, C.M.; Chang, Y.T.; Wang, J.Y. Robotic-assisted total mesorectal excision with the single-docking technique for patients with rectal cancer. BMC Surg. 2017, 17, 126. [Google Scholar] [CrossRef]
- Huang, C.W.; Su, W.C.; Yin, T.C.; Chen, P.J.; Chang, T.K.; Chen, Y.C.; Li, C.C.; Hsieh, Y.C.; Tsai, H.L.; Wang, J.Y. Time interval between the completion of radiotherapy and robotic-assisted surgery among patients with stage I-III rectal cancer undergoing preoperative chemoradiotherapy. PLoS ONE 2020, 15, e0240742. [Google Scholar] [CrossRef]
- Huang, C.W.; Su, W.C.; Chang, T.K.; Ma, C.J.; Yin, T.C.; Tsai, H.L.; Chen, P.J.; Chen, Y.C.; Li, C.C.; Hsieh, Y.C.; et al. Impact of previous abdominal surgery on robotic-assisted rectal surgery in patients with locally advanced rectal adenocarcinoma: A propensity score matching study. World J. Surg. Oncol. 2020, 18, 308. [Google Scholar] [CrossRef]
- Chen, P.J.; Su, W.C.; Chang, T.K.; Chen, Y.C.; Li, C.C.; Yin, T.C.; Tsai, H.L.; Ma, C.J.; Huang, C.W.; Wang, J.Y. Oncological outcomes of robotic-assisted total mesorectal excision after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Asian J. Surg. 2021, 44, 957–963. [Google Scholar] [CrossRef]
- Su, W.C.; Huang, C.W.; Ma, C.J.; Chen, P.J.; Tsai, H.L.; Chang, T.K.; Chen, Y.C.; Li, C.C.; Yeh, Y.S.; Wang, J.Y. Feasibility of robot-assisted surgery in elderly patients with rectal cancer. J. Minim. Access Surg. 2021, 17, 165–174. [Google Scholar] [CrossRef]
- Yin, T.C.; Su, W.C.; Chen, P.J.; Chang, T.K.; Chen, Y.C.; Li, C.C.; Hsieh, Y.C.; Tsai, H.L.; Huang, C.W.; Wang, J.Y. Oncological Outcomes of Robotic-Assisted Surgery With High Dissection and Selective Ligation Technique for Sigmoid Colon and Rectal Cancer. Front. Oncol. 2020, 10, 570376. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Tortti, A., III. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; pp. 143–164. [Google Scholar]
- Huang, Y.M.; Huang, Y.J.; Wei, P.L. Outcomes of robotic versus laparoscopic surgery for mid and low rectal cancer after neoadjuvant chemoradiation therapy and the effect of learning curve. Medicine 2017, 96, e8171. [Google Scholar] [CrossRef]
- Kuo, L.J.; Lin, Y.K.; Chang, C.C.; Tai, C.J.; Chiou, J.F.; Chang, Y.J. Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: Comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int. J. Colorectal Dis. 2014, 29, 555–562. [Google Scholar] [CrossRef]
- Inoue, Y.; Ng, J.Y.; Chu, C.H.; Lai, Y.L.; Huang, I.P.; Yang, S.H.; Chen, C.C. Robotic or transanal total mesorectal excision (TaTME) approach for rectal cancer, how about both? Feasibility and outcomes from a single institution. J. Robot. Surg. 2022, 16, 149–157. [Google Scholar] [CrossRef]
