Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Bone Metabolism and Physiopathology of Osteoporosis
3. Physiopathology of Osteoporosis in CCS: General Risk Factors
4. The Contribution of Cancer Therapies to Bone Mass Loss in CCS
4.1. Chemotherapy
4.2. Radiotherapy
4.3. Hematopoietic Stem-Cell Transplantation
5. Bone Loss in CCS with a Different Cancer Diagnosis
5.1. ALL and Lymphoma
5.2. Brain Tumors and Neuroblastoma
5.3. OS and Ewing’s Sarcoma
5.4. Wilm’s Tumor
6. Therapy and Prevention
7. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Isaksson, S.; Bogefors, K.; Akesson, K.; Ora, I.; Egund, L.; Bobjer, J.; Leijonhufvud, I.; Giwercman, A. Low bone mineral density is associated with hypogonadism and cranial irradiation in male childhood cancer survivors. Osteoporos. Int. 2020, 31, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Skinner, R.; Wallace, W.H.; Hjorth, L.; Mulder, R.L.; Wong, F.L.; Yasui, Y.; Bhakta, N.; Constine, L.S.; Bhatia, S.; et al. Surveillance for Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Wakefield, C.E.; Cohn, R.J. Nutritional interventions for survivors of childhood cancer. Cochrane Database Syst. Rev. 2016, 2016, CD009678. [Google Scholar] [CrossRef]
- Bloomhardt, H.M.; Sint, K.; Ross, W.L.; Rotatori, J.; Ness, K.; Robinson, C.; Carpenter, T.O.; Chow, E.J.; Kadan-Lottick, N.S. Severity of reduced bone mineral density and risk of fractures in long-term survivors of childhood leukemia and lymphoma undergoing guideline-recommended surveillance for bone health. Cancer 2020, 126, 202–210. [Google Scholar] [CrossRef] [PubMed]
- den Hoed, M.A.; Klap, B.C.; te Winkel, M.L.; Pieters, R.; van Waas, M.; Neggers, S.J.; Boot, A.M.; Blijdorp, K.; van Dorp, W.; Pluijm, S.M.; et al. Bone mineral density after childhood cancer in 346 long-term adult survivors of childhood cancer. Osteoporos. Int. 2015, 26, 521–529. [Google Scholar] [CrossRef]
- Im, C.; Li, N.; Moon, W.; Liu, Q.; Morton, L.M.; Leisenring, W.M.; Howell, R.M.; Chow, E.J.; Sklar, C.A.; Wilson, C.L.; et al. Genome-wide Association Studies Reveal Novel Locus With Sex-/Therapy-Specific Fracture Risk Effects in Childhood Cancer Survivors. J. Bone Miner. Res. 2021, 36, 685–695. [Google Scholar] [CrossRef]
- Jin, H.Y.; Lee, J.A. Low bone mineral density in children and adolescents with cancer. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 137–144. [Google Scholar] [CrossRef]
- Marcucci, G.; Beltrami, G.; Tamburini, A.; Body, J.J.; Confavreux, C.B.; Hadji, P.; Holzer, G.; Kendler, D.; Napoli, N.; Pierroz, D.D.; et al. Bone health in childhood cancer: Review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann. Oncol. 2019, 30, 908–920. [Google Scholar] [CrossRef]
- van Atteveld, J.E.; Mulder, R.L.; van den Heuvel-Eibrink, M.M.; Hudson, M.M.; Kremer, L.C.M.; Skinner, R.; Wallace, W.H.; Constine, L.S.; Higham, C.E.; Kaste, S.C.; et al. Bone mineral density surveillance for childhood, adolescent, and young adult cancer survivors: Evidence-based recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Diabetes Endo 2021, 9, 622–637. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid. Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Ameri, P.; Cadeddu, C.; Ghigo, A.; Madonna, R.; Marone, G.; Mercurio, V.; Monte, I.; Novo, G.; Parrella, P.; et al. Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Nylander, V.; Ingerslev, L.R.; Zhong, L.; Fabre, O.; Clifford, B.; Johnston, K.; Cohn, R.J.; Barres, R.; Simar, D. T cell epigenetic remodeling and accelerated epigenetic aging are linked to long-term immune alterations in childhood cancer survivors. Clin. Epigenetics 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Sulicka-Grodzicka, J.; Surdacki, A.; Seweryn, M.; Mikolajczyk, T.; Rewiuk, K.; Guzik, T.; Grodzicki, T. Low-grade chronic inflammation and immune alterations in childhood and adolescent cancer survivors: A contribution to accelerated aging? Cancer Med.-Us 2021, 10, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Manem, V.S.K.; Grassberger, C.; Paganetti, H. Predicting Organ-Specific Risk Interactions between Radiation and Chemotherapy in Secondary Cancer Survivors. Cancers 2017, 9, 119. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, S.Y.; Cho, W.K.; Lee, J.W.; Cho, K.S.; Park, S.H.; Hahn, S.H.; Jung, M.H.; Chung, N.G.; Cho, B.; et al. Factors related to decreased bone mineral density in childhood cancer survivors. J. Korean Med. Sci. 2013, 28, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Anam, A.K.; Insogna, K. Update on Osteoporosis Screening and Management. Med. Clin. N. Am. 2021, 105, 1117–1134. [Google Scholar] [CrossRef]
- Goyal, L.; Ajmera, K. Osteoporosis: A Step-by-Step Case-Based Study. Cureus 2022, 14, e23900. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, H.; Liu, T.; Yang, H.; He, F. Insights into the Role of Macrophage Polarization in the Pathogenesis of Osteoporosis. Oxid. Med. Cell. Longev. 2022, 2022, 2485959. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Falchetti, A.; Gennari, L.; Cairoli, E.; Bertoldo, F.; Vescini, F.; Scillitani, A.; Chiodini, I. Evaluation of bone fragility in endocrine disorders. Eur. J. Endocrinol. 2019, 180, R213–R232. [Google Scholar] [CrossRef]
- Faulkner, R.A.; Bailey, D.A. Osteoporosis: A pediatric concern? Med. Sport Sci. 2007, 51, 1–12. [Google Scholar]
- Bonjour, J.P.; Chevalley, T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr. Rev. 2014, 35, 820–847. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, D.M.; Coutinho, F.L.; Toledo, R.A.; Montenegro, F.L.M.; Correia-Deur, J.E.M.; Toledo, S.P.A. Early-Onset, Progressive, Frequent, Extensive, and Severe Bone Mineral and Renal Complications in Multiple Endocrine Neoplasia Type 1-Associated Primary Hyperparathyroidism. J. Bone Miner. Res. 2010, 25, 2382–2391. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H. Factors influencing peak bone mass gain. Front. Med. 2021, 15, 53–69. [Google Scholar] [CrossRef]
