An Orthotopic Model of Glioblastoma Is Resistant to Radiodynamic Therapy with 5-AminoLevulinic Acid
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Spectroscopic Dosage
2.3. Porphyrin Concentrations Were Measured by Cytometry
2.4. Spheroid Growth and Viability
2.5. Cell Culture for Radiotherapy
2.6. Mouse Model
2.7. Radiotherapy
2.8. Statistical Analyses
3. Results
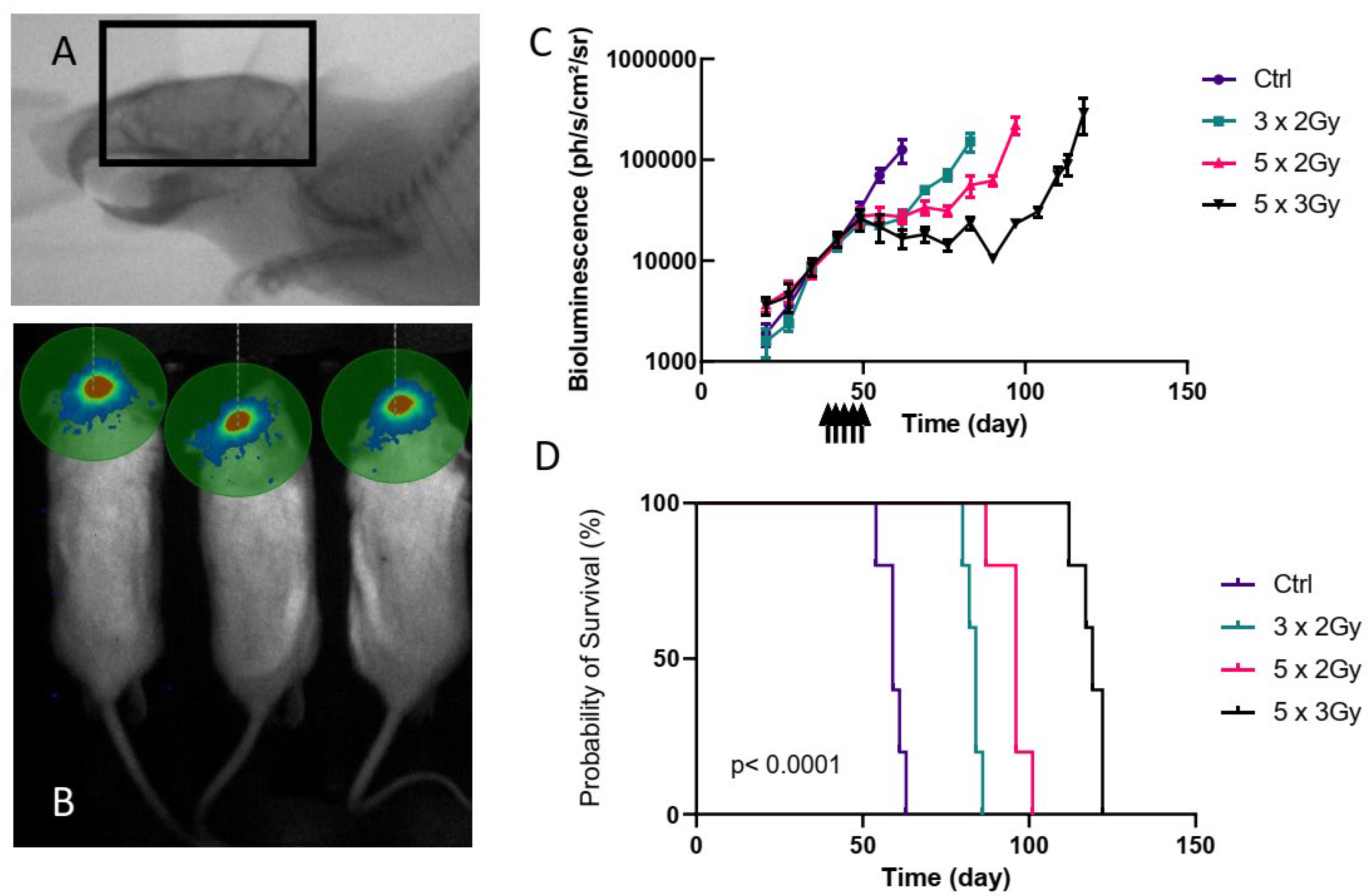
3.1. Orthotopic PDX Treated by Radiotherapy
3.2. Defining the Time to Inject 5-ALA before Irradiation
3.3. 5-ALA Does Not Radiosensitize the P3 Orthotopic PDX Model Treated by the Whole Brain Fractionated Radiotherapy
3.4. 5-ALA Treatment Radiosensitization Evaluation on P3 Cell-Derived Spheroids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Brandes, A.A.; Rampling, R.; Kouwenhoven, M.C.M.; Kros, J.M.; Carpentier, A.F.; Clement, P.M.; Frenay, M.; Campone, M.; Baurain, J.-F.; et al. Randomized Phase II Trial of Erlotinib versus Temozolomide or Carmustine in Recurrent Glioblastoma: EORTC Brain Tumor Group Study 26034. J. Clin. Oncol. 2009, 27, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with Temozolomide for Patients with Newly Diagnosed, EGFRvIII-Expressing Glioblastoma (ACT IV): A Randomised, Double-Blind, International Phase 3 Trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the Blood–Brain Barrier Really Disrupted in All Glioblastomas? A Critical Assessment of Existing Clinical Data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Richard, E.; Ged, C.; Bedel, A.; Moreau-Gaudry, F.; de Verneuil, H. Cellular and Gene Therapy for Erythropoietic Porphyrias. In Handbook of Porphyrin Science; Porphyrias and Sideroblastic Anemias; World Scientific: Singapore, 2013; Volume 29. [Google Scholar]
- Stummer, W.; Goetz, C. Fluorescence-Guided Resection of Glioblastoma Multiforme by Using 5-Aminolevulinic Acid-Induced Porphyrins: A Prospective Study in 52 Consecutive Patients. J. Neurosurg. 2000, 93, 11. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. ALA-Glioma Study Group Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA Fluorescence-Guided Resection of High-Grade Glioma Leads to Greater Extent of Resection with Better Outcomes: A Systematic Review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef]