- Li, Z.; Coleman, J.; D’Adamo, C.R.; Wolf, J.; Katlic, M.; Ahuja, N.; Blumberg, D.; Ahuja, V. Operative Mortality Prediction for Primary Rectal Cancer: Age Matters. J. Am. Coll. Surg. 2019, 228, 627–633. [Google Scholar] [CrossRef]
- Chang, W.; Wei, Y.; Ren, L.; Jian, M.; Chen, Y.; Chen, J.; Liu, T.; Huang, W.; Peng, S.; Xu, J. Short-term and long-term outcomes of robotic rectal surgery-from the real word data of 1145 consecutive cases in China. Surg. Endosc. 2020, 34, 4079–4088. [Google Scholar] [CrossRef]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA 2017, 318, 1569–1580. [Google Scholar] [CrossRef]
- Katsuno, H.; Hanai, T.; Masumori, K.; Koide, Y.; Matsuoka, H.; Tajima, Y.; Endo, T.; Mizuno, M.; Chong, Y.; Maeda, K.; et al. Short- and long-term outcomes of robotic surgery for rectal cancer: A single-center retrospective cohort study. Surg. Today 2020, 50, 240–247. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kinugasa, Y.; Shiomi, A.; Kagawa, H.; Yamakawa, Y.; Furuatni, A.; Manabe, S.; Yamaoka, Y.; Hino, H. Short- and long-term outcomes of robotic-assisted laparoscopic surgery for rectal cancer: Results of a single high-volume center in Japan. Int. J. Colorectal Dis. 2018, 33, 1755–1762. [Google Scholar] [CrossRef]
- Hyde, L.Z.; Baser, O.; Mehendale, S.; Guo, D.; Shah, M.; Kiran, R.P. Impact of surgical approach on short-term oncological outcomes and recovery following low anterior resection for rectal cancer. Colorectal Dis. 2019, 21, 932–942. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D.H.; Kim, B.R.; Kim, Y.W. The American Society of Anesthesiologists score influences on postoperative complications and total hospital charges after laparoscopic colorectal cancer surgery. Medicine 2018, 97, e0653. [Google Scholar] [CrossRef]
- Kim, C.N.; Bae, S.U.; Lee, S.G.; Yang, S.H.; Hyun, I.G.; Jang, J.H.; Cho, B.S.; Park, J.S. Clinical and oncologic outcomes of totally robotic total mesorectal excision for rectal cancer: Initial results in a center for minimally invasive surgery. Int. J. Colorectal Dis. 2016, 31, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.C.; Park, J.W.; Chang, H.J.; Kim, D.Y.; Nam, B.H.; Sohn, D.K.; Oh, J.H. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann. Surg. 2018, 267, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, C.; Li, C.; Chen, J.; Luo, H.; Zeng, D.; Yu, P. Robotic Total Mesorectal Excision for Rectal Cancer: A Series of 392 Cases and Mid-Term Outcomes from A Single Center in China. J. Gastrointest. Surg. 2017, 21, 569–576. [Google Scholar] [CrossRef]
