A Subset of Secreted Proteins in Ascites Can Predict Platinum-Free Interval in Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Sample Characteristics
2.2. Multiplexed Bead-Based Immunoassays
2.3. Data Preprocessing and Lasso Modeling
3. Results
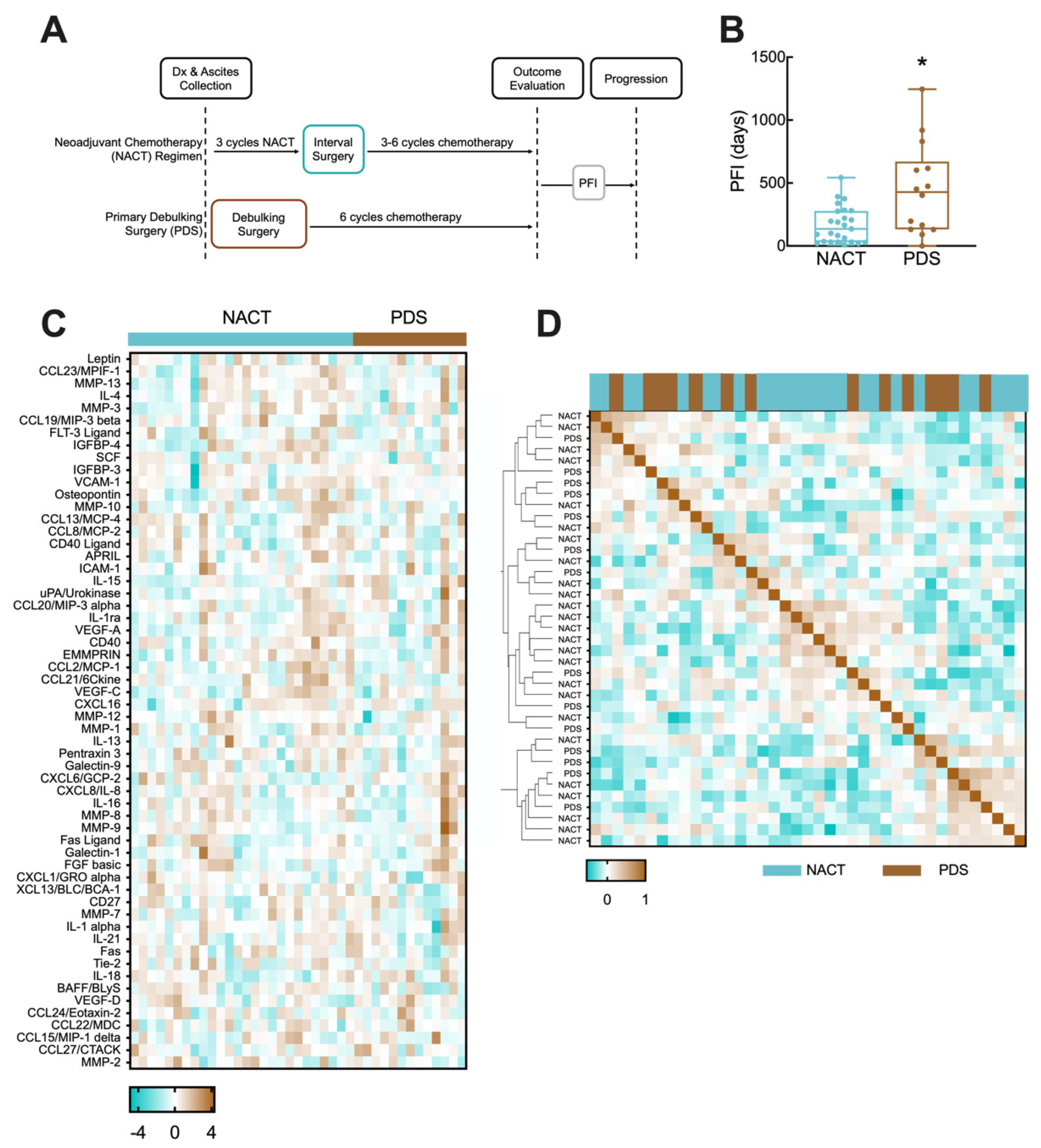
3.1. NACT and PDS Patients in Cohort Have Differential Time to Disease Recurrence
3.2. Regression Model Predicts Disease Recurrence from Ascites Protein Levels for NACT Cohort
3.3. Balancing Model Error Tolerance and Sparsity Increases Model Robustness for NACT Cohort
3.4. PFI Model of Analyte Levels in Ascites from PDS Cohort Depends Less Heavily on Sparsity to Produce Robust Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, H.; Yang, L.; Deng, Y.; Yan, Y.; Wang, S.; Yang, G.; Ma, H. Improved survival in ovarian cancer, with widening survival gaps of races and socioeconomic status: A period analysis, 1983–2012. J. Cancer 2018, 9, 3548–3556. [Google Scholar] [CrossRef] [PubMed]
- Blagden, S.P.; Nicum, S. A source of hope for platinum-resistant ovarian cancer? Lancet 2021, 397, 254–256. [Google Scholar] [CrossRef]
- van Zyl, B.; Tang, D.; Bowden, N.A. Biomarkers of platinum resistance in ovarian cancer: What can we use to improve treatment. Endocr. Relat. Cancer 2018, 25, R303–R318. [Google Scholar] [CrossRef]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Friedlander, M.; Trimble, E.; Tinker, A.; Alberts, D.; Avall-Lundqvist, E.; Brady, M.; Harter, P.; Pignata, S.; Pujade-Lauraine, E.; Sehouli, J.; et al. Gynecologic Cancer I Clinical trials in recurrent ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 771–775. [Google Scholar] [CrossRef]
- Tomao, F.; D’Incalci, M.; Biagioli, E.; Peccatori, F.A.; Colombo, N. Restoring platinum sensitivity in recurrent ovarian cancer by extending the platinum-free interval: Myth or reality? Cancer 2017, 123, 3450–3459. [Google Scholar] [CrossRef]
- Szender, J.B.; Emmons, T.; Belliotti, S.; Dickson, D.; Khan, A.; Morrell, K.; Khan, A.; Singel, K.L.; Mayor, P.C.; Moysich, K.B.; et al. Impact of ascites volume on clinical outcomes in ovarian cancer: A cohort study. Gynecol. Oncol. 2017, 146, 491–497. [Google Scholar] [CrossRef]
- Hwangbo, S.; Kim, S.I.; Kim, J.H.; Eoh, K.J.; Lee, C.; Kim, Y.T.; Suh, D.S.; Park, T.; Song, Y.S. Development of Machine Learning Models to Predict Platinum Sensitivity of High-Grade Serous Ovarian Carcinoma. Cancers 2021, 13, 1875. [Google Scholar] [CrossRef]
- Mankoo, P.K.; Shen, R.; Schultz, N.; Levine, D.A.; Sander, C. Time to recurrence and survival in serous ovarian tumors predicted from integrated genomic profiles. PLoS ONE 2011, 6, e24709. [Google Scholar] [CrossRef]
- Sun, C.Y.; Su, T.F.; Li, N.; Zhou, B.; Guo, E.S.; Yang, Z.Y.; Liao, J.; Ding, D.; Xu, Q.; Lu, H.; et al. chemotherapy response classifier based on support vector machines for high-grade serous ovarian carcinoma. Oncotarget 2016, 7, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.P.; Kuo, K.T.; Chen, C.H.; Chang, M.C.; Lin, H.P.; Hu, Y.H.; Chiang, Y.C.; Cheng, W.F.; Chen, C.A. Developing a Prognostic Gene Panel of Epithelial Ovarian Cancer Patients by a Machine Learning Model. Cancers 2019, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Kapur, A.; Felder, M.; Patankar, M.S.; Kreeger, P.K. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget 2016, 7, 86608–86620. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res. 2018, 78, 3560–3573. [Google Scholar] [CrossRef]