- Guler-Yuksel, M.; Hoes, J.N.; Bultink, I.E.M.; Lems, W.F. Glucocorticoids, Inflammation and Bone. Calcif. Tissue Int. 2018, 102, 592–606. [Google Scholar] [CrossRef]
- McLaughlin, F.; Mackintosh, J.; Hayes, B.P.; McLaren, A.; Uings, I.J.; Salmon, P.; Humphreys, J.; Meldrum, E.; Farrow, S.N. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone 2002, 30, 924–930. [Google Scholar] [CrossRef]
- Rossi, F.; Perrotta, S.; Bellini, G.; Luongo, L.; Tortora, C.; Siniscalco, D.; Francese, M.; Torella, M.; Nobili, B.; Di Marzo, V.; et al. Iron overload causes osteoporosis in thalassemia major patients through interaction with transient receptor potential vanilloid type 1 (TRPV1) channels. Haematologica 2014, 99, 1876–1884. [Google Scholar] [CrossRef]
- Wang, F.S.; Ko, J.Y.; Weng, L.H.; Yeh, D.W.; Ke, H.J.; Wu, S.L. Inhibition of glycogen synthase kinase-3beta attenuates glucocorticoid-induced bone loss. Life Sci. 2009, 85, 685–692. [Google Scholar] [CrossRef]
- Wang, F.S.; Ko, J.Y.; Yeh, D.W.; Ke, H.C.; Wu, H.L. Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 2008, 149, 1793–1801. [Google Scholar] [CrossRef]
- Wilson-Barnes, S.L.; Lanham-New, S.A.; Lambert, H. Modifiable risk factors for bone health & fragility fractures. Best Pract. Res. Clin. Rheumatol. 2022, 2022, 101758. [Google Scholar]
- Whittier, X.; Saag, K.G. Glucocorticoid-induced Osteoporosis. Rheum Dis. Clin. N. Am. 2016, 42, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Tu, J.; Zhang, Z. Ferroptosis: A New Regulatory Mechanism in Osteoporosis. Oxid. Med. Cell. Longev. 2022, 2022, 2634431. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, D.; Luo, X.; Zhou, J.; Shang, P.; Zhang, H. Iron Overload-Induced Osteocyte Apoptosis Stimulates Osteoclast Differentiation Through Increasing Osteocytic RANKL Production In Vitro. Calcif. Tissue Int. 2020, 107, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, W.; Shoaib, S.; Fatma, R.; Zaka-Ur-Rab, Z.; Afzal, M. Beta-thalassemia and the advent of new interventions beyond transfusion and iron chelation. Br. J. Clin. Pharmacol. 2022, 88, 3610–3626. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar]
- Rizzoli, R.; Body, J.J.; Brandi, M.L.; Cannata-Andia, J.; Chappard, D.; El Maghraoui, A.; Gluer, C.C.; Kendler, D.; Napoli, N.; Papaioannou, A.; et al. International Osteoporosis Foundation Committee of Scientific Advisors Working Group on Cancer-Induced Bone, D. Cancer-associated bone disease. Osteoporos. Int. 2013, 24, 2929–2953. [Google Scholar] [CrossRef]
- Kann, P.H.; Bartsch, D.; Langer, P.; Waldmann, J.; Hadji, P.; Pfutzner, A.; Klusener, J. Peripheral bone mineral density in correlation to disease-related predisposing conditions in patients with multiple endocrine neoplasia type 1. J. Endocrinol. Investig. 2012, 35, 573–579. [Google Scholar]
- Cooper, C.; Westlake, S.; Harvey, N.; Javaid, K.; Dennison, E.; Hanson, M. Review: Developmental origins of osteoporotic fracture. Osteoporos. Int. 2006, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lim, J.S. Bone mineral density deficits in childhood cancer survivors: Pathophysiology, prevalence, screening, and management. Korean J. Pediatr. 2013, 56, 60–67. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Freeman, C.; Bernstein, M.L.; Lawrence, S.; Rodd, C. Osteopenia in children who have undergone posterior fossa or craniospinal irradiation for brain tumors. Arch. Pediat. Adol. Med. 2004, 158, 491–496. [Google Scholar] [CrossRef]
- Arikoski, P.; Kroger, H.; Riikonen, P.; Parviainen, M.; Voutilainen, R.; Komulainen, J. Disturbance in bone turnover in children with a malignancy at completion of chemotherapy. Med. Pediatr. Oncol. 1999, 33, 455–461. [Google Scholar] [CrossRef]
- Mora, S.; Gilsanz, V. Establishment of peak bone mass. Endocrin. Metab. Clin. 2003, 32, 39–63. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Hwang, Y.C.; Chung, H.Y.; Woo, J.T. Vitamin D and diabetes in Koreans: Analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2009. Diabetic. Med. 2012, 29, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, V.; Darlington, A.S.; Eiser, C.; Bishop, N.J.; Davies, H.A. Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J. Bone Miner. Res. 2002, 17, 1073–1080. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Hudson, M.M.; Landier, W. Survivorship: Childhood Cancer Survivors. Prim. Care 2009, 36, 743–780. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A. Normal acquisition and loss of bone mass. Horm. Res. 2003, 60 (Suppl. 3), 71–76. [Google Scholar] [CrossRef] [PubMed]
- Modan-Moses, D.; Pinhas-Hamiel, O.; Munitz-Shenkar, D.; Temam, V.; Kanety, H.; Toren, A. Vitamin D status in pediatric patients with a history of malignancy. Pediatr. Res. 2012, 72, 620–624. [Google Scholar] [CrossRef]
- Bhandari, R.; Teh, J.B.; Herrera, C.; Echevarria, M.; Lindenfeld, L.; Wong, F.L.; Wilson, K.; Armenian, S.H. Prevalence and risk factors for vitamin D deficiency in long-term childhood cancer survivors. Pediatr. Blood Cancer 2021, 68, e29048. [Google Scholar] [CrossRef]
- Anderson, P.H.; Atkins, G.J.; Turner, A.G.; Kogawa, M.; Findlay, D.M.; Morris, H.A. Vitamin D metabolism within bone cells: Effects on bone structure and strength. Mol. Cell Endocrinol. 2011, 347, 42–47. [Google Scholar] [CrossRef]
- Anderson, P.H.; Lam, N.N.; Turner, A.G.; Davey, R.A.; Kogawa, M.; Atkins, G.J.; Morris, H.A. The pleiotropic effects of vitamin D in bone. J. Steroid Biochem. Mol. Biol. 2013, 136, 190–194. [Google Scholar] [CrossRef]