- Lim, D.R.; Bae, S.U.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg. Endosc. 2017, 31, 1728–1737. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Ashwin, K.R.; Rajashekhar, J.; Zaveri, S. Prospective Randomized Study Comparing Robotic-Assisted Surgery with Traditional Laparotomy for Rectal Cancer-Indian Study. Indian J. Surg. 2015, 77 (Suppl. 3), 788–794. [Google Scholar] [CrossRef]
- Sujatha-Bhaskar, S.; Jafari, M.D.; Gahagan, J.V.; Inaba, C.S.; Koh, C.Y.; Mills, S.D.; Carmichael, J.C.; Stamos, M.; Pigazzi, A. Defining the Role of Minimally Invasive Proctectomy for Locally Advanced Rectal Adenocarcinoma. Ann. Surg. 2017, 266, 574–581. [Google Scholar] [CrossRef]
- Masoomi, H.; Moghadamyeghaneh, Z.; Mills, S.; Carmichael, J.C.; Pigazzi, A.; Stamos, M.J. Risk factors for conversion of laparoscopic colorectal surgery to open surgery: Does conversion worsen outcome? World J. Surg. 2015, 39, 1240–1247. [Google Scholar] [CrossRef]
- Finochi, M.; Menahem, B.; Eid, Y.; Lubrano, J.; Alves, A. Does conversion during laparoscopic rectal oncological surgery increases postoperative complications and anastomotic leakage rates? A meta-analysis. J. Visc. Surg. 2020, 157, 277–287. [Google Scholar] [CrossRef]
- Allaix, M.E.; Furnée, E.J.; Mistrangelo, M.; Arezzo, A.; Morino, M. Conversion of laparoscopic colorectal resection for cancer: What is the impact on short-term outcomes and survival? World J. Gastroenterol. 2016, 22, 8304–8313. [Google Scholar] [CrossRef]
- Parascandola, S.A.; Hota, S.; Sparks, A.D.; Boulos, S.; Cavallo, K.; Kim, G.; Obias, V. Trends in utilization, conversion rates, and outcomes for minimally invasive approaches to non-metastatic rectal cancer: A national cancer database analysis. Surg. Endosc. 2021, 5, 3154–3165. [Google Scholar] [CrossRef]
- Kidner, T.B.; Ozao-Choy, J.J.; Yoon, J.; Bilchik, A.J. Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am. J. Surg. 2012, 204, 843–847. [Google Scholar] [CrossRef]
- Mechera, R.; Schuster, T.; Rosenberg, R.; Speich, B. Lymph node yield after rectal resection in patients treated with neoadjuvant radiation for rectal cancer: A systematic review and meta-analysis. Eur. J. Cancer 2017, 72, 84–94. [Google Scholar] [CrossRef]
- Fleshman, J.; Branda, M.; Sargent, D.J.; Boller, A.M.; George, V.; Abbas, M.; Peters, W.R., Jr.; Maun, D.; Chang, G.; Herline, A.; et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015, 314, 1346–1355. [Google Scholar] [CrossRef]