- Cardenas, C.; Montagna, M.K.; Pitruzzello, M.; Lima, E.; Mor, G.; Alvero, A.B. Adipocyte microenvironment promotes Bclxl expression and confers chemoresistance in ovarian cancer cells. Apoptosis 2017, 22, 558–569. [Google Scholar] [CrossRef]
- Saif, M.W.; Siddiqui, I.A.; Sohail, M.A. Management of ascites due to gastrointestinal malignancy. Ann. Saudi Med. 2009, 29, 369–377. [Google Scholar] [CrossRef]
- Fogg, K.C.; Olson, W.R.; Miller, J.N.; Khan, A.; Renner, C.; Hale, I.; Weisman, P.S.; Kreeger, P.K. Alternatively activated macrophage-derived secretome stimulates ovarian cancer spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett. 2019, 458, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fogg, K.C.; Renner, C.M.; Christian, H.; Walker, A.; Marty-Santos, L.; Khan, A.; Olson, W.R.; Parent, C.; O’Shea, A.; Wellik, D.M.; et al. Ovarian Cells Have Increased Proliferation in Response to Heparin-Binding Epidermal Growth Factor as Collagen Density Increases. Tissue Eng. Part A 2020, 26, 747–758. [Google Scholar] [CrossRef]
- Shender, V.O.; Pavlyukov, M.S.; Ziganshin, R.H.; Arapidi, G.P.; Kovalchuk, S.I.; Anikanov, N.A.; Altukhov, I.A.; Alexeev, D.G.; Butenko, I.O.; Shavarda, A.L.; et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol. Cell Proteomics 2014, 13, 3558–3571. [Google Scholar] [CrossRef]
- Bery, A.; Leung, F.; Smith, C.R.; Diamandis, E.P.; Kulasingam, V. Deciphering the ovarian cancer ascites fluid peptidome. Clin. Proteom. 2014, 11, 13. [Google Scholar] [CrossRef]
- Finkernagel, F.; Reinartz, S.; Schuldner, M.; Malz, A.; Jansen, J.M.; Wagner, U.; Worzfeld, T.; Graumann, J.; von Strandmann, E.P.; Müller, R. Dual-platform affinity proteomics identifies links between the recurrence of ovarian carcinoma and proteins released into the tumor microenvironment. Theranostics 2019, 9, 6601–6617. [Google Scholar] [CrossRef]
- Kolomeyevskaya, N.; Eng, K.H.; Khan, A.N.H.; Grzankowski, K.S.; Singel, K.L.; Moysich, K.; Segal, B.H. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-α and interleukin-6 in predicting reduced progression-free survival in epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 352–357. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Rustin, G.J.; Vergote, I.; Eisenhauer, E.; Pujade-Lauraine, E.; Quinn, M.; Thigpen, T.; du Bois, A.; Kristensen, G.; Jakobsen, A.; Sagae, S.; et al. Gynecological Cancer I Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int. J. Gynecol. Cancer 2011, 21, 419–423. [Google Scholar] [CrossRef]