- Delvin, E.; Alos, N.; Rauch, F.; Marcil, V.; Morel, S.; Boisvert, M.; Lecours, M.A.; Laverdiere, C.; Sinnett, D.; Krajinovic, M.; et al. Vitamin D nutritional status and bone turnover markers in childhood acute lymphoblastic leukemia survivors: A PETALE study. Clin. Nutr. 2019, 38, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L.; Ness, K.K. Bone mineral density deficits and fractures in survivors of childhood cancer. Curr. Osteoporos. Rep. 2013, 11, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, W.D.; Clipp, E.C.; Miller, P.E.; Jones, L.W.; Ward, D.S.; Bonner, M.J.; Rosoff, P.M.; Snyder, D.C.; Demark-Wahnefried, W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol. Nurs. Forum. 2008, 35, 121–130. [Google Scholar] [CrossRef]
- Nathan, P.C.; Ford, J.S.; Henderson, T.O.; Hudson, M.M.; Emmons, K.M.; Casillas, J.N.; Lown, E.A.; Ness, K.K.; Oeffinger, K.C. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2363–2373. [Google Scholar] [CrossRef] [Green Version]
- Tota-Maharaj, R.; Defilippis, A.P.; Blumenthal, R.S.; Blaha, M.J. A practical approach to the metabolic syndrome: Review of current concepts and management. Curr. Opin. Cardiol. 2010, 25, 502–512. [Google Scholar] [CrossRef]
- Ferrari, S.; Bianchi, M.L.; Eisman, J.A.; Foldes, A.J.; Adami, S.; Wahl, D.A.; Stepan, J.J.; de Vernejoul, M.C.; Kaufman, J.M.; IOF Committee of Scientific Advisors Working Group on Osteoporosis Pathophysiology. Osteoporosis in young adults: Pathophysiology, diagnosis, and management. Osteoporos. Int. 2012, 23, 2735–2748. [Google Scholar] [CrossRef] [PubMed]
- Makitie, O.; Heikkinen, R.; Toiviainen-Salo, S.; Henriksson, M.; Puukko-Viertomies, L.R.; Jahnukainen, K. Long-term skeletal consequences of childhood acute lymphoblastic leukemia in adult males: A cohort study. Eur. J. Endocrinol. 2013, 168, 281–288. [Google Scholar] [CrossRef]
- Fontana, A.; Matthey, S.; Mayor, C.; Dufour, C.; Destaillats, A.; Ballabeni, P.; Maeder, S.; Newman, C.J.; Beck Popovic, M.; Renella, R.; et al. PASTEC—A prospective, single-center, randomized, cross-over trial of pure physical versus physical plus attentional training in children with cancer. Pediatr. Hematol. Oncol. 2021, 39, 329–342. [Google Scholar] [CrossRef]
- Beller, R.; Bennstein, S.B.; Gotte, M. Effects of Exercise Interventions on Immune Function in Children and Adolescents With Cancer and HSCT Recipients—A Systematic Review. Front. Immunol. 2021, 12, 746171. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yoo, S.; Yeon, S.J.; Min, J.H.; Kim, D.I.; Lee, J.W.; Han, J.W.; Lyu, C.J.; Jeon, J.Y. Physical activity levels, exercise preferences, and exercise barriers in Korean children and adolescents after cancer treatment. Support. Care Cancer 2022, 30, 1787–1796. [Google Scholar] [CrossRef]
- te Winkel, M.L.; de Muinck Keizer-Schrama, S.M.; de Jonge, R.; van Beek, R.D.; van der Sluis, I.M.; Hop, W.C.; Pieters, R.; van den Heuvel-Eibrink, M.M. Germline variation in the MTHFR and MTRR genes determines the nadir of bone density in pediatric acute lymphoblastic leukemia: A prospective study. Bone 2011, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- den Hoed, M.A.; Pluijm, S.M.; Stolk, L.; Uitterlinden, A.G.; Pieters, R.; van den Heuvel-Eibrink, M.M. Genetic variation and bone mineral density in long-term adult survivors of childhood cancer. Pediatr. Blood Cancer 2016, 63, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- van Atteveld, J.E.; Pluijm, S.M.F.; Ness, K.K.; Hudson, M.M.; Chemaitilly, W.; Kaste, S.C.; Robison, L.L.; Neggers, S.; Yasui, Y.; van den Heuvel-Eibrink, M.M.; et al. Prediction of Low and Very Low Bone Mineral Density Among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2019, 37, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Lequin, M.H.; van der Shuis, I.M.; Van Rijn, R.R.; Hop, W.C.; van ven Huevel-Eibrink, M.M.; MuinckKeizer-Schrama, S.M.; van Kuijk, C. Bone mineral assessment with tibial ultrasonometry and dual-energy X-ray absorptiometry in long-term survivors of acute lymphoblastic leukemia in childhood. J. Clin. Densitom. 2002, 5, 167–173. [Google Scholar] [CrossRef]
- Thomas, I.H.; Donohue, J.E.; Ness, K.K.; Dengel, D.R.; Baker, K.S.; Gurney, J.G. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer 2008, 113, 3248–3256. [Google Scholar] [CrossRef]
- Oskarsson, T.; Duun-Henriksen, A.K.; Bautz, A.; Montgomery, S.; Harila-Saari, A.; Petersen, C.; Niinimaki, R.; Madanat-Harjuoja, L.; Tryggvadottir, L.; Holmqvist, A.S.; et al. Skeletal adverse events in childhood cancer survivors: An Adult Life after Childhood Cancer in Scandinavia cohort study. Int. J. Cancer 2021, 149, 1863–1876. [Google Scholar] [CrossRef] [PubMed]
- Aisenberg, J.; Hsieh, K.; Kalaitzoglou, G.; Whittam, E.; Heller, G.; Schneider, R.; Sklar, C. Bone mineral density in young adult survivors of childhood cancer. J. Pediatr. Hematol. Oncol. 1998, 20, 241–245. [Google Scholar] [CrossRef]
- French, S.A.; Fulkerson, J.A.; Story, M. Increasing weight-bearing physical activity and calcium intake for bone mass growth in children and adolescents: A review of intervention trials. Prev. Med. 2000, 31, 722–731. [Google Scholar] [CrossRef]
- Inaba, H.; Cao, X.; Han, A.Q.; Panetta, J.C.; Ness, K.K.; Metzger, M.L.; Rubnitz, J.E.; Ribeiro, R.C.; Sandlund, J.T.; Jeha, S.; et al. Bone mineral density in children with acute lymphoblastic leukemia. Cancer 2018, 124, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, I.M.; van den Heuvel-Eibrink, M.M.; Hahlen, K.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med. Pediatr. Oncol. 2000, 35, 415–420. [Google Scholar] [CrossRef]
- Al-Tonbary, Y.A.; El-Ziny, M.A.; Elsharkawy, A.A.; El-Hawary, A.K.; El-Ashry, R.; Fouda, A.E. Bone mineral density in newly diagnosed children with neuroblastoma. Pediatr. Blood Cancer 2011, 56, 202–205. [Google Scholar] [CrossRef]