- Saadat, L.V.; Fields, A.C.; Lyu, H.; Urman, R.D.; Whang, E.E.; Goldberg, J.; Bleday, R.; Melnitchouk, N. National Surgical Quality Improvement Program analysis of unplanned reoperation in patients undergoing low anterior resection or abdominoperineal resection for rectal cancer. Surgery 2019, 165, 602–607. [Google Scholar] [CrossRef]
- Ryuk, J.P.; Choi, G.S.; Park, J.S.; Kim, H.J.; Park, S.Y.; Yoon, G.S.; Jun, S.H.; Kwon, Y.C. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann. Surg. Treat. Res. 2014, 86, 143–151. [Google Scholar] [CrossRef]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef]
- Tan, W.J.; Tan, H.J.; Dorajoo, S.R.; Foo, F.J.; Tang, C.L.; Chew, M.H. Rectal Cancer Surveillance-Recurrence Patterns and Survival Outcomes from a Cohort Followed up Beyond 10 Years. J. Gastrointest. Cancer 2018, 49, 422–428. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Huang, Y.; Lu, X.; Huang, Z.; Chi, P. Defining and predicting early recurrence in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur. J. Surg. Oncol. 2020, 46, 2057–2063. [Google Scholar] [CrossRef]
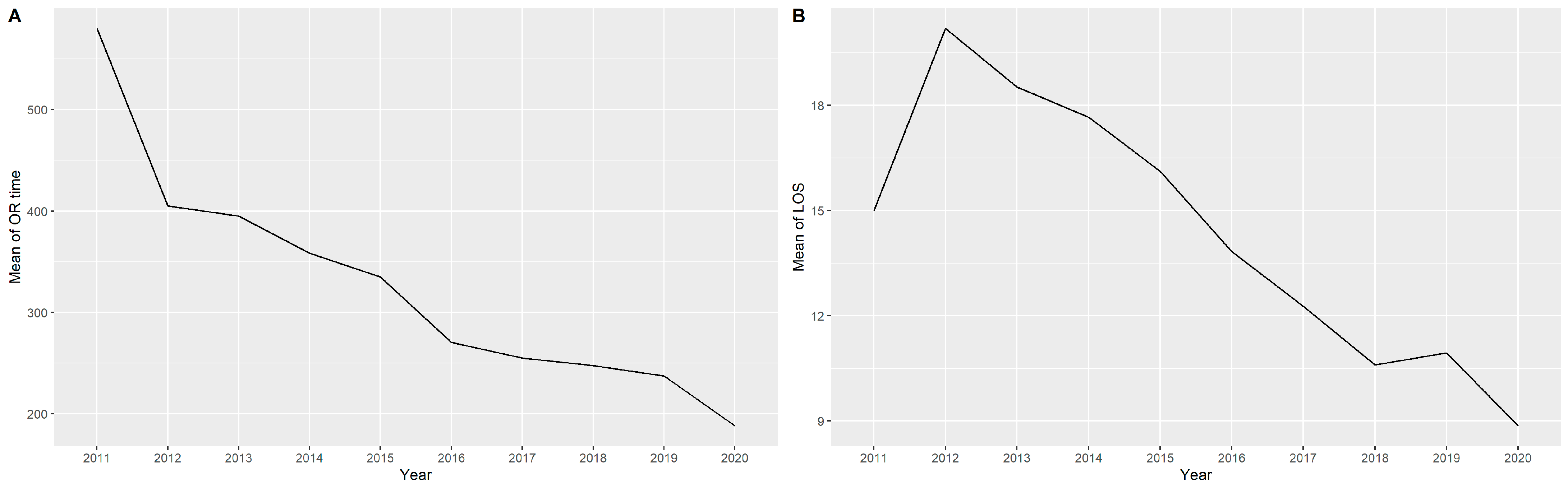
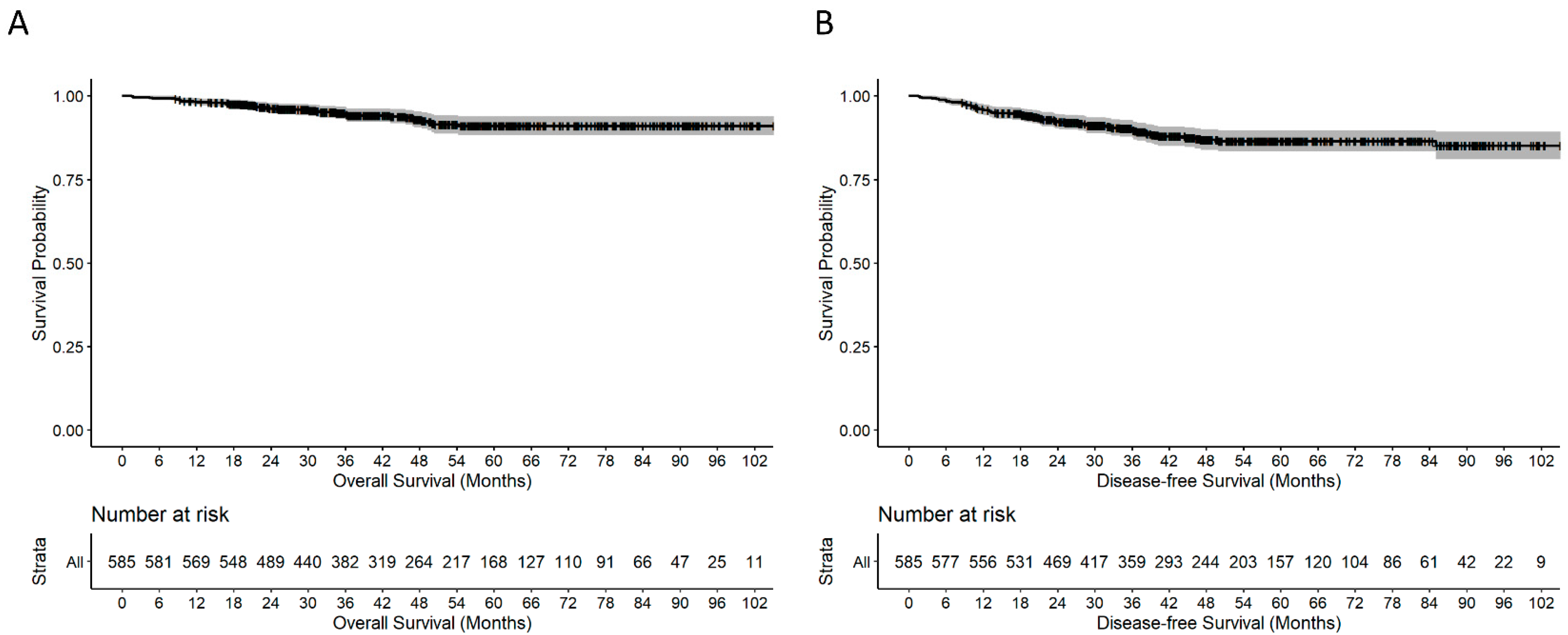
| Characteristic | Median (IQR a or %) |
|---|---|
| Age (years, median) (range) | 60 (51–67) |
| Gender | |
| Female | 255 (42.1%) |
| Male | 350 (57.9%) |
| Tumor distance from anal verge (cm) | |
| ≤5 (Lower) | 301 (49.7%) |
| 6–10 (Middle) | 176 (29.1%) |
| 11–15 (Upper) | 116 (19.2%) |
| Unknown | 12 (2.0%) |
| AJCC Stage b | |
| 0 | 1 (0.2%) |
| I | 281 (46.4%) |
| II | 111 (18.4%) |
| III | 194 (32.1%) |
| IV | 13 (2.1%) |
| NA c | 5 (0.8%) |
| Pre-operation treatment | |
| CCRT d | 429 (70.8%) |
| Chemotherapy | 7 (1.2%) |
| Radiation | 18 (3.0%) |
| None | 151 (25.0) |
| CCI e scores | |
| 0, 1 | 536 (88.6%) |
| 2 | 28 (4.6%) |
| ≥3 | 41 (6.8%) |
| ASA f classification | |
| I | 21 (3.5%) |
| II | 422 (70.1%) |
| III | 157 (26.1%) |
| IV | 2 (0.3%) |
| BMI g kg/m2 | 23.7 (21.6–26.7) |
| Procedure | |
| LAR h | 377 (62.3%) |
| ISR i | 200 (33.1%) |
| APR j | 28 (4.6%) |
| Intraoperative Safety | |
|---|---|
| Characteristic | Median (IQR a or %) |
| Conversions to open surgery | 1 (0.2%) |
| Console Time (min, median) (range) | 211 (172–256) |
| Operation Time (min, median) (range) | 270 (210–335) |
| Estimated blood loss (mL, Median) | 50 (30–100) |
| Blood transfusion during surgery | 10 (1.7%) |
| Rehospitalization within the 30-day postoperative period | 8 (1.3%) |
| Reoperation within the 30-day postoperative period | 10 (1.7%) |
| Death during surgery | 0 (0.0%) |
| Death within the 30-day postoperative period | 0 (0.0%) |
| Pathological outcomes and Oncological outcomes | |