- Leroy, H.-A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.-S.; Mordon, S.; Reyns, N. Is Interstitial Photodynamic Therapy for Brain Tumors Ready for Clinical Practice? A Systematic Review. Photodiagnosis Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Vermandel, M.; Leroy, H.-A.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative PhotoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2019, 84, E414–E419. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Nagasawa, S.; Doi, M.; Takahashi, M.; Narita, Y.; Yamamoto, J.; Ikemoto, M.J.; Iwahashi, H. In Vivo Study of the Efficacy and Safety of 5-Aminolevulinic Radiodynamic Therapy for Glioblastoma Fractionated Radiotherapy. Int. J. Mol. Sci. 2021, 22, 9762. [Google Scholar] [CrossRef] [PubMed]
- Panetta, J.V.; Cvetkovic, D.; Chen, X.; Chen, L.; Ma, C.-M.C. Radiodynamic Therapy Using 15-MV Radiation Combined with 5-Aminolevulinic Acid and Carbamide Peroxide for Prostate Cancer in Vivo. Phys. Med. Biol. 2020, 65, 165008. [Google Scholar] [CrossRef]
- Nordmann, N.J.; Michael, A.P. 5-Aminolevulinic Acid Radiodynamic Therapy for Treatment of High-Grade Gliomas: A Systematic Review. Clin. Neurol. Neurosurg. 2021, 201, 106430. [Google Scholar] [CrossRef]
- Ito, E.; Yue, S.; Moriyama, E.H.; Hui, A.B.; Kim, I.; Shi, W.; Alajez, N.M.; Bhogal, N.; Li, G.; Datti, A.; et al. Uroporphyrinogen Decarboxylase Is a Radiosensitizing Target for Head and Neck Cancer. Sci. Transl. Med. 2011, 3, 67ra7. [Google Scholar] [CrossRef]
- Luksiene, Z.; Juzenas, P.; Moan, J. Radiosensitization of Tumours by Porphyrins. Cancer Lett. 2006, 235, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Misawa, M.; Murakami, M.; Mori, T.; Nomura, K.; Iwahashi, H. 5-Aminolevulinic Acid Enhances Cancer Radiotherapy in a Mouse Tumor Model. SpringerPlus 2013, 2, 602. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Combined Treatment with X-Ray Irradiation and 5-Aminolevulinic Acid Elicits Better Transcriptomic Response of Cell Cycle-Related Factors than X-Ray Irradiation Alone. Int. J. Radiat. Biol. 2016, 92, 774–789. [Google Scholar] [CrossRef]
- Yamamoto, J.; Ogura, S.-I.; Shimajiri, S.; Nakano, Y.; Akiba, D.; Kitagawa, T.; Ueta, K.; Tanaka, T.; Nishizawa, S. 5-Aminolevulinic Acid-Induced Protoporphyrin IX with Multi-Dose Ionizing Irradiation Enhances Host Antitumor Response and Strongly Inhibits Tumor Growth in Experimental Glioma in Vivo. Mol. Med. Rep. 2015, 11, 1813–1819. [Google Scholar] [CrossRef] [Green Version]
