Association between Serum Level of Multiple Trace Elements and Esophageal Squamous Cell Carcinoma Risk: A Case–Control Study in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Data Collection and Variable Definition
2.2. Serum Trace Element Assay
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Study Population
3.2. Serum Trace Element Concentrations
3.3. Associations of Trace Element Concentrations with Risk for ESCC
3.4. Bayesian Kernel Machine Regression (BKMR) Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thrift, A.P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; McCormack, V.; Abedi-Ardekani, B.; Arnold, M.; Camargo, M.C.; Dar, N.A.; Dawsey, S.M.; Etemadi, A.; Fitzgerald, R.C.; Fleischer, D.E.; et al. International cancer seminars: A focus on esophageal squamous cell carcinoma. Ann. Oncol. 2017, 28, 2086–2093. [Google Scholar] [CrossRef]
- Chen, F.; Cole, P.; Mi, Z.; Xing, L. Dietary trace elements and esophageal cancer mortality in Shanxi, China. Epidemiology 1992, 3, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.Q.; Abnet, C.C.; Qiao, Y.L.; Dawsey, S.M.; Dong, Z.W.; Sun, X.D.; Fan, J.H.; Gunter, E.W.; Taylor, P.R.; Mark, S.D. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am. J. Clin. Nutr. 2004, 79, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Steevens, J.; van den Brandt, P.A.; Goldbohm, R.A.; Schouten, L.J. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology 2010, 138, 1704–1713. [Google Scholar] [CrossRef]
- Pritchett, N.R.; Burgert, S.L.; Murphy, G.A.; Brockman, J.D.; White, R.E.; Lando, J.; Chepkwony, R.; Topazian, M.D.; Abnet, C.C.; Dawsey, S.M.; et al. Cross sectional study of serum selenium concentration and esophageal squamous dysplasia in western Kenya. BMC Cancer 2017, 17, 835. [Google Scholar] [CrossRef]
- Li, P.; Xu, J.; Shi, Y.; Ye, Y.; Chen, K.; Yang, J.; Wu, Y. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin. Nutr. 2014, 33, 415–420. [Google Scholar] [CrossRef]
- Li, Q.; Cui, L.; Tian, Y.; Cui, H.; Li, L.; Dou, W.; Li, H.; Wang, L. Protective Effect of Dietary Calcium Intake on Esophageal Cancer Risk: A Meta-Analysis of Observational Studies. Nutrients 2017, 9, 510. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium exposure and risk of lung cancer: A meta-analysis of cohort and case-control studies among general and occupational populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Nikkhah, M.; Sohrabi, M.; Rezaee Farimani, A.; Mirasgari Shahi, M.; Ziaie, H.; Shirmardi, S.; Kohi, Z.; Salehpour, D.; Safarnezhad Tameshkel, F.; et al. Evaluating tissue levels of the eight trace elements and heavy metals among esophagus and gastric cancer patients: A comparison between cancerous and non-cancerous tissues. J. Trace. Elem. Med. Biol. 2021, 68, 126761. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, J.; Chen, H.; Conigrave, K.M.; Hao, W. Alcohol use in China. Alcohol Alcohol. 2003, 38, 537–542. [Google Scholar] [CrossRef]