| Characteristic | Median (IQR a or %) |
| Harvested Lymph Node | 14 (10–20) |
| Distal resection margin | |
| Free | 584 (96.5%) |
| Positive | 21 (3.5%) |
| Circumferential resection margin | |
| Free | 575 (95.0%) |
| Positive | 30 (5.0%) |
| Relapse | 113 (18.7%) |
| Local recurrence | 18 (3.0%) |
| Distant metastasis | 95 (15.7) |
| Cancer-specific death during follow-up period | 39 (6.4%) |
| Complications | Number (%) |
|---|---|
| Post-operative bleeding | 1 (0.2%) |
| Anastomosis leakage | 18 (3.0%) |
| Ileus | 20 (3.3%) |
| Infection events a | 22 (3.6%) |
| Urinary retention | 6 (1.0%) |
| Urinary infection | 14 (2.3%) |
| Pulmonary complication | 6 (1.0%) |
| Total | 87 (14.4%) |
| Author (Year, Design) | Country | Patient Number | Surgery Type | Cancer Stage | OR Time (Minutes) | Lymph Node Yields | LOS (Days) | Conversion |
|---|---|---|---|---|---|---|---|---|
| Present study | Taiwan | R: 605 | LAR, APR, ISR | I, II, III, IV | 284.11 | 15.35 | 13.5 | 0.17% |
| Katsuno [30] (2020, Cohort) | Japan | R: 115 | LAR, APR, ISR | I, II | 341 | NA | 11 * | 0 |
| Yamaguchi [31] (2018, Cohort) | Japan | R: 551 | HAR, LAR, ISR, APR, Hartmann | I, II, III, IV | 257 | NA | 7 * | 0 |
| Kim [34] (2016, Cohort) | Korea | R: 60 | LAR, APR | I, II, III, IV | 466.8 | 20.1 | 8.6 | 0% |
| Tang [36] (2016, Cohort) | China | R: 392 | LAR, APR, Hartmann | I, II, III, IV | 297 | 14.6 | 12.1 | 1.80% |
| Lim [37] (2017, Cohort) | Korea | R: 74 | LAR, ISR, CAA, APR | CR, I, II, III | 365.2 | 11.6 | NA | 1.40% |
| L: 64 | 311.6 | 14.7 | NA | 6.30% | ||||
| Chen [11] (2020, Cohort) | Taiwan | R: 88 | TME | CR, I, II, III | NA | NA | NA | NA |
| L: 37 | NA | NA | NA | NA | ||||
| O: 175 | NA | NA | NA | NA | ||||
| Huang [24] (2017, Cohort) | Taiwan | R: 40 | LAR, ISR | I, II, III | 274.4 | NA | 12.9 | NA |
| L: 38 | 235.4 | NA | 11.7 | NA | ||||
| Somashekhar [38] (2015, RCT) | India | R: 25 | LAR, AR | NA | R: 310.3 | 16.88 | 7.52 | NA |
| O: 25 | L: 246.9 | 15.2 | 13.24 | NA | ||||
| Jayne [29] (2017, RCT) ROLARR | Multinational (Ten countries) | R: 237 | LAR, APR, HAR, Hartmann (High anterior resection) | I, II, III, IV | R: 298.5 | 24.1 | 8.2 | 8.10% |
| L: 234 | L: 261 | 23.2 | 8 | 12.20% | ||||
| Kim [35] (2018, RCT) | Korea | R: 66 | LAR, APR, Hartmann | I, II, III, IV | R: 339.2 | 18 | 10.3 | 1.50% |
| L: 73 | L: 227.8 | 15 | 10.8 | 0% | ||||
| Sujatha-Bhaskar [39] (2017, Database) | United States | R: 905 | APR, Proctectomy (incl. LAR) | I, II, III | NA | 15.7 | NA | 7% |
| O: 3399 | NA | 14.8 | NA | NA | ||||
| L: 2009 | NA | 15.2 | NA | 14% | ||||