- Holzer, G.; Krepler, P.; Koschat, M.A.; Grampp, S.; Dominkus, M.; Kotz, R. Bone mineral density in long-term survivors of highly malignant osteosarcoma. J. Bone Joint Surg. Br. 2003, 85, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Winter, C.C.; Rosenbaum, D.; Boos, J.; Gosheger, G.; Hardes, J.; Vieth, V. Early decrements in bone density after completion of neoadjuvant chemotherapy in pediatric bone sarcoma patients. BMC Musculoskelet. Disord. 2010, 11, 287. [Google Scholar] [CrossRef]
- Ruza, E.; Sierrasesumaga, L.; Azcona, C.; Patino-Garcia, A. Bone mineral density and bone metabolism in children treated for bone sarcomas. Pediatr. Res. 2006, 59, 866–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, Y.M.; Fatayerji, D.; Eastell, R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: Relative contributions of bone size and volumetric bone mineral density. Osteoporos. Int. 2004, 15, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.A.; Ma, J.; Fernandez, C.V.; Halton, J.; Alos, N.; Miettunen, P.M.; Jaremko, J.L.; Ho, J.; Shenouda, N.; Matzinger, M.A.; et al. Incident Vertebral Fractures in Children With Leukemia During the Four Years Following Diagnosis. J. Clin. Endocrinol. Metab. 2015, 100, 3408–3417. [Google Scholar] [CrossRef]
- Wilson, C.L.; Dilley, K.; Ness, K.K.; Leisenring, W.L.; Sklar, C.A.; Kaste, S.C.; Stovall, M.; Green, D.M.; Armstrong, G.T.; Robison, L.L.; et al. Fractures among long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2012, 118, 5920–5928. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Di Paola, A.; Pota, E.; Argenziano, M.; Di Pinto, D.; Marrapodi, M.M.; Di Leva, C.; Di Martino, M.; Tortora, C. Biological Aspects of Inflamm-Aging in Childhood Cancer Survivors. Cancers 2021, 13, 4933. [Google Scholar] [CrossRef]
- van der Sluis, I.M.; van den Heuvel-Eibrink, M.M.; Hahlen, K.; Krenning, E.P.; de Muinck Keizer-Schrama, S.M. Altered bone mineral density and body composition, and increased fracture risk in childhood acute lymphoblastic leukemia. J. Pediatr. 2002, 141, 204–210. [Google Scholar] [CrossRef]
- Mancuso, S.; Scaturro, D.; Santoro, M.; Di Gaetano, G.; Vitagliani, F.; Falco, V.; Siragusa, S.; Gonnelli, S.; Mauro, G.L. Bone damage after chemotherapy for lymphoma: A real-world experience. BMC Musculoskelet Disord. 2021, 22, 1024. [Google Scholar] [CrossRef]
- Kendler, D.L.; Body, J.J.; Brandi, M.L.; Broady, R.; Cannata-Andia, J.; Cannata-Ortiz, M.J.; El Maghraoui, A.; Guglielmi, G.; Hadji, P.; Pierroz, D.D.; et al. Bone management in hematologic stem cell transplant recipients. Osteoporos. Int. 2018, 29, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H. Update on Management of the Oral and Maxillofacial Surgery Patient on Corticosteroids. Oral. Maxillofac. Surg. Clin. N. Am. 2022, 34, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, M.; Zhou, H.; Seibel, M. MECHANISMS IN ENDOCRINOLOGY: Local and systemic effects of glucocorticoids on metabolism: New lessons from animal models. Eur. J. Endocrinol. 2021, 185, R113–R129. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Jones-Wallace, D.; Rose, S.R.; Boyett, J.M.; Lustig, R.H.; Rivera, G.K.; Pui, C.H.; Hudson, M.M. Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: Frequency of occurrence and risk factors for their development. Leukemia 2001, 15, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Rizzoli, R.; Biver, E. Glucocorticoid-induced osteoporosis: Who to treat with what agent? Nat. Rev. Rheumatol. 2015, 11, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Frieze, D.A. Musculoskeletal pain associated with corticosteroid therapy in cancer. Curr. Pain Headache Rep. 2010, 14, 256–260. [Google Scholar] [CrossRef]
- Klein, G.L.; Xie, Y.; Qin, Y.X.; Lin, L.; Hu, M.; Enkhbaatar, P.; Bonewald, L.F. Preliminary evidence of early bone resorption in a sheep model of acute burn injury: An observational study. J. Bone Miner. Metab. 2014, 32, 136–141. [Google Scholar] [CrossRef]
- Fiscaletti, M.; Samoilenko, M.; Dubois, J.; Miron, M.C.; Lefebvre, G.; Krajinovic, M.; Laverdiere, C.; Sinnett, D.; Alos, N. Predictors of Vertebral Deformity in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia: The PETALE Study. J. Clin. Endocrinol. Metab. 2021, 106, 512–525. [Google Scholar] [CrossRef]
- Davies, J.H.; Evans, B.A.; Jenney, M.E.; Gregory, J.W. Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin. Endocrinol. (Oxf.) 2005, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Stovall, M.; Robison, L.L. Long-Term Effects of Radiation Exposure among Adult Survivors of Childhood Cancer: Results from the Childhood Cancer Survivor Study. Radiat. Res. 2010, 174, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J.; Diel, I.J. Osteoporosis due to cancer treatment: Pathogenesis and management. J. Clin. Oncol. 2000, 18, 1570–1593. [Google Scholar] [CrossRef] [PubMed]
- van Santen, H.M.; Van den Heuvel-Eibrink, M.M.; van de Wetering, M.D.; Wallace, W.H. Hypogonadism in Children with a Previous History of Cancer: Endocrine Management and Follow-Up. Horm. Res. Paediat. 2019, 91, 93–103. [Google Scholar] [CrossRef]
- Pollock, N.I.; Cohen, L.E. Growth Hormone Deficiency and Treatment in Childhood Cancer Survivors. Front. Endocrinol. 2021, 12, 745932. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.J.; Sangiao-Alvarellos, S.; Cordido, F. Treatment with Growth Hormone for Adults with Growth Hormone Deficiency Syndrome: Benefits and Risks. Int. J. Mol. Sci. 2018, 19, 893. [Google Scholar] [CrossRef] [Green Version]
- Song, A.L.; Fish, J.D. Caring for survivors of childhood cancer: It takes a village. Curr. Opin. Pediatr. 2018, 30, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Late Effects of Treatment for Childhood Cancer (PDQ(R)): Patient Version. In PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2002.