- Lin, T.; Liu, T.; Lin, Y.; Zhang, C.; Yan, L.; Chen, Z.; He, Z.; Wang, J. Serum levels of chemical elements in esophageal squamous cell carcinoma in Anyang, China: A case-control study based on machine learning methods. BMJ Open 2017, 7, e015443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, H.B.; Cheng, M.X.; Wang, L.; Gao, C.B.; Huang, F. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 20560–20572. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef]
- Yang, Y.W.; Dai, C.M.; Chen, X.H.; Feng, J.F. The Relationship between Serum Trace Elements and Oxidative Stress of Patients with Different Types of Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 4846951. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Fu, W.; Lu, Y.; Wei, W.; Chen, W.; Wu, X.; Meng, H.; Feng, Y.; Liu, Y.; et al. Circulating essential metals and lung cancer: Risk assessment and potential molecular effects. Environ. Int. 2019, 127, 685–693. [Google Scholar] [CrossRef]
- Lossow, K.; Schwarz, M.; Kipp, A.P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol. 2021, 42, 101900. [Google Scholar] [CrossRef] [PubMed]
- Abnet, C.C.; Lai, B.; Qiao, Y.L.; Vogt, S.; Luo, X.M.; Taylor, P.R.; Dong, Z.W.; Mark, S.D.; Dawsey, S.M. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J. Natl. Cancer Inst. 2005, 97, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Taccioli, C.; Chen, H.; Jiang, Y.; Liu, X.P.; Huang, K.; Smalley, K.J.; Farber, J.L.; Croce, C.M.; Fong, L.Y. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene 2012, 31, 4550–4558. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, J.; Pan, X.; Quimby, D.; Zanesi, N.; Druck, T.; Pfeifer, G.P.; Croce, C.M.; Fong, L.Y.; Huebner, K. Effect of zinc supplementation on N-nitrosomethylbenzylamine-induced forestomach tumor development and progression in tumor suppressor-deficient mouse strains. Carcinogenesis 2011, 32, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Alder, H.; Taccioli, C.; Chen, H.; Jiang, Y.; Smalley, K.J.; Fadda, P.; Ozer, H.G.; Huebner, K.; Farber, J.L.; Croce, C.M.; et al. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis 2012, 33, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Taccioli, C.; Palamarchuk, A.; Tagliazucchi, G.M.; Jing, R.; Smalley, K.J.; Fan, S.; Altemus, J.; Fiehn, O.; Huebner, K.; et al. Abrogation of esophageal carcinoma development in miR-31 knockout rats. Proc. Natl. Acad. Sci. USA 2020, 117, 6075–6085. [Google Scholar] [CrossRef]
- Liu, C.M.; Liang, D.; Jin, J.; Li, D.J.; Zhang, Y.C.; Gao, Z.Y.; He, Y.T. Research progress on the relationship between zinc deficiency, related microRNAs, and esophageal carcinoma. Thorac. Cancer 2017, 8, 549–557. [Google Scholar] [CrossRef]