| Hyde [32] (2019, Database) | United States | R: 6035 | LAR | I, II, III, IV | NA | 17 | 6.3 | 7.45 |
| O: 21,421 | NA | 16.4 | 7.8 | NA | ||||
| L: 13,826 | NA | 16.8 | 6.8 | 14.95 | ||||
| Chang [28] (2020, Database) | China | R: 1145 | APR, LAR, APR, Hartmann | Benign, I, II, III, IV | NA | 17 | NA | NA |
| Author (year, design) | Country | Patient number | Reoperation | Transfusion | Blood loss (mL) | Positive CRM | Recurrence | |
| Present study | Taiwan | R: 605 | 1.70% | 1.65% | 72.58 | 4.96% | Local: 2.96% Systemic: 15.67% | |
| Katsuno [30] (2020, Cohort) | Japan | R: 115 | NA | 0 | 20 | NA | Local: 3.5% Systemic: 20.0% | |
| Yamaguchi [31] (2018, Cohort) | Japan | R: 551 | NA | 0 | 10 | NA | NA | |
| Kim [34] (2016, Cohort) | Korea | R: 60 | NA | NA | 74.2 | 11.70% | Local: 1.9% Systemic: 26.4% | |
| Tang [36] (2016, Cohort) | China | R:392 | 1.8% | NA | 67.5 | 2.30% | Local: 2.3% | |
| Lim [37] (2017, Cohort) | Korea | R: 74 | NA | NA | NA | NA | Local: 2.7% Systemic: 18.9% | |
| L: 64 | NA | NA | NA | NA | Local: 6.3 Systemic: 15.6% | |||
| Chen [11] (2020, Cohort) | Taiwan | R: 88 | NA | NA | NA | 3.40% | Local: 2.30% Systemic: 21,6% | |
| L: 37 | NA | NA | NA | 16.20% | Local: 21.60% Systemic: 35.1% | |||
| O: 175 | NA | NA | NA | 12% | Local:6.90% Systemic: 20.6% | |||
| Huang [24] (2017, Cohort) | Taiwan | R: 40 | NA | NA | 41.9 | NA | NA | |
| L: 38 | NA | NA | 55.1 | NA | NA | |||
| Somashekhar [38] (2015, RCT) | India | R: 25 | NA | NA | 165.14 | 0% | NA | |
| O: 25 | NA | NA | 406.04 | 0% | NA | |||
| Jayne [29] (2017, RCT) ROLARR | Multinational (Ten countries) | R: 237 | NA | NA | NA | 5.10% | NA | |
| L: 234 | NA | NA | NA | 6.30% | NA | |||
| Kim [35] (2018, RCT) | Korea | R: 66 | 3.03% | NA | 100 | 6.10% | NA | |
| L: 73 | 2.74% | NA | 50 | 5.50% | NA | |||
| Sujatha-Bhaskar [39] (2017, Database) | United States | R: 905 | NA | NA | NA | 4.75% | NA | |
| O: 3399 | NA | NA | NA | 7.62% | NA | |||
| L: 2009 | NA | NA | NA | 4.87% | NA | |||
| Hyde [32] (2019, Database) | United States | R: 6035 | NA | NA | NA | NA | NA | |
| O: 21,421 | NA | NA | NA | NA | NA | |||
| L: 13,826 | NA | NA | NA | NA | NA | |||
| Chang [28] (2020, Database) | China | R: 1145 | 0.80% | NA | NA | 1.30% | NA | |
| Author (year, design) | Country | Patient Number | 30 Day Readmission | 30 Day Mortality | Disease Free Survival (DFS) | |||
| Present study | Taiwan | R: 605 | 1.32% | 0% | 5y: 86.3% | |||
| Katsuno [30] (2020, Cohort) | Japan | R: 115 | NA | NA | I: 93.5% II: 100% III: 83.8% | |||
| Yamaguchi [31] (2018, Cohort) | Japan | R: 551 | NA | NA | I: 93.6% II: 75% III: 77.6% | |||