- Chemaitilly, W.; Cohen, L.E.; Mostoufi-Moab, S.; Patterson, B.C.; Simmons, J.H.; Meacham, L.R.; van Santen, H.M.; Sklar, C.A. Endocrine Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Remes, T.M.; Arikoski, P.M.; Lahteenmaki, P.M.; Arola, M.O.; Pokka, T.M.L.; Riikonen, V.P.; Sirkia, K.H.; Rantala, H.M.J.; Harila-Saari, A.H.; Ojaniemi, M.K. Bone mineral density is compromised in very long-term survivors of irradiated childhood brain tumor. Acta Oncol. 2018, 57, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Ott, S.M.; Lewiecki, E.M.; Sarafoglou, K.; Wu, J.Y.; Thompson, M.J.; Vaux, J.J.; Dean, D.R.; Saag, K.G.; Hashmi, S.K.; et al. Bone Health Management After Hematopoietic Cell Transplantation: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Tr. 2020, 26, 1784–1802. [Google Scholar] [CrossRef]
- Anandi, P.; Jain, N.A.; Tian, X.; Wu, C.O.; Pophali, P.A.; Koklanaris, E.; Ito, S.; Savani, B.N.; Barrett, J.; Battiwalla, M. Factors influencing the late phase of recovery after bone mineral density loss in allogeneic stem cell transplantation survivors. Bone Marrow Transpl. 2016, 51, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Savani, B.N.; Donohue, T.; Kozanas, E.; Shenoy, A.; Singh, A.K.; Childs, R.W.; Barett, A.J. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol. Blood Marrow Tr. 2007, 13, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; McCarthy, P.L.; Dunford, L.M.; Roy, D.M.; Brown, K.; Paplham, P.; Syta, M.; Lamonica, D.; Smiley, S.; Battiwalla, M.; et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transpl. 2008, 41, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Georges, G.E.; Bar, M.; Onstad, L.; Yi, J.C.; Shadman, M.; Flowers, M.E.; Carpenter, P.A.; Stewart, S.; Lee, S.J.; Holmberg, L.A. Survivorship after Autologous Hematopoietic Cell Transplantation for Lymphoma and Multiple Myeloma: Late Effects and Quality of Life. Biol. Blood Marrow Tr. 2020, 26, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.; Helg, C.; Collao, C.; Chapuis, B.; Slosman, D.; Bonjour, J.P.; Rizzoli, R. Allogeneic bone marrow transplantation is associated with a preferential femoral neck bone loss. Osteoporos. Int. 2001, 12, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Moesch, M.; Zumsteg, M.; Struja, T.; Bernet, S.; Medinger, M.; Mueller, B.; Passweg, J.; Bargetzi, M.; Schuetz, P. Predictors of impaired bone health in long-term survivors after allogeneic stem cell transplantation. Bone Marrow Transpl. 2019, 54, 1651–1661. [Google Scholar] [CrossRef]
- Lin, J.N.; Chen, H.J.; Yang, C.H.; Lai, C.H.; Lin, H.H.; Chang, C.S.; Liang, J.A. Risk of osteoporosis and pathologic fractures in cancer patients who underwent hematopoietic stem cell transplantation: A nationwide retrospective cohort study. Oncotarget 2017, 8, 34811–34819. [Google Scholar] [CrossRef]
- Mielcarek, M.; Furlong, T.; O’Donnell, P.V.; Storer, B.E.; McCune, J.S.; Storb, R.; Carpenter, P.A.; Flowers, M.E.; Appelbaum, F.R.; Martin, P.J. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016, 127, 1502–1508. [Google Scholar] [CrossRef]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef]
- Pundole, X.; Murphy, W.A.; Ebede, C.C.; Karim, E.; Manocha, S.; Don-Pedro, D.; Rondon, G.; Leung, C.H.; Liu, S.Y.; Du, X.L.L.; et al. Fracture risk prediction using FRAX in patients following hematopoietic stem cell transplantation. Arch. Osteoporos. 2018, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.A.; Halton, J.M.; Bradley, C.; Wu, B.; Barr, R.D. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: Influence of disease, drugs and nutrition. Int. J. Cancer Suppl. 1998, 11, 35–39. [Google Scholar] [CrossRef]
- Warner, J.T.; Evans, W.D.; Webb, D.K.; Bell, W.; Gregory, J.W. Relative osteopenia after treatment for acute lymphoblastic leukemia. Pediatr. Res. 1999, 45 Pt 1, 544–551. [Google Scholar] [CrossRef]
- Bhojwani, D.; Yang, J.J.; Pui, C.H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef]
- Halton, J.; Gaboury, I.; Grant, R.; Alos, N.; Cummings, E.A.; Matzinger, M.; Shenouda, N.; Lentle, B.; Abish, S.; Atkinson, S.; et al. Advanced Vertebral Fracture Among Newly Diagnosed Children With Acute Lymphoblastic Leukemia: Results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) Research Program. J. Bone Miner. Res. 2009, 24, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Hogler, W.; Wehl, G.; van Staa, T.; Meister, B.; Klein-Franke, A.; Kropshofer, G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: Comparison of fracture risk with the General Practice Research Database. Pediatr. Blood Cancer 2007, 48, 21–27. [Google Scholar] [CrossRef]
- Jarfelt, M.; Fors, H.; Lannering, B.; Bjarnason, R. Bone mineral density and bone turnover in young adult survivors of childhood acute lymphoblastic leukaemia. Eur. J. Endocrinol. 2006, 154, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Marinovic, D.; Dorgeret, S.; Lescoeur, B.; Alberti, C.; Noel, M.; Czernichow, P.; Sebag, G.; Vilmer, E.; Leger, J. Improvement in bone mineral density and body composition in survivors of childhood acute lymphoblastic leukemia: A 1-year prospective study. Pediatrics 2005, 116, e102–e108. [Google Scholar] [CrossRef]
- Mostoufi-Moab, S.; Kelly, A.; Mitchell, J.A.; Baker, J.; Zemel, B.S.; Brodsky, J.; Long, J.; Leonard, M.B. Changes in pediatric DXA measures of musculoskeletal outcomes and correlation with quantitative CT following treatment of acute lymphoblastic leukemia. Bone 2018, 112, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.; Marshall, J.A.; Baron, A.E.; Krebs, N.F.; Hambidge, K.M.; Albano, E. Normal bone mineral density after treatment for childhood acute lymphoblastic leukemia diagnosed between 1991 and 1998. J. Pediatr. 2001, 138, 898–904. [Google Scholar] [CrossRef]