- Carroll, M.J.; Parent, C.R.; Page, D.; Kreeger, P.K. Tumor cell sensitivity to vemurafenib can be predicted from protein expression in a BRAF-V600E basket trial setting. BMC Cancer 2019, 19, 1025. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- Kaplonek, P.; Cizmeci, D.; Fischinger, S.; Collier, A.R.; Suscovich, T.; Linde, C.; Broge, T.; Mann, C.; Amanat, F.; Dayal, D.; et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci. Transl. Med. 2022, 14, eabm2311. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Yang, X.; Chen, C.; Fan, D.; Wu, X.; Si, C.; Xu, Y.; Shao, B.; Chen, Z.; et al. Immune Landscape Refines the Classification of Colorectal Cancer with Heterogeneous Prognosis, Tumor Microenvironment and Distinct Sensitivity to Frontline Therapies. Front. Cell Dev. Biol. 2021, 9, 784199. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Ge, X.; Weng, S.; Dang, Q.; Han, X. Gene Expression Profile Reveals a Prognostic Signature of Non-MSI-H/pMMR Colorectal Cancer. Front. Cell Dev. Biol. 2022, 10, 790214. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Meng, L.; Li, L.; Gao, J.; Yu, S.; Hu, B.; Yang, H.; Guo, W.; Zhang, S. An Integrated Fibrosis Signature for Predicting Survival and Immunotherapy Efficacy of Patients with Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 766609. [Google Scholar] [CrossRef]
- Yeo, I.-K. A new family of power transformations to improve normality or symmetry. Biometrika 2000, 87, 954–959. [Google Scholar] [CrossRef]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Cerne, K.; Hadzialjevic, B.; Skof, E.; Verdenik, I.; Kobal, B. Potential of osteopontin in the management of epithelial ovarian cancer. Radiol. Oncol. 2019, 53, 105–115. [Google Scholar] [CrossRef]
- Kenny, H.A.; Lengyel, E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 2009, 8, 683–688. [Google Scholar] [CrossRef]
- Fu, Z.; Xu, S.; Xu, Y.; Ma, J.; Li, J.; Xu, P. The expression of tumor-derived and stromal-derived matrix metalloproteinase 2 predicted prognosis of ovarian cancer. Int. J. Gynecol. Cancer 2015, 25, 356–362. [Google Scholar] [CrossRef]
- Szubert, S.; Szpurek, D.; Moszynski, R.; Nowicki, M.; Frankowski, A.; Sajdak, S.; Michalak, S. Extracellular matrix metalloproteinase inducer (EMMPRIN) expression correlates positively with active angiogenesis and negatively with basic fibroblast growth factor expression in epithelial ovarian cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Zeng, X.; Gu, H.; Li, M.; Tan, H.; Jin, Z.; Hua, T.; Shi, R.; Wang, H. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32804. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Okuno, T.; Miki, Y.; Tsujio, G.; Sera, T.; Yamamoto, Y.; Kushiyama, S.; Nishimura, S.; Kuroda, K.; Togano, S.; et al. EMMPRIN in extracellular vesicles from peritoneal mesothelial cells stimulates the invasion activity of diffuse-type gastric cancer cells. Cancer Lett. 2021, 521, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nikas, I.P.; Lee, C.; Song, M.J.; Kim, B.; Ryu, H.S. Biomarkers expression among paired serous ovarian cancer primary lesions and their peritoneal cavity metastases in treatment-naive patients: A single-center study. Cancer Med. 2022, 11, 2193–2203. [Google Scholar] [CrossRef]
- Konecny, G.; Untch, M.; Pihan, A.; Kimmig, R.; Gropp, M.; Stieber, P.; Hepp, H.; Slamon, D.; Pegram, M. Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin. Cancer Res. 2001, 7, 1743–1749. [Google Scholar]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef]