- Mark, S.D.; Qiao, Y.L.; Dawsey, S.M.; Wu, Y.P.; Katki, H.; Gunter, E.W.; Fraumeni, J.F., Jr.; Blot, W.J.; Dong, Z.W.; Taylor, P.R. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J. Natl. Cancer Inst. 2000, 92, 1753–1763. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Selenium and kidney disease. J. Nephrol. 2013, 26, 266–272. [Google Scholar] [CrossRef]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in Research on the Toxicological Effects of Selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Cooper, C.; Harvey, N.C. Cardiovascular safety of calcium, magnesium and strontium: What does the evidence say? Aging Clin. Exp. Res. 2021, 33, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejska, B.; Stepien, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Chen, L.J.; Tang, L.Y.; He, J.R.; Su, Y.; Cen, Y.L.; Yu, D.D.; Wu, B.H.; Lin, Y.; Chen, W.Q.; Song, E.W.; et al. Urinary strontium and the risk of breast cancer: A case-control study in Guangzhou, China. Environ. Res. 2012, 112, 212–217. [Google Scholar] [CrossRef]
- Wach, S.; Weigelt, K.; Michalke, B.; Lieb, V.; Stoehr, R.; Keck, B.; Hartmann, A.; Wullich, B.; Taubert, H.; Chaudhri, A. Diagnostic potential of major and trace elements in the serum of bladder cancer patients. J. Trace Elem. Med. Biol. 2018, 46, 150–155. [Google Scholar] [CrossRef]
- Mirzaee, M.; Semnani, S.; Roshandel, G.; Nejabat, M.; Hesari, Z.; Joshaghani, H. Strontium and antimony serum levels in healthy individuals living in high- and low-risk areas of esophageal cancer. J. Clin. Lab. Anal. 2020, 34, e23269. [Google Scholar] [CrossRef] [PubMed]
- Jamakala, O.; Rani, U.A. Amelioration Effect of Zinc and Iron Supplementation on Selected Oxidative Stress Enzymes in Liver and Kidney of Cadmium-Treated Male Albino Rat. Toxicol. Int. 2015, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Byrne, A.R.; Kosta, L. Vanadium in foods and in human body fluids and tissues. Sci. Total Environ. 1978, 10, 17–30. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, V.A.; Nersesyan, A.K.; Atefie, K.; Hoelzl, C.; Ferk, F.; Bichler, J.; Valic, E.; Schaffer, A.; Schulte-Hermann, R.; Fenech, M.; et al. Inhalative exposure to vanadium pentoxide causes DNA damage in workers: Results of a multiple end point study. Environ. Health Perspect. 2008, 116, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Cobalt in hard metals and cobalt sulfate, gallium arsenide, indium phosphide and vanadium pentoxide. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 86, 1–294. [Google Scholar]
- Rondini, E.A.; Walters, D.M.; Bauer, A.K. Vanadium pentoxide induces pulmonary inflammation and tumor promotion in a strain-dependent manner. Part. Fibre Toxicol. 2010, 7, 9. [Google Scholar] [CrossRef]
- Clancy, H.A.; Sun, H.; Passantino, L.; Kluz, T.; Munoz, A.; Zavadil, J.; Costa, M. Gene expression changes in human lung cells exposed to arsenic, chromium, nickel or vanadium indicate the first steps in cancer. Metallomics 2012, 4, 784–793. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Xiu, Q.; Lu, H.; Han, H. Cyclooxygenase-2 induction requires activation of nuclear factor of activated T-cells in Beas-2B cells after vanadium exposure and plays an anti-apoptotic role. Arch. Biochem. Biophys. 2007, 468, 92–99. [Google Scholar] [CrossRef]