- Mandel, K.; Atkinson, S.; Barr, R.D.; Pencharz, P. Skeletal morbidity in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2004, 22, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Bunch, K.J.; Kendall, G.M.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Case-control study of paternal occupational exposures and childhood lymphoma in Great Britain, 1962–2010. Br. J. Cancer 2019, 120, 1153–1161. [Google Scholar] [CrossRef]
- Benmiloud, S.; Steffens, M.; Beauloye, V.; de Wandeleer, A.; Devogelaer, J.P.; Brichard, B.; Vermylen, C.; Maiter, D. Long-term effects on bone mineral density of different therapeutic schemes for acute lymphoblastic leukemia or non-Hodgkin lymphoma during childhood. Horm. Res. Paediatr. 2010, 74, 241–250. [Google Scholar] [CrossRef]
- Sala, A.; Talsma, D.; Webber, C.; Posgate, S.; Atkinson, S.; Barr, R. Bone mineral status after treatment of malignant lymphoma in childhood and adolescence. Eur. J. Cancer Care (Engl.) 2007, 16, 373–379. [Google Scholar] [CrossRef] [PubMed]
- van Beek, R.D.; van den Heuvel-Eibrink, M.M.; Hakvoort-Cammel, F.G.; van den Bos, C.; van der Pal, H.J.; Krenning, E.P.; de Rijke, Y.B.; Pieters, R.; de Muinck Keizer-Schrama, S.M. Bone mineral density, growth, and thyroid function in long-term survivors of pediatric Hodgkin’s lymphoma treated with chemotherapy only. J. Clin. Endocrinol. Metab. 2009, 94, 1904–1909. [Google Scholar] [CrossRef]
- Pollack, I.F.; Agnihotri, S.; Broniscer, A. Childhood brain tumors: Current management, biological insights, and future directions. J. Neurosurg. Pediatr. 2019, 23, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef]
- Pietila, S.; Sievanen, H.; Ala-Houhala, M.; Koivisto, A.M.; Liisa Lenko, H.; Makipernaa, A. Bone mineral density is reduced in brain tumour patients treated in childhood. Acta Paediatr. 2006, 95, 1291–1297. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, S.M.; Lee, Y.A.; Shin, C.H.; Yang, S.W.; Lim, J.S. Risk factors for osteoporosis in long-term survivors of intracranial germ cell tumors. Osteoporos. Int. 2012, 23, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.E.; Gordon, J.H.; Popovsky, E.Y.; Sainath, N.N.; Feldman, H.A.; Kieran, M.W.; Gordon, C.M. Bone density in post-pubertal adolescent survivors of childhood brain tumors. Pediatr. Blood Cancer 2012, 58, 959–963. [Google Scholar] [CrossRef]
- Gurney, J.G.; Kadan-Lottick, N.S.; Packer, R.J.; Neglia, J.P.; Sklar, C.A.; Punyko, J.A.; Stovall, M.; Yasui, Y.; Nicholson, H.S.; Wolden, S.; et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 2003, 97, 663–673. [Google Scholar] [CrossRef]
- Utriainen, P.; Vatanen, A.; Toiviainen-Salo, S.; Saarinen-Pihkala, U.; Makitie, O.; Jahnukainen, K. Skeletal outcome in long-term survivors of childhood high-risk neuroblastoma treated with high-dose therapy and autologous stem cell rescue. Bone Marrow Transpl. 2017, 52, 711–716. [Google Scholar] [CrossRef]
- Gawade, P.L.; Hudson, M.M.; Kaste, S.C.; Neglia, J.P.; Wasilewski-Masker, K.; Constine, L.S.; Robison, L.L.; Ness, K.K. A systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr. Pediatr. Rev. 2014, 10, 249–262. [Google Scholar] [CrossRef]
- Argenziano, M.; Tortora, C.; Pota, E.; Di Paola, A.; Di Martino, M.; Di Leva, C.; Di Pinto, D.; Rossi, F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals 2021, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Bellini, G.; Di Pinto, D.; Tortora, C.; Manzo, I.; Punzo, F.; Casale, F.; Rossi, F. The Role of Mifamurtide in Chemotherapy-induced Osteoporosis of Children with Osteosarcoma. Curr. Cancer Drug. Targets 2017, 17, 650–656. [Google Scholar] [CrossRef]
- Punzo, F.; Tortora, C.; Argenziano, M.; Pinto, D.D.; Pota, E.; Martino, M.D.; Paola, A.D.; Rossi, F. Can Denosumab be used in combination with Doxorubicin in Osteosarcoma? Oncotarget 2020, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Manzo, I.; Bellini, G.; Casale, F.; Rossi, F. Anti-proliferative, pro-apoptotic and anti-invasive effect of EC/EV system in human osteosarcoma. Oncotarget 2017, 8, 54459–54471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punzo, F.; Tortora, C.; Di Pinto, D.; Pota, E.; Argenziano, M.; Di Paola, A.; Casale, F.; Rossi, F. Bortezomib and endocannabinoid/endovanilloid system: A synergism in osteosarcoma. Pharmacol. Res. 2018, 137, 25–33. [Google Scholar] [CrossRef]
- Grunewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Alava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing sarcoma. Nat. Rev. Dis. Primers 2018, 4, 5. [Google Scholar] [CrossRef]
- Luetke, A.; Meyers, P.A.; Lewis, I.; Juergens, H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 2014, 40, 523–532. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, D.H.; Lee, J.A.; Kim, D.H.; Cho, J.; Cho, W.H.; Lee, S.Y.; Jeon, D.G. Young age at diagnosis, male sex, and decreased lean mass are risk factors of osteoporosis in long-term survivors of osteosarcoma. J. Pediatr. Hematol. Oncol. 2013, 35, 54–60. [Google Scholar] [CrossRef]