- Sakariassen, P.Ø.; Prestegarden, L.; Wang, J.; Skaftnesmo, K.O.; Mahesparan, R.; Molthoff, C. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 16466–16471. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, E.; Rosland, G.V.; Talasila, K.M.; Knappskog, S.; Keunen, O.; Sottoriva, A. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro-Oncol. 2016, 18, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Daubon, T.; Léon, C.; Clarke, K.; Andrique, L.; Salabert, L.; Darbo, E. Deciphering the complex role of thrombospondin-1 in glioblastoma development. Nat. Commun. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Guyon, J.; Andrique, L.; Pujol, N.; Røsland, G.V.; Recher, G.; Bikfalvi, A.; Daubon, T. A 3D Spheroid Model for Glioblastoma. J. Vis. Exp. 2020, 60998. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Magaut, C.R.; Storevik, S.; Geraldo, L.H.; Mathivet, T.; Latif, M.A. TGF-β promotes microtube formation in glioblastoma through thrombospondin 1. Neuro-Oncology 2022, 24, 541–553. [Google Scholar] [CrossRef]
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S. TGF-β is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 2014, 2, e1443. [Google Scholar] [CrossRef]
- Lafitte, M.; Rousseau, B.; Moranvillier, I.; Taillepierre, M.; Peuchant, E.; Guyonnet-Dupérat, V.; Bedel, A.; Dubus, P.; de Verneuil, H.; Moreau-Gaudry, F.; et al. In Vivo Gene Transfer Targeting in Pancreatic Adenocarcinoma with Cell Surface Antigens. Mol. Cancer 2012, 11, 81. [Google Scholar] [CrossRef]
- Colucci, F.; Soudais, C.; Rosmaraki, E.; Vanes, L.; Tybulewicz, V.L.; Di Santo, J.P. Dissecting NK Cell Development Using a Novel Alymphoid Mouse Model: Investigating the Role of the c-Abl Proto-Oncogene in Murine NK Cell Differentiation. J. Immunol. 1950 1999, 162, 2761–2765. [Google Scholar]
- Kang, H. Sample Size Determination and Power Analysis Using the G*Power Software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Yamada, K.; Murayama, Y.; Kamada, Y.; Arita, T.; Kosuga, T.; Konishi, H.; Morimura, R.; Shiozaki, A.; Kuriu, Y.; Ikoma, H.; et al. Radiosensitizing Effect of 5-aminolevulinic Acid in Colorectal Cancer in Vitro and in Vivo. Oncol. Lett. 2019, 17, 5132–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.; Nagasawa, S.; Ikemoto, M.J.; Sato, C.; Sato, M.; Iwahashi, H. Verification of 5-Aminolevurinic Radiodynamic Therapy Using a Murine Melanoma Brain Metastasis Model. Int. J. Mol. Sci. 2019, 20, 5155. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roman, N.; Stevenson, K.; Gilmour, L.; Hamilton, G.; Chalmers, A.J. A Novel 3D Human Glioblastoma Cell Culture System for Modeling Drug and Radiation Responses. Neuro-Oncology 2017, 19, 229–241. [Google Scholar] [CrossRef] [PubMed]