- Miller, E.C.; Miller, J.A. Mechanisms of chemical carcinogenesis. Cancer 1981, 47, 1055–1064. [Google Scholar] [CrossRef]
- Jennette, K.W. The role of metals in carcinogenesis: Biochemistry and metabolism. Environ. Health Perspect. 1981, 40, 233–252. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Z.; Lin, X.; Liao, J.; Zhang, Y.; Xie, B.; Huang, Y.; Peng, L. Environmental Cadmium Exposure Promotes the Development, Progression and Chemoradioresistance of Esophageal Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2022, 10, 792933. [Google Scholar] [CrossRef]
- Abbas, G.; Krasna, M. Overview of esophageal cancer. Ann. Cardiothorac. Surg. 2017, 6, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.S.; Chen, X.; Tu, S. Etiology and Prevention of Esophageal Cancer. Gastrointest. Tumors 2016, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
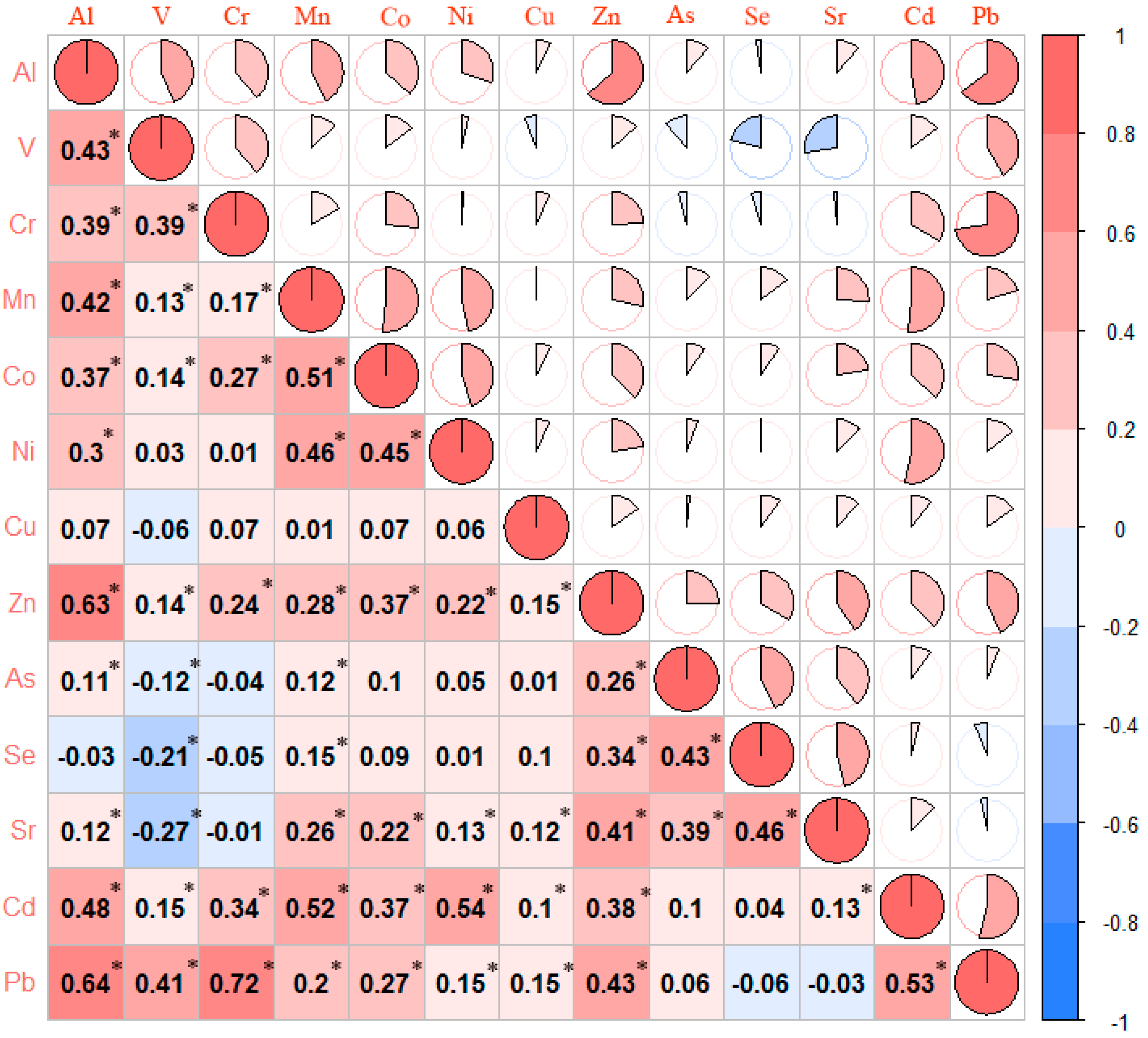




| Variables | Controls (n = 191) | Cases (n = 185) | p |
|---|---|---|---|
| Age, mean ± SD | 62.23 ± 8.86 | 62.65 ± 7.36 | 0.908 |
| Sex, n (%) | |||
| Male | 137 (71.73) | 133 (71.89) | 0.972 |
| Female | 54 (28.27) | 52 (28.11) | |
| Smoking, n (%) | |||
| No | 126 (65.97) | 70 (38.25) | <0.001 |
| Yes | 65 (34.03) | 113 (61.75) | |
| Drinking, n (%) | |||
| No | 144 (75.39) | 129 (70.49) | 0.200 |
| Yes | 47 (24.61) | 54 (29.51) | |
| Family history of EC, n (%) | |||
| No | 189 (98.95) | 163 (89.07) | <0.001 |
| Yes | 2 (1.05) | 20 (10.93) |
| Elements a | Controls (n = 191) | Cases (n = 185) | p |
|---|---|---|---|
| Al | 1097.5 (722.0, 1543.0) | 857.2 (461.2, 1576.4) | 0.015 |
| V | 0.788 (0.566, 1.141) | 0.849 (0.673, 1.076) | 0.030 |
| Cr | 29.1 (20.3, 36.0) | 28.9 (19.6, 39.4) | 0.833 |
| Mn | 14.5 (11.0, 20.0) | 11.7 (9.2, 15.5) | <0.001 |
| Co | 0.370 (0.278, 0.472) | 0.295 (0.219, 0.440) | 0.001 |
| Ni | 7.7 (3.8, 24.5) | 7.9 (4.6, 14.0) | 0.953 |
| Cu | 1067.5 (973.8, 1203.6) | 1094.0 (960.9, 1258.9) | 0.239 |
| Zn | 1186.3 (1033.5, 1635.4) | 904.5 (743.7, 1184.6) | <0.001 |
| As | 4.4 (2.0, 7.4) | 2.5 (1.4, 5.3) | <0.001 |
| Se | 131.5 (116.6, 144.4) | 107.4 (96.4, 122.4) | <0.001 |
| Sr | 83.1 (61.2, 98.3) | 48.8 (41.0, 58.1) | <0.001 |
| Cd | 0.384 (0.242, 0.670) | 0.331 (0.205, 0.569) | 0.026 |
| Pb | 105.5 (63.4, 136.7) | 105.0 (63.0, 171.3) | 0.382 |
| Elements | Variables | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for Trend |
|---|---|---|---|---|---|---|
| Al | Case/control | 58/36 | 49/45 | 31/63 | 47/47 | |
| Model 1 | 1 (Ref.) | 0.71 (0.19, 2.62) | 0.26 (0.06, 1.09) | 2.73 (0.49, 15.39) | 0.419 | |
| Model 2 | 1 (Ref.) | 0.58 (0.14, 2.41) | 0.15 (0.03, 0.75) | 1.92 (0.30, 12.52) | 0.537 | |
| V | Case/control | 27/67 | 55/39 | 61/33 | 42/52 | |
| Model 1 | 1 (Ref.) | 3.07 (0.92, 10.32) | 6.33 (1.67, 24.02) | 0.53 (0.14, 2.00) | 0.338 | |
| Model 2 | 1 (Ref.) | 3.72 (0.94, 14.70) | 7.16 (1.70, 30.15) | 0.50 (0.12, 2.15) | 0.243 | |