- Pirker-Fruhauf, U.M.; Friesenbichler, J.; Urban, E.C.; Obermayer-Pietsch, B.; Leithner, A. Osteoporosis in children and young adults: A late effect after chemotherapy for bone sarcoma. Clin. Orthop. Relat. Res. 2012, 470, 2874–2885. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, W.H.; Lee, J.A.; Kim, D.H.; Seo, J.H.; Lim, J.S. Bone mineral density change during adjuvant chemotherapy in pediatric osteosarcoma. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Servaes, S.E.; Hoffer, F.A.; Smith, E.A.; Khanna, G. Imaging of Wilms tumor: An update. Pediatr. Radiol. 2019, 49, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Othman, F.; Guo, C.Y.; Webber, C.; Atkinson, S.A.; Barr, R.D. Osteopenia in survivors of Wilms tumor. Int. J. Oncol. 2002, 20, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C.A.K.; Murphy-Alford, A.J.; Cohen, J.; Fleming, M.R.; Wakefield, C.E.; Naumann, F. Poor diet quality and adverse eating behaviors in young survivors of childhood cancer. Pediatr. Blood Cancer 2022, 69, e29408. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Abrams, S.A.; Committee on Nutrition; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; de Ferranti, S.D.; Golden, N.H.; Magge, S.N.; Schwarzenberg, S.J.; et al. Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, F.; Bishop, N. Osteogenesis imperfecta in children. Bone 2021, 148, 115914. [Google Scholar] [CrossRef]
- Soares, A.P.; do Espirito Santo, R.F.; Line, S.R.; Pinto, M.; Santos Pde, M.; Toralles, M.B.; do Espirito Santo, A.R. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ. Toxicol. Pharmacol. 2016, 42, 212–217. [Google Scholar] [CrossRef]
- Nasomyont, N.; Hornung, L.N.; Gordon, C.M.; Wasserman, H. Outcomes following intravenous bisphosphonate infusion in pediatric patients: A 7-year retrospective chart review. Bone 2019, 121, 60–67. [Google Scholar] [CrossRef]
- August, K.J.; Dalton, A.; Katzenstein, H.M.; George, B.; Olson, T.A.; Wasilewski-Masker, K.; Rapkin, L.B. The Use of Zoledronic Acid in Pediatric Cancer Patients. Pediatr. Blood Cancer 2011, 56, 610–614. [Google Scholar] [CrossRef]
- Liu, L.; Geng, H.; Mei, C.J.; Chen, L.B. Zoledronic Acid Enhanced the Antitumor Effect of Cisplatin on Orthotopic Osteosarcoma by ROS-PI3K/AKT Signaling and Attenuated Osteolysis. Oxid. Med. Cell Longev. 2021, 2021, 6661534. [Google Scholar] [CrossRef]
- Lim, S.W.; Ahn, J.H.; Choi, A.; Cho, W.H.; Lee, J.A.; Kim, D.H.; Seo, J.H.; Lim, J.S. Efficacy of pamidronate in pediatric osteosarcoma patients with low bone mineral density. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 21–25. [Google Scholar] [CrossRef]
- Park, P.G.; Hong, C.R.; Kang, E.; Park, M.; Lee, H.; Kang, H.J.; Shin, H.Y.; Ha, I.S.; Cheong, H.I.; Yoon, H.J.; et al. Acute Kidney Injury in Pediatric Cancer Patients. J. Pediatr. 2019, 208, 243–250.e3. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garach, A.; Garcia-Fontana, B.; Munoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Hildebrand, G.K.; Kasi, A. Denosumab; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Temrikar, Z.H.; Suryawanshi, S.; Meibohm, B. Pharmacokinetics and Clinical Pharmacology of Monoclonal Antibodies in Pediatric Patients. Paediatr. Drugs 2020, 22, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Boyce, A.M. Denosumab: An Emerging Therapy in Pediatric Bone Disorders. Curr. Osteoporos. Rep. 2017, 15, 283–292. [Google Scholar] [CrossRef]
- Murphy-Alford, A.J.; White, M.; Lockwood, L.; Hallahan, A.; Davies, P.S.W. Body composition, dietary intake and physical activity of young survivors of childhood cancer. Clin. Nutr. 2019, 38, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.F.C.; Maia-Lemos, P.D.S.; Pisani, L.P. Nutritional Characteristics of the Diets of Child and Adolescent Cancer Survivors. J. Adolesc. Young Adult. Oncol. 2018, 7, 230–237. [Google Scholar] [CrossRef]
- Marcoux, S.; Drouin, S.; Laverdiere, C.; Alos, N.; Andelfinger, G.U.; Bertout, L.; Curnier, D.; Friedrich, M.G.; Kritikou, E.A.; Lefebvre, G.; et al. The PETALE study: Late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr. Blood Cancer 2017, 64, e26361. [Google Scholar] [CrossRef]
- Dieffenbach, B.V.; Liu, Q.; Murphy, A.J.; Stein, D.R.; Wu, N.; Madenci, A.L.; Leisenring, W.M.; Kadan-Lottick, N.S.; Christison-Lagay, E.R.; Goldsby, R.E.; et al. Late-onset kidney failure in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur. J. Cancer 2021, 155, 216–226. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocr. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Fouda, A.; Kandil, S.; Boujettif, K.; Fayea, N. Hypovitamininosis D in Childhood Cancer Survivors: Importance of Vitamin D Supplementation and Measurement Over Different Points of Time. J. Pediatr. Hematol. Oncol. 2018, 40, e83–e90. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Naciu, A.M.; Tabacco, G.; Manfrini, S.; Trimboli, P.; Vescini, F.; Falchetti, A. Calcium citrate: From biochemistry and physiology to clinical applications. Rev. Endocr. Metab. Disord. 2019, 20, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kalra, E.K. Nutraceutical—Definition and introduction. Aaps. Pharmsci. 2003, 5, E25. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chen, K.M.; Hassanshahi, M.; Tang, Q.; Howe, P.R.; Xian, C.J. Childhood cancer chemotherapy-induced bone damage: Pathobiology and protective effects of resveratrol and other nutraceuticals. Ann. N. Y. Acad. Sci. 2017, 1403, 109–117. [Google Scholar] [CrossRef]
- Feng, Y.L.; Jiang, X.T.; Ma, F.F.; Han, J.; Tang, X.L. Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. Int. J. Mol. Med. 2018, 41, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Shandala, T.; Soo, P.P.; Su, Y.W.; King, T.J.; Chen, K.M.; Howe, P.R.; Xian, C.J. Effects of Resveratrol Supplementation on Methotrexate Chemotherapy-Induced Bone Loss. Nutrients 2017, 9, 255. [Google Scholar] [CrossRef]
- King, T.J.; Shandala, T.; Lee, A.M.; Foster, B.K.; Chen, K.M.; Howe, P.R.; Xian, C.J. Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats. Int. J. Mol. Sci. 2015, 16, 18293–18311. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Zhang, F. Icariin promotes the proliferation and differentiation of osteoblasts from the rat mandible by the Wnt/betacatenin signalling pathway. Mol. Med. Rep. 2018, 18, 3445–3450. [Google Scholar] [CrossRef]