- Munakata, S.; Enomoto, T.; Tsujimoto, M.; Otsuki, Y.; Miwa, H.; Kanno, H.; Aozasa, K. Expressions of Fas ligand and other apoptosis-related genes and their prognostic significance in epithelial ovarian neoplasms. Br. J. Cancer 2000, 82, 1446–1452. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Straszewski, S.L.; Kamsteeg, M.; Hanczaruk, B.; Schwartz, P.E.; Rutherford, T.J.; Mor, G. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003, 63, 5573–5581. [Google Scholar]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Akbani, R.; Liu, W.; Ju, Z.; Ling, S.; Vellano, C.P.; Roebuck, P.; Yu, Q.; Eterovic, A.K.; et al. Characterization of Human Cancer Cell Lines by Reverse-phase Protein Arrays. Cancer Cell 2017, 31, 225–239. [Google Scholar] [CrossRef]
- Krstajic, D.; Buturovic, L.J.; Leahy, D.E.; Thomas, S. Cross-validation pitfalls when selecting and assessing regression and classification models. J. Cheminform. 2014, 6, 10. [Google Scholar] [CrossRef]
- Zamarin, D. Novel therapeutics: Response and resistance in ovarian cancer. Int. J. Gynecol. Cancer 2019, 29 (Suppl. S2), s16–s21. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.A. EUS-guided paracentesis and ascitic fluid analysis. Endosc. Ultrasound 2018, 7, 223–227. [Google Scholar] [CrossRef]



| NACT | PDS | Statistical Comparison | |
|---|---|---|---|
| N | 25 | 14 | |
| Age (years) | 65.4 ± 7.0 | 66.1 ± 8.5 | p = 0.73, 2-sided t-test, equal variance |
| Stage III | 14 | 6 | p = 0.51, Fisher’s exact test |
| Stage IV | 11 | 8 | |
| 0–10 mm residual 1 | 16 | 9 | p = 0.47, Fisher’s exact test |
| >10 mm residual | 5 | 5 | |
| <1 L ascites 2 | 5 | 4 | p = 0.68, Fisher’s exact test |
| CA-125 (serum) | 1900 ± 2900 | 2800 ± 4600 | p = 0.53, 2-sided t-test, unequal variance; R2 = −0.16 vs. PFI |
| Total chemotherapy 3 cycles | 6.1 ± 2.5 | 6.8 ± 1.4 | p = 0.26, 2-sided t-test, equal variance |
| Carboplatin 4 | 4 | 1 | p = 0.37, Fisher’s exact test |
| Bevacizumab maintenance | 4 | 3 | p > 0.99, Fisher’s exact test |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carroll, M.J.; Kaipio, K.; Hynninen, J.; Carpen, O.; Hautaniemi, S.; Page, D.; Kreeger, P.K. A Subset of Secreted Proteins in Ascites Can Predict Platinum-Free Interval in Ovarian Cancer. Cancers 2022, 14, 4291. https://doi.org/10.3390/cancers14174291
Carroll MJ, Kaipio K, Hynninen J, Carpen O, Hautaniemi S, Page D, Kreeger PK. A Subset of Secreted Proteins in Ascites Can Predict Platinum-Free Interval in Ovarian Cancer. Cancers. 2022; 14(17):4291. https://doi.org/10.3390/cancers14174291
Chicago/Turabian StyleCarroll, Molly J., Katja Kaipio, Johanna Hynninen, Olli Carpen, Sampsa Hautaniemi, David Page, and Pamela K. Kreeger. 2022. "A Subset of Secreted Proteins in Ascites Can Predict Platinum-Free Interval in Ovarian Cancer" Cancers 14, no. 17: 4291. https://doi.org/10.3390/cancers14174291
APA StyleCarroll, M. J., Kaipio, K., Hynninen, J., Carpen, O., Hautaniemi, S., Page, D., & Kreeger, P. K. (2022). A Subset of Secreted Proteins in Ascites Can Predict Platinum-Free Interval in Ovarian Cancer. Cancers, 14(17), 4291. https://doi.org/10.3390/cancers14174291