| Kim [34] (2016, Cohort) | Korea | R: 60 | NA | NA | 4y: 72.8% | |||
| Tang [36] (2016, Cohort) | China | R: 392 | NA | 0.5% | 3y: 74.3% | |||
| Lim [37] (2017, Cohort) | Korea | R: 74 | NA | NA | NA | |||
| L: 64 | NA | NA | NA | |||||
| Chen [11] (2020, Cohort) | Taiwan | R: 88 | NA | NA | NA | |||
| L: 37 | NA | NA | NA | |||||
| O: 175 | NA | NA | NA | |||||
| Huang [24] (2017, Cohort) | Taiwan | R: 40 | NA | NA | NA | |||
| L: 38 | NA | NA | NA | |||||
| Somashekhar [38] (2015, RCT) | India | R: 25 | NA | NA | NA | |||
| O: 25 | NA | NA | NA | |||||
| Jayne [29] (2017, RCT) ROLARR | Multinational (Ten countries) | R: 237 | NA | 0.80% | NA | |||
| L: 234 | NA | 0.90% | NA | |||||
| Kim [35] (2018, RCT) | Korea | R: 66 | NA | NA | NA | |||
| L: 73 | NA | NA | NA | |||||
| Sujatha-Bhaskar [39] (2017, Database) | United States | R: 905 | NA | 0% | NA | |||
| O: 3399 | NA | 0% | NA | |||||
| L: 2009 | NA | 0.16% | NA | |||||
| Hyde [32] (2019, Database) | United States | R: 6035 | NA | 0.9 | NA | |||
| O: 21,421 | NA | 1.1 | NA | |||||
| L: 13,826 | NA | 1.5 | NA | |||||
| Chang [28] (2020, Database) | China | R: 1145 | 2.30% | 0.10% | NA | |||
| Author (Year, Design) | Country | Patient Number | Overall Complication Rate | Anastomotic Leakage | Incisional Hernia | Surgical Site Infection | Ileus |
|---|---|---|---|---|---|---|---|
| Present study | Taiwan | R: 605 | 13.39% | 2.98% | 0% | 3.64% | 3.31% |
| Katsuno [30] (2020, Cohort) | Japan | R: 115 | 14.80% | 6.10% | NA | 1.70% | NA |
| Yamaguchi [31] (2018, Cohort) | Japan | R: 551 | 15.50% | 2.20% | NA | NA | NA |
| Kim [34] (2016, Cohort) | Korea | R: 60 | 15% | 5% | NA | NA | 3% |
| Tang [36] (2016, Cohort) | China | R:392 | 9.9% | 4.10% | NA | NA | NA |
| Huang [24] (2017, Cohort) | Taiwan | R: 40 | 15.00% | 7.50% | NA | NA | 0.00% |
| L: 38 | 18% | 5% | NA | NA | 13% | ||
| Somashekhar [38] (2015, RCT) | India | R: 25 | 0.00% | NA | NA | NA | NA |
| O: 25 | 20.00% | NA | NA | NA | NA | ||
| Jayne [29] (2017, RCT) ROLARR | Multinational (Ten countries) | R: 237 | 33% | 15% | NA | 9% | NA |
| L: 234 | 31.70% | 17.40% | NA | 8.30% | NA | ||
| Kim [35] (2018, RCT) | Korea | R: 66 | 34.80% | 12.10% | NA | NA | 9.10% |
| L: 73 | 23% | 7% | NA | NA | 12% | ||
| Sujatha-Bhaskar [39] (2017, Database) | United States | R: 905 | NA | NA | NA | NA | NA |
| O: 3399 | NA | NA | NA | NA | NA | ||
| L: 2009 | NA | NA | NA | NA | NA | ||
| Chang [28] (2020, Database) | China | R: 1145 | 16.30% | 4.20% | NA | NA | 1.30% |
| Author (year, design) | Country | Patient number | Abdominal bleeding | Urinary retention | Urinary infection | Pneumonia | Fecal incontinence |