- Bielecki, J.E.; Tadi, P. Therapeutic Exercise; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chen, L.R.; Hou, P.H.; Chen, K.H. Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients 2019, 11, 2848. [Google Scholar] [CrossRef]
- Chaput, J.P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef]
- Zurcher, S.J.; Jung, R.; Monnerat, S.; Schindera, C.; Eser, P.; Meier, C.; Rueegg, C.S.; von der Weid, N.X.; Kriemler, S. High impact physical activity and bone health of lower extremities in childhood cancer survivors: A cross-sectional study of SURfit. Int. J. Cancer 2020, 147, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.; te Winkel, M.L.; van Beek, R.D.; de Muinck Keizer-Schrama, S.M.; Kemper, H.C.; Hop, W.C.; van den Heuvel-Eibrink, M.M.; Pieters, R. A randomized trial investigating an exercise program to prevent reduction of bone mineral density and impairment of motor performance during treatment for childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 53, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.A.; van Dulmen-den Broeder, E.; Kaspers, G.J. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, 3, CD008796. [Google Scholar] [CrossRef]
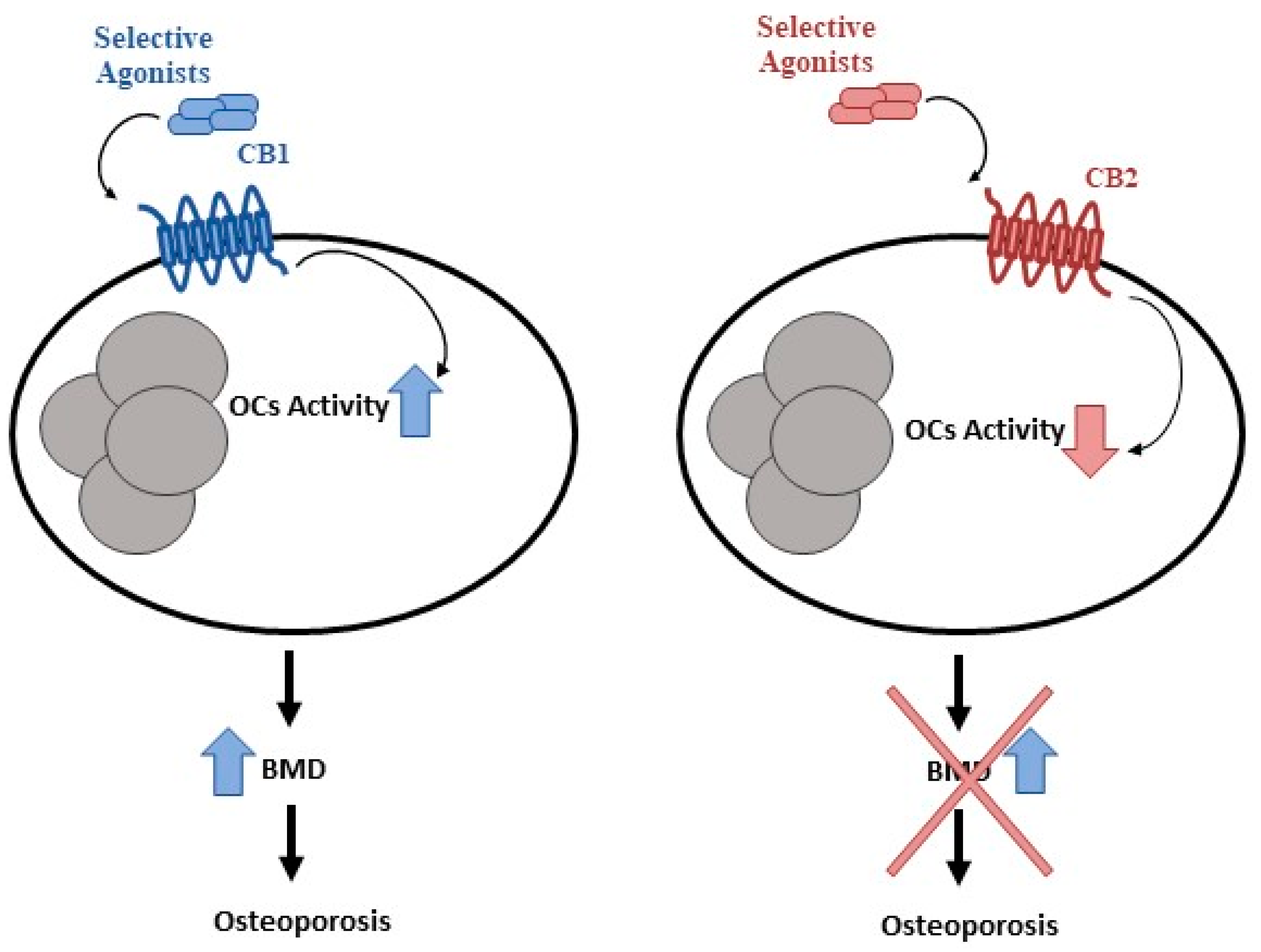
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; van’t Hof, R.J.; Ralston, S.H. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009, 10, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Tortora, C.; Bernardo, M.E.; Luongo, L.; Conforti, A.; Starc, N.; Manzo, I.; Nobili, B.; Locatelli, F.; et al. CB2 and TRPV1 receptors oppositely modulate in vitro human osteoblast activity. Pharmacol. Res. 2015, 99, 194–201. [Google Scholar] [CrossRef]
- Rossi, F.; Siniscalco, D.; Luongo, L.; De Petrocellis, L.; Bellini, G.; Petrosino, S.; Torella, M.; Santoro, C.; Nobili, B.; Perrotta, S.; et al. The endovanilloid/endocannabinoid system in human osteoclasts: Possible involvement in bone formation and resorption. Bone 2009, 44, 476–484. [Google Scholar] [CrossRef]
- Robin, F.; Cadiou, S.; Albert, J.D.; Bart, G.; Coiffier, G.; Guggenbuhl, P. Methotrexate osteopathy: Five cases and systematic literature review. Osteoporos. Int. 2021, 32, 225–232. [Google Scholar] [CrossRef]
- Raghu Nadhanan, R.; Fan, C.M.; Su, Y.W.; Howe, P.R.; Xian, C.J. Fish oil in comparison to folinic acid for protection against adverse effects of methotrexate chemotherapy on bone. J. Orthop. Res. 2014, 32, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.L.; Hudson, M.M. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef]
- Vuotto, S.C.; Krull, K.R.; Li, C.; Oeffinger, K.C.; Green, D.M.; Patel, S.K.; Srivastava, D.; Stovall, M.; Ness, K.K.; Armstrong, G.T.; et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2017, 123, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Ferguson, D.W.; Gill, J.; Paul, J.; Symonds, P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 721–732. [Google Scholar] [CrossRef]
- Hawkins, N.A.; Soman, A.; Lunsford, N.B.; Leadbetter, S.; Rodriguez, J.L. Use of Medications for Treating Anxiety and Depression in Cancer Survivors in the United States. J. Clin. Oncol. 2017, 35, 78–85. [Google Scholar] [CrossRef]
- Nipp, R.D.; Shui, A.M.; Perez, G.K.; Kirchhoff, A.C.; Peppercorn, J.M.; Moy, B.; Kuhlthau, K.; Park, E.R. Patterns in Health Care Access and Affordability Among Cancer Survivors During Implementation of the Affordable Care Act. JAMA Oncol. 2018, 4, 791–797. [Google Scholar] [CrossRef]
- de Boer, A.G.E.M.; Taskila, T.; Ojajarvi, A.; van Dijk, F.J.H.; Verbeek, J.H.A.M. Cancer Survivors and Unemployment A Meta-analysis and Meta-regression. JAMA-J. Am. Med. Assoc. 2009, 301, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.C.; Kuhlthau, K.; Pajolek, H.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Park, E.R. Employer-sponsored health insurance coverage limitations: Results from the Childhood Cancer Survivor Study. Supportive Care Cancer 2013, 21, 377–383. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Lawrence, W.F.; Clauser, S.; Davis, W.W.; Brown, M.L. Burden of illness in cancer survivors: Findings from a population-based national sample. JNCI-J. Natl. Cancer I 2004, 96, 1322–1330. [Google Scholar] [CrossRef] [PubMed]

| Type of Cancer | Risk Factor for Osteoporosis |
|---|---|
| Acute Lymphoblastic Leukemia (ALL) | ALL treatments (corticosteroids and methotrexate) |
| Lymphoma | Lymphoma treatments (prednisone, vincristine, procarbazine, low dose of corticosteroids, and mechlorethamine) |
| Brain Tumor | GH deficiency and craniospinal high doses of corticosteroids and alkylating agent administration |
| Neuroblastoma | High doses of therapy and hematopoietic stem cell transplantation (HSCT) |
| Osteosarcoma | Neoadjuvant chemotherapy, lean mass reduction, male sex, and young age at diagnosis |
| Ewing’s Sarcoma | Neoadjuvant chemotherapy |
| Wilm’s Tumor | Chemotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, F.; Tortora, C.; Paoletta, M.; Marrapodi, M.M.; Argenziano, M.; Di Paola, A.; Pota, E.; Di Pinto, D.; Di Martino, M.; Iolascon, G. Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives. Cancers 2022, 14, 4349. https://doi.org/10.3390/cancers14184349
Rossi F, Tortora C, Paoletta M, Marrapodi MM, Argenziano M, Di Paola A, Pota E, Di Pinto D, Di Martino M, Iolascon G. Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives. Cancers. 2022; 14(18):4349. https://doi.org/10.3390/cancers14184349
Chicago/Turabian StyleRossi, Francesca, Chiara Tortora, Marco Paoletta, Maria Maddalena Marrapodi, Maura Argenziano, Alessandra Di Paola, Elvira Pota, Daniela Di Pinto, Martina Di Martino, and Giovanni Iolascon. 2022. "Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives" Cancers 14, no. 18: 4349. https://doi.org/10.3390/cancers14184349