- An ad hoc committee of the National Cancer Research Institute; Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Ali, M.Y.; Oliva, C.R.; Noman, A.S.M.; Allen, B.G.; Goswami, P.C.; Zakharia, Y. Radioresistance in Glioblastoma and the Development of Radiosensitizers. Cancers 2020, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, A.; Stevenson, K.; Tulk, A.; Chalmers, A.J. Evaluation of Four Different Small Animal Radiation Plans on Tumour and Normal Tissue Dosimetry in a Glioblastoma Mouse Model. Br. J. Radiol. 2019, 92, 20180469. [Google Scholar] [CrossRef]
- Schipper, M.L.; Patel, M.R.; Gambhir, S.S. Evaluation of Firefly Luciferase Bioluminescence Mediated Photodynamic Toxicity in Cancer Cells. Mol. Imaging Biol. 2006, 8, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef]
- To-Figueras, J.; Millet, O.; Herrero, C. Congenital Erythropoietic Porphyria. In Handbook of Porphyrin Science (Chapter 29); World Scientific: Singapore, 2013; pp. 151–217. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupin, C.; Sutter, J.; Amintas, S.; Derieppe, M.-A.; Lalanne, M.; Coulibaly, S.; Guyon, J.; Daubon, T.; Boutin, J.; Blouin, J.-M.; et al. An Orthotopic Model of Glioblastoma Is Resistant to Radiodynamic Therapy with 5-AminoLevulinic Acid. Cancers 2022, 14, 4244. https://doi.org/10.3390/cancers14174244
Dupin C, Sutter J, Amintas S, Derieppe M-A, Lalanne M, Coulibaly S, Guyon J, Daubon T, Boutin J, Blouin J-M, et al. An Orthotopic Model of Glioblastoma Is Resistant to Radiodynamic Therapy with 5-AminoLevulinic Acid. Cancers. 2022; 14(17):4244. https://doi.org/10.3390/cancers14174244
Chicago/Turabian StyleDupin, Charles, Jade Sutter, Samuel Amintas, Marie-Alix Derieppe, Magalie Lalanne, Soule Coulibaly, Joris Guyon, Thomas Daubon, Julian Boutin, Jean-Marc Blouin, and et al. 2022. "An Orthotopic Model of Glioblastoma Is Resistant to Radiodynamic Therapy with 5-AminoLevulinic Acid" Cancers 14, no. 17: 4244. https://doi.org/10.3390/cancers14174244
APA StyleDupin, C., Sutter, J., Amintas, S., Derieppe, M.-A., Lalanne, M., Coulibaly, S., Guyon, J., Daubon, T., Boutin, J., Blouin, J.-M., Richard, E., Moreau-Gaudry, F., Bedel, A., Vendrely, V., & Dabernat, S. (2022). An Orthotopic Model of Glioblastoma Is Resistant to Radiodynamic Therapy with 5-AminoLevulinic Acid. Cancers, 14(17), 4244. https://doi.org/10.3390/cancers14174244