| Present study | Taiwan | R: 605 | 0.17% | 0.99% | 2.31% | 0.99% | 0.17% |
| Katsuno [30] (2020, Cohort) | Japan | R: 115 | NA | 3.50% | NA | NA | NA |
| Yamaguchi [31] (2018, Cohort) | Japan | R: 551 | NA | 2.20% | 1.80% | 1.30% | NA |
| Kim [34] (2016, Cohort) | Korea | R: 60 | NA | NA | NA | NA | NA |
| Tang [36] (2016, Cohort) | China | R:392 | NA | NA | NA | 0.00% | NA |
| Huang [24] (2017, Cohort) | Taiwan | R: 40 | NA | NA | NA | NA | NA |
| L: 38 | NA | NA | NA | NA | NA | ||
| Somashekhar [38] (2015, RCT) | India | R: 25 | NA | 8.00% | NA | NA | NA |
| O: 25 | NA | 20.00% | NA | NA | NA | ||
| Jayne [29] (2017, RCT) ROLARR | Multinational (Ten countries) | R: 237 | NA | NA | NA | NA | NA |
| L: 234 | NA | NA | NA | NA | NA | ||
| Kim [35] (2018, RCT) | Korea | R: 66 | 0.70% | NA | NA | NA | NA |
| L: 73 | 0% | NA | NA | NA | NA | ||
| Sujatha-Bhaskar [39] (2017, Database) | L: 5935 | NA | NA | NA | NA | NA | |
| United States | R: 905 | NA | NA | NA | NA | NA | |
| O: 3399 | NA | NA | NA | NA | NA | ||
| Chang [28] (2020, Database) | L: 13,826 | NA | NA | NA | NA | NA | |
| China | R: 1145 | NA | 2.50% | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-W.; Wei, P.-L.; Chen, C.-C.; Kuo, L.-J.; Wang, J.-Y. Clinical Safety and Effectiveness of Robotic-Assisted Surgery in Patients with Rectal Cancer: Real-World Experience over 8 Years of Multiple Institutions with High-Volume Robotic-Assisted Surgery. Cancers 2022, 14, 4175. https://doi.org/10.3390/cancers14174175
Huang C-W, Wei P-L, Chen C-C, Kuo L-J, Wang J-Y. Clinical Safety and Effectiveness of Robotic-Assisted Surgery in Patients with Rectal Cancer: Real-World Experience over 8 Years of Multiple Institutions with High-Volume Robotic-Assisted Surgery. Cancers. 2022; 14(17):4175. https://doi.org/10.3390/cancers14174175
Chicago/Turabian StyleHuang, Ching-Wen, Po-Li Wei, Chien-Chih Chen, Li-Jen Kuo, and Jaw-Yuan Wang. 2022. "Clinical Safety and Effectiveness of Robotic-Assisted Surgery in Patients with Rectal Cancer: Real-World Experience over 8 Years of Multiple Institutions with High-Volume Robotic-Assisted Surgery" Cancers 14, no. 17: 4175. https://doi.org/10.3390/cancers14174175
APA StyleHuang, C.-W., Wei, P.-L., Chen, C.-C., Kuo, L.-J., & Wang, J.-Y. (2022). Clinical Safety and Effectiveness of Robotic-Assisted Surgery in Patients with Rectal Cancer: Real-World Experience over 8 Years of Multiple Institutions with High-Volume Robotic-Assisted Surgery. Cancers, 14(17), 4175. https://doi.org/10.3390/cancers14174175