| Mn | Case/control | 58/36 | 51/43 | 44/50 | 32/62 | |
| Model 1 | 1 (Ref.) | 1.39 (0.44, 4.44) | 0.73 (0.21, 2.56) | 0.21 (0.05, 0.95) | 0.075 | |
| Model 2 | 1 (Ref.) | 1.11 (0.31, 3.97) | 0.74 (0.19, 2.94) | 0.12 (0.03, 0.95) | 0.099 | |
| Co | Case/control | 58/36 | 48/46 | 38/56 | 41/53 | |
| Model 1 | 1 (Ref.) | 1.16 (0.39, 3.43) | 1.27 (0.38, 4.20) | 2.55 (0.73, 8.89) | 0.939 | |
| Model 2 | 1 (Ref.) | 1.46 (0.45, 4.75) | 1.81 (0.46, 7.11) | 3.90 (0.93, 16.44) | 0.674 | |
| Ni | Case/control | 54/40 | 51/43 | 56/38 | 38/56 | |
| Model 1 | 1 (Ref.) | 5.43 (1.74, 16.94) | 6.48 (1.95, 21.49) | 5.59 (1.30, 23.95) | 0.003 | |
| Model 2 | 1 (Ref.) | 5.95 (1.74, 20.33) | 4.60 (1.31, 16.22) | 3.60 (0.74, 17.48) | 0.008 | |
| Zn | Case/control | 81/13 | 47/47 | 23/71 | 34/60 | |
| Model 1 | 1 (Ref.) | 0.22 (0.06, 0.75) | 0.04 (0.01, 0.17) | 0.07 (0.01, 0.41) | 0.001 | |
| Model 2 | 1 (Ref.) | 0.39 (0.10, 1.57) | 0.04 (0.01, 0.21) | 0.12 (0.02, 0.86) | 0.004 | |
| As | Case/control | 63/31 | 52/42 | 37/57 | 15/79 | |
| Model 1 | 1 (Ref.) | 1.00 (0.33, 3.04) | 1.13 (0.35, 3.59) | 2.94 (0.78, 11.12) | 0.023 | |
| Model 2 | 1 (Ref.) | 0.80 (0.24, 2.63) | 0.92 (0.25, 3.32) | 2.86 (0.63, 12.96) | 0.046 | |
| Se | Case/control | 81/13 | 52/42 | 37/57 | 15/79 | |
| Model 1 | 1 (Ref.) | 0.27 (0.08, 0.92) | 0.22 (0.06, 0.75) | 0.05 (0.01, 0.20) | <0.001 | |
| Model 2 | 1 (Ref.) | 0.18 (0.05, 0.71) | 0.19 (0.05, 0.74) | 0.06 (0.01, 0.27) | <0.001 | |
| Sr | Case/control | 75/19 | 67/27 | 41/53 | 2/92 | |
| Model 1 | 1 (Ref.) | 1.32 (0.44, 3.96) | 0.15 (0.05, 0.47) | 0.00 (0.00, 0.01) | <0.001 | |
| Model 2 | 1 (Ref.) | 1.11 (0.33, 3.72) | 0.11 (0.03, 0.40) | 0.00 (0.00, 0.01) | <0.001 | |
| Cd | Case/control | 56/38 | 47/47 | 39/55 | 43/51 | |
| Model 1 | 1 (Ref.) | 0.46 (0.12, 1.73) | 1.24 (0.29, 5.29) | 1.49 (0.34, 6.45) | 0.381 | |
| Model 2 | 1 (Ref.) | 0.43 (0.10, 1.79) | 1.12 (0.23, 5.53) | 1.66 (0.35, 7.80) | 0.308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, G.; Huang, A.; Cao, K.; Tan, W.; Geng, H.; Lin, X.; Zhan, F.; Wu, K.; Zheng, S.; et al. Association between Serum Level of Multiple Trace Elements and Esophageal Squamous Cell Carcinoma Risk: A Case–Control Study in China. Cancers 2022, 14, 4239. https://doi.org/10.3390/cancers14174239
Zhang J, Wang G, Huang A, Cao K, Tan W, Geng H, Lin X, Zhan F, Wu K, Zheng S, et al. Association between Serum Level of Multiple Trace Elements and Esophageal Squamous Cell Carcinoma Risk: A Case–Control Study in China. Cancers. 2022; 14(17):4239. https://doi.org/10.3390/cancers14174239
Chicago/Turabian StyleZhang, Jingbing, Geng Wang, Anyan Huang, Kexin Cao, Wei Tan, Hui Geng, Xiaosheng Lin, Fulan Zhan, Kusheng Wu, Shukai Zheng, and et al. 2022. "Association between Serum Level of Multiple Trace Elements and Esophageal Squamous Cell Carcinoma Risk: A Case–Control Study in China" Cancers 14, no. 17: 4239. https://doi.org/10.3390/cancers14174239









